Published online May 16, 2015. doi: 10.4253/wjge.v7.i5.481
Peer-review started: November 2, 2014
First decision: December 26, 2014
Revised: January 13, 2015
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: May 16, 2015
Processing time: 201 Days and 15.5 Hours
Over the past 30 years, the field of endoscopy has witnessed several advances. With the advent of endoscopic mucosal resection, removal of large mucosal lesions have become possible. Thereafter, endoscopic submucosal resection was refined, permitting en bloc removal of large superficial neoplasms. Such techniques have facilitated the development of antireflux mucosectomy, a promising novel treatment for gastroesophageal reflux. The introduction and use of over the scope clips has allowed for endoscopic closure of defects in the gastrointestinal tract, which were traditionally treated with surgical intervention. With the development of per-oral endoscopic myotomy (POEM), the treatment of achalasia and spastic disorders of the esophagus have been revolutionized. From the submucosal tunnelling technique developed for POEM, Per oral endoscopic tumor resection of subepithelial tumors was made possible. Simultaneously, advances in biotechnology have expanded esophageal stenting capabilities with the introduction of fully covered metal and plastic stents, as well as biodegradable stents. Once deemed a primarily diagnostic tool, endoscopy has quickly transcended to a minimally invasive intervention and therapeutic tool. These techniques are reviewed with regards to their application to benign disease of the esophagus.
Core tip: Antireflux mucosectomy is an endoscopic antireflux procedure showing promising results in patients with refractory gastroesophageal reflux. Over the scope clips and esophageal stents permit safe endoscopic closure of esophagogastric defects, decreasing the requirement for surgical intervention. Per-oral endoscopic myotomy allows the precise performance of endoscopic myotomy for the treatment of spastic esophageal motility disorders with the efficacy of a surgical myotomy without the associated surgical morbidity. Per-oral endoscopic tumor resection enables en bloc endoscopic removal of subepithelial tumors (SETs) and is both a diagnostic and therapeutic intervention for esophageal SETs. These techniques will expand the boundaries of therapeutic endoscopy, decrease the need for surgical intervention, and improve patient outcomes.
- Citation: Bechara R, Inoue H. Recent advancement of therapeutic endoscopy in the esophageal benign diseases. World J Gastrointest Endosc 2015; 7(5): 481-495
- URL: https://www.wjgnet.com/1948-5190/full/v7/i5/481.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i5.481
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal problems with an estimated increasing prevalence of over 25% in North America[1,2]. Consequently, it is a source of significant morbidity as well as considerable healthcare costs. In the United states alone, an estimated 9.3 billion dollars was incurred in direct healthcare cost as a result of GERD[3].
The standard surgical treatment for GERD is the Nissen fundoplication, where the fundus is wrapped around the lower esophagus to reinforce the lower esophageal sphincter (LES). This produces excellent short-term results and is generally safe with a post-operative complication rate of approximately 2%[4]. A recent multicenter randomized trial showed that there was no significant difference in symptom remissions at five years follow-up between oral esomeprazole therapy and laparoscopic Nissen fundoplication[5,6]. Studies with longer follow-up, have reported relapse rates of up to 50% at 12 years post-laparoscopic Nissen fundoplication[7,8]. Furthermore, reoperations in these patients has increased morbidity and relapse is still a possibility[9,10].
Recently, there has been great interest in pursuing endoscopic alternatives to laparoscopic antireflux surgery. There are three categories of such procedures; endoscopic devices for gastric plication, injection/implantable substances at the gastroesophageal junction (GEJ) and ablative therapies.
Endoscopic suturing devices allow plication 1-2 cm below the GEJ with the goal of reinforcing the LES, mimicking laparoscopic anti-reflux surgery. Depending on the device used, total procedure times vary from 30-60 min. However, due to safety, cost, and questionable long-term efficacy, many of these devices are no longer available. One currently available device is EsophyX® (EndoGastric Solutions, Washington, United States) which is marketed to deliver transoral incisionless fundoplication. Due to the fact that long-term efficacy data are not available, significant cost of the device, and the need to confirm safety and define optimal technique, it has not become widely used[11].
Injectable treatments where liquid chemical polymers are directly injected into the LES result in bulking and reinforcement of the natural barrier to reflux. These treatments demonstrated promising early results, but have been removed from the market due to safety concerns related to transmural injection resulting in mediastinits, pericarditis, and death[12-14].
Ablative therapy consists of thermal energy delivered to the GEJ, which results in tissue remodeling that provides reinforcement to the LES. Stretta® (Mederi Therapeutics Inc., Connecticut, United States) is a currently available device which delivers low radiofrequency energy. The Stretta device has been available in the United States since 2000 and has good safety data, contrary to many of the previously mentioned therapies. In short and mid-term follow-up, there is evidence of significant improvement in subjective and objective indicators of GERD. Long-term efficacy has not been consistently demonstrated with some series showing 60% of patients proceed to antireflux surgery, while other series have shown a more durable response[15-19].
Many of the studies on endoscopic antireflux procedures are limited to small single-center case series demonstrating good short-term improvement in symptoms. However, consistent long-term durable efficacy has not been shown, with the few randomized control studies failing to show improvement over sham control arms. Due to the lack of convincing evidence for adequate long-term symptom control, associated high-cost and some safety concerns endoscopic antireflux procedures have failed to become widely used.
With the introduction of strip biopsy by Tada et al[20] in 1984, endoscopic resection with local injection of hypertonic saline injection (ERHSE) by Hirao et al[21] 1988, cap EMR by Inoue et al[22] in 1990 and subsequent development of ESD in Japan, resection of superficial gastrointestinal neoplasia was revolutionized[20-24]. The safety and efficacy of EMR/ESD have been well reported and are now widely applied by endoscopists around the world[23-25]. A known complication of esophageal EMR/ESD, particularly when more than two-thirds circumferential, is stricture development[26-28]. The exact mechanism of stricture formation is unknown. However, from experimental models it has been shown to involve acute inflammation, angiogenesis, fibrous hyperplasia with replacement of the submucosa with dense collagen fibers, and ultimately, atrophy of the muscularis propria[29,30]. In 2003, Inoue et al[22] reported a case of circumferential EMR for short-segment Barretts with high-grade dysplasia that was found on endoscopy performed for objectively confirmed (24-h esophageal pH testing) reflux symptoms. A circumferential EMR was performed extending to include a 2 cm wide portion of the gastric cardia. It was hypothesized that this would improve the reflux symptoms by causing fibrosis at the gastric cardia resulting in reinforcement of the LES. As expected, excellent symptomatic and objective (normalization of 24-h esophageal pH testing) improvement resulted and the patient has remained off of PPI for over 10 years[31]. Then in 2014, Inoue et al[32] published a series of 10 patients that received the antireflux mucosectomy (ARMs) procedure for refractory GERD showing excellent results both subjectively and objectively.
Patients with GERD that are considered for ARMs are those without a large sliding hiatus hernia that have been objectively confirmed to be PPI refractory on 24-h esophageal pH testing. The presence of Barrett’s esophagus does not preclude the performance of ARMs.
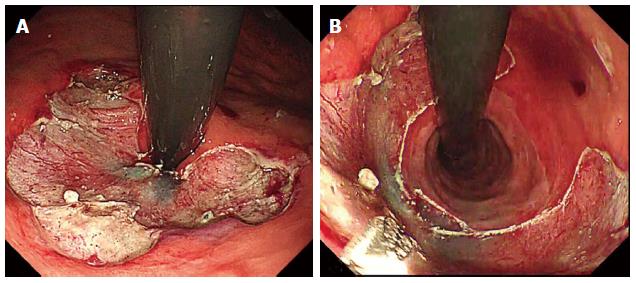
The ARMs procedure can be performed with ESD or EMR and is generally as follows: Step 1: Marking of area for mucosectomy. Mucosal reduction is planned along lesser curve of the gastric cardia in crescentic fashion (Figure 1A). When retroflexed in the stomach, the length of preserved mucosa on the side of the greater curve is estimated at twice the diameter of the endoscope (approximately 2 cm); Step 2: Submucosal injection. Both EMR and ESD can be used depending on the experience of the operator and the presence of mucosal lesions. Submucosal injection is made along the markings to ensure adequate lift to prevent deep injury or perforation; and Step 3: Mucosectomy. The mucosectomy is performed via EMR or ESD (Figure 1B).
In the first two cases of ARMs, circumferential mucosectomy was performed which resulted in stricture formation, however these were successfully treated with balloon dilation. Subsequently, all ARMS were performed in a hemi-circumferential or crescentic fashion that produced adequate fibrosis to alleviate GERD without stricture formation[32].
All patients had significant improvement in subjective and objective indicators of GERD. The DeMeester, heartburn and regurgitation scores all showed significant impressive improvement. Twenty-four hours esophageal pH testing showed the mean fraction of time at pH < 4 improved from 29.1% to 3.1%[32].
This series of ARMs showed promising safety and efficacy, however, the sample size was small, owing to the low incidence of GERD in Japan. Larger randomized sham-controlled studies with long-term follow-up are required to confirm these findings. Unique aspects of ARMs as an endoscopic treatment for GERD is that the safety of EMR/ESD has already been established, and endoscopists are already familiar with these techniques. These facts would allow ARMs to potentially be performed by most endoscopist with expertise in esophogastric EMR/ESD. In addition, there is no requirement for new, expensive specialized equipment. Thus, if future studies confirm the early promising results of ARMs, it has the potential to become a widely used endoscopic treatment for GERD, as it would meet the demands of safety, efficacy and cost-effectiveness.
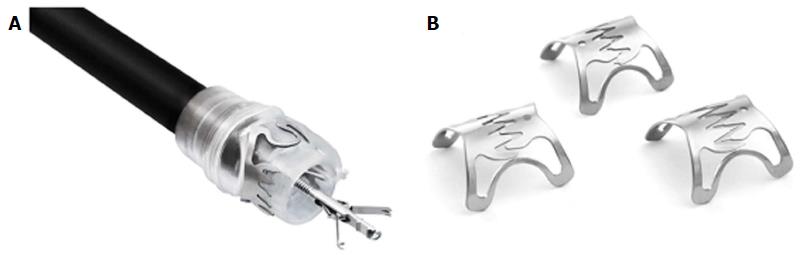
The over the scope clips (OTSCs) were initially introduced for closure of perforations and for mechanical hemostasis of complicated arterial bleeds of the gastrointestinal tract. The OTSC consist of a nitinol alloy with a similar shape to a bear trap. The clip, is preloaded on a clear applicator hood which is mounted onto the scope tip. The deployment system is analogous to that of a variceal banding device with the string running through the working channel of the endoscope and is fastened to a rotatable handle that is attached to the port of the working channel.
Specifically pertaining to the esophagus, the OTSC has successfully been used for refractory bleeds (non-variceal), closer of iatrogenic perforations, Boerhaave’s syndrome, anastomotic leaks, tracheaesophageal fistula and securing fully covered self-expandable metal stents (SEMS)[33-43].
After mounting of the OTSC, the target area is identified, suctioned into the hood and the clip is deployed bringing the tissue into apposition. Alternatively, one of the available graspers or anchor can be used, allowing for dimproved apposition of the defect and better visualization of the tissue prior to clip deployment (Figure 2). Once the clip is deployed a permanent closing force of 8-9 Newtons (N) is applied to the tissue without causing necrosis[43]. Depending on the indication, different teeth are available; rounded (type a, Figure 2B left) for atraumatic application, pointed (type t, Figure 2B middle) and long pointed (type gc, Figure 2B right) for more tissue apposition. Some of the challenges with the OTSC device are that it limits sharp angulation which can make maneuverability in the esophagus more challenging and the attached OTSC device slightly impairs the endoscopic view.
Complications with the OTSC have been uniformly rare in all the published series, the majority reporting no or few complications[33-41,43-52]. However, isolated cases of esophageal perforation, inadvertent tongue piercing and intestinal obstruction (from accidental inclusion of opposing walls into the OTSC) have been reported[44,51,53].
The OTSC device has been shown to be safe and effective for refractory arterial GI bleeding and closure of iatrogenic perforations 20 mm and smaller[47,51]. The successful closure of anastomotic leaks and fistulas in case series has been largely favorable, but efficacy has varied widely between 38%-100% in published series, due to heterogeneity of cases, series size and operators experience[36-38,40-43,45,48-51,54,55]. However, two recent meta-analysis showed success rates of 80%-100% for both perforation and fistula closure, with failure usually associated with chronic fibrotic fistulas[52,56]. Most recently the European Society of Gastrointestinal Endoscopy released its position statement on iatrogenic endoscopic perforations and endorsed the use of the OTSC device for closure of iatrogenic esophageal perforations[57].
Multiple studies have reported that the OTSC device has good clinical efficacy for closure of esophageal, perforations, fistula and anastomotic leaks with few complications. Depending on the expertise available the OTSC device can be considered an early treatment option for esophageal perforation, leaks and fistula.
Achalasia is an esophageal motor disorder resulting from inhibitory neuron dysfunction causing loss of peristalsis and impaired LES relaxation. This leads to impaired food bolus propulsion and stasis in the esophagus. Patients may experience dysphagia, regurgitation, chest pain, weight loss and heartburn[58-60]. The conventional treatments are laparoscopic Heller myotomy (LHM) and pneumatic dilation (PD). The first account of an endoscopic myotomy dates back to 1980 by Ortega et al[61] in Venezuela, where they described two 1cm long myotomies to a depth of 3 mm performed at the LES in 17 patients. In 1997, Pasricha et al[62] in the United States, described an experimental technique on a bovine model, where a mucosal incision was made five centimetres above the GEJ and a balloon was placed into the submucosal space to create a tunnel down to the GEJ, where a myotomy of the circular muscle was performed[62]. In 2010, Inoue et al[32] in Japan modified the endoscopic myotomy procedure such that it permitted safe and effective human application. Since the introduction of POEM, there has been an dramatic increase in POEM studies and the procedure is now being performed worldwide.
Currently, there are no universal guidelines for the indication of POEM. It is the opinion of the authors of this review that with the reported efficacy and safety from our center, that POEM can be considered a first line treatment for achalasia. POEM has been safety performed in patients with previous PD, LHM, Botox injection, and even previous POEM. In our center, it has also been safely performed in patients with type 1 and type 2 sigmoid achalasia as well as octogenarians. Other motility disorders such as diffuse esophageal spasm (DES), nutcracker esophagus, Jack-hammer esophagus, and hypertensive LES have also been successfully and safely treated with POEM.
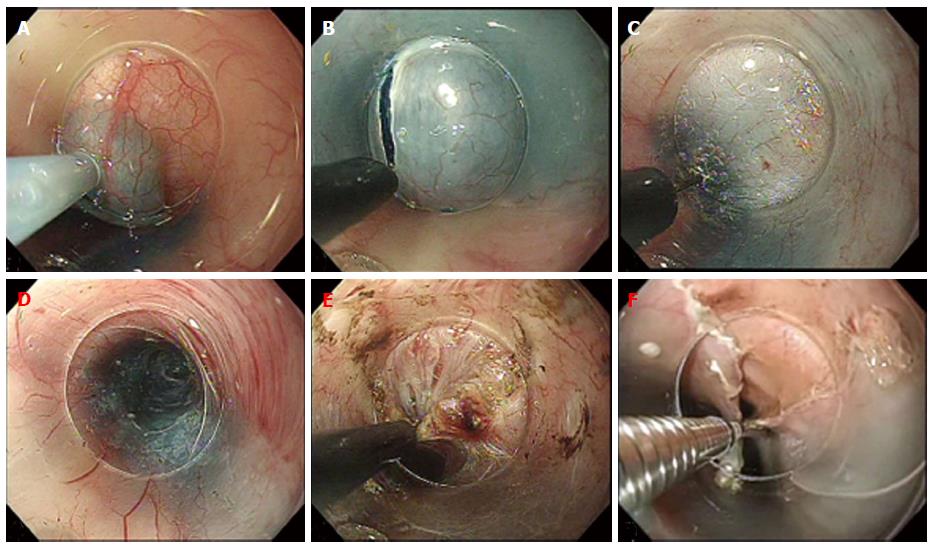
The first successful case of POEM in a human was performed September, 2008 by Haruhiro Inoue. Since then, it has been widely accepted and performed with many slight variations to the original technique. The procedure as performed at our center is as follows (Figure 3): Step 1: Submucosal Injection and Incision. After the area of mucosal incision is chosen (approximately 13 cm above the GEJ for standard myotomy) 10 cc of saline with indigocarmine is injected into the submucosa and a 1.5-2 cm longitudinal incision is made with a triangle-tip knife (KD-640 L; Olympus). To avoid mucosal injury, the submucosal tunnel is dissected as close as possible to the circular muscle; Step 2: Creation of the submucosal tunnel. After enough space is created in the submucosa, mucosal entry is achieved and the tunnel is carefully extended down to the gastric side for approximately 3 cm; Step 3: Endoscopic myotomy. The circular muscle fibers are carefully dissected with the Triangle tip knife. When there is no abnormal contraction of the esophageal body or symptoms of chest pain, the standard myotomy is 8-10 cm; and Step 4: Closure of Mucosal entry. After completion of the myotomy and good hemostasis is confirmed, prophylactic antibiotic is instilled into submucosal tunnel and the mucosal entry site is clipped closed.
The main technical limitation to the performance of POEM is the presence of severe submucosal fibrosis which limits the ability to safety perform the submucosal tunnel and can occur when patients have had severe esophagitis, multiple previous endoscopic treatments, extensive esophageal EMR/ESD in the POEM field or radiation therapy.
Complications include; capnomediastinum, capnoperitoneum, intraprocedural and delayed bleeding, mucosal laceration/ischemia and GERD. The vast majority of complications reported have been treated conservatively and there have been no mortalities reported or requirement for conversion to open surgical procedure[63-73]. The most robust data comes from the international POEM survey (iPOEMS) database, reporting major complications occurred in 3.2% of 841 cases[74] which were treated conservatively without sequelae. In comparison, the large European trial comparing PD and LHM showed a 4% perforation rate for PD and a 12% rate of mucosal tear for LHM[75].
There is heterogeneity in reporting and classification of complications, partially accounting for the variability in reported complication rates (Table 1). Therefore, a standardized, internationally agreed upon adverse event reporting system for POEM is required. However, it is important to note that all the reported complications have been treated successfully endoscopically, with needle decompression or conservative management without any significant sequelae.
| Ref. | Country | No. of patients | Success rate (%) | Complications (%) | Mean follow-up (mo) |
| inoue et al[82] 2010a | Japan | 17 | 100 | 0 | 5 |
| von Renteln et al[79] 2012 | Germany | 16 | 94 | 12.5 | 3 |
| Swanstrom et al[67] 2012 | United States | 18 | 100 | 16.7 | 11 |
| Ren et al[85] 2012 | China | 119 | 94 | 55 | 3 |
| Costamagna et al[65] 2012 | Italy | 11 | 100 | 0 | 3 |
| Lee et al[66] 2013 | South Korea | 13 | 100 | 0 | 7 |
| Hungness et al[76] 2013 | United States | 18 | 89 | 22 | 6 |
| Teitelbaum et al[77] 2013 | United States | 12 | 100 | NR | 9 |
| Zhou et al[83] 2013b | China | 12 | 92 | 16.7 | 10 |
| Von Renteln et al[64] 2013c | International | 70 | 82.4 | 14.3 | 12 |
| Sharata et al[84] 2013d | United States | 31 | 100 | 12.5 | 6 |
| Freidel et al[68] 2013 | United States | 45 | 95 | 33 | 3 |
| Inoue et al[80] 2013 | Japan | 300 | 100 | 6 | 12 |
| Sharata et al[73] 2014 | United States | 75 | 98 | 11 | 16 |
| Bhayani et al[78] 2014 | United States | 37 | 100 | 13.5 | 6 |
| Minami et al[63] 2014 | Japan | 28 | 96 | 0 | 3 |
POEM is now being performed globally with excellent clinical results, with patients showing improvement of mean Eckardt scores from 5.4-8.8 pre-POEM to 0.4-1.7 post-POEM[63-68,76-81]. In addition, many series have reported decreases in LES pressure and barium column height[63-67,76-79,82]. Success rates, defined by a post-POEM Eckardt score ≤ 3, are summarized in Table 1. Multiple comparative studies have shown that POEM is at least as effective as LHM with shorter hospital stay and decreased post-procedure pain[76-78].
POEM has also been shown to be effective in patients with previous LHM. Zhou et al[83] reported a mean improvement in Eckardt score of 9.2 to 1.3, and Onimaru et al[81] reported a mean improvement in Eckardt score of 6.5 to 1.1. Patients who have failed Botox injections or PD have also seen comparable improvements post POEM[84].
Generally, other spastic disorders of the esophagus that have been treated surgically require a longer myotomy necessitating thoracotomy. This is another advantage of POEM, where a long myotomy can be performed without increased invasiveness or complications. From the iPOEMS database, the POEM procedure was performed in 25 DES patients, 106 Nutcracker patients, and 58 Hypertensive LES (HTLES) patients. Compared to achalasia, POEM was equally effective in Nutcracker esophagus and HTLES, but less effective for DES[74]. In the recent series by Sharata et al[73] that included 12 Nutcracker esophagus, 5 DES, and 8 HTLES patients, complete dysphagia relief was achieved in 70.8% of non-achalasia cases, while chest pain was relieved in 91.5%[73]. There are also two case reports demonstrating successful application of POEM for Jackhammer esophagus[86,87].
In our center, the majority of POEM cases were performed at 2 o’clock (anterior-lesser curve) or 5 o’clock (posterior-lesser curve) positions. In some cases, previous procedures such as LHM, POEM, or ESD (for esophageal lesion) had been performed, precluding safe submucosal tunnelling in the normal location and alternate positions were used. At present there are no studies to guide which site of standard myotomy is most optimal. This will hopefully be addressed with a large multicenter, randomized trial in the near future.
A selective circular muscle myotomy is normally performed in our center. Nevertheless, some centers prefer a full thickness myotomy. Li et al[88] compared full thickness myotomy with selective circular muscle myotomy and found no difference in either efficacy or adverse events. However, shorter operative times are observed with full thickness myotomy[88]. Until there is more evidence, we suggest an isolated circular myotomy to prevent potential damage to adjacent structures and.
Over 2000 POEM procedures have been performed worldwide. Most of the of the studies show excellent efficacy with low rate of major complications, all of which have been managed without sequelae. There is also growing evidence for the use of POEM for other spastic disorders of esophagus. Over time, POEM may arguably become the standard of care for achalasia and other spastic disorders of the esophagus.
Subepithelial tumors (SETs) of the upper gastrointestinal tract are generally uncommon with an incidence of about 0.4% of all routine esophagogastric endoscopic examinations[89]. Gastric SETs have a malignancy rate of approximately 50%, in contrast, esophageal SETs are usually benign leiomyomas and only 1%-3% harbor malignancy[89-91]. Generally, SETs are asymptomatic and found incidentally on endoscopic or radiologic examination for unrelated symptoms or screening. However, larger SETs can cause dysphagia, chest pain, regurgitation and bleeding[92,93]. Traditionally, excision of symptomatic SETs has been performed with open surgical, laparoscopic or thoracoscopic techniques. These procedures are invasive, associated with significant health-care cost and morbidity[94-96]. In addition, if the lesion in question is benign it is difficult to justify surgical excision with associated surgical morbidity. With the introduction of POEM, the submucosal tunnelling technique has been subsequently applied for Per-oral endoscopic tumor (POET) resection by Inoue et al[97] in 2012. The technique has allowed SETs to be removed from the esophagus and gastric cardia, safety and effectively. Since its first description, multiple series have been published supporting its safety and efficacy.
Most of the SETs removed via POET have been benign. The presumptive diagnoses were made using a combination of endoscopy, endoscopic ultrasound (EUS) and CT scan. Indications for resection were presence of symptoms, enlarging tumor or unclear diagnosis in which resection was diagnostic.
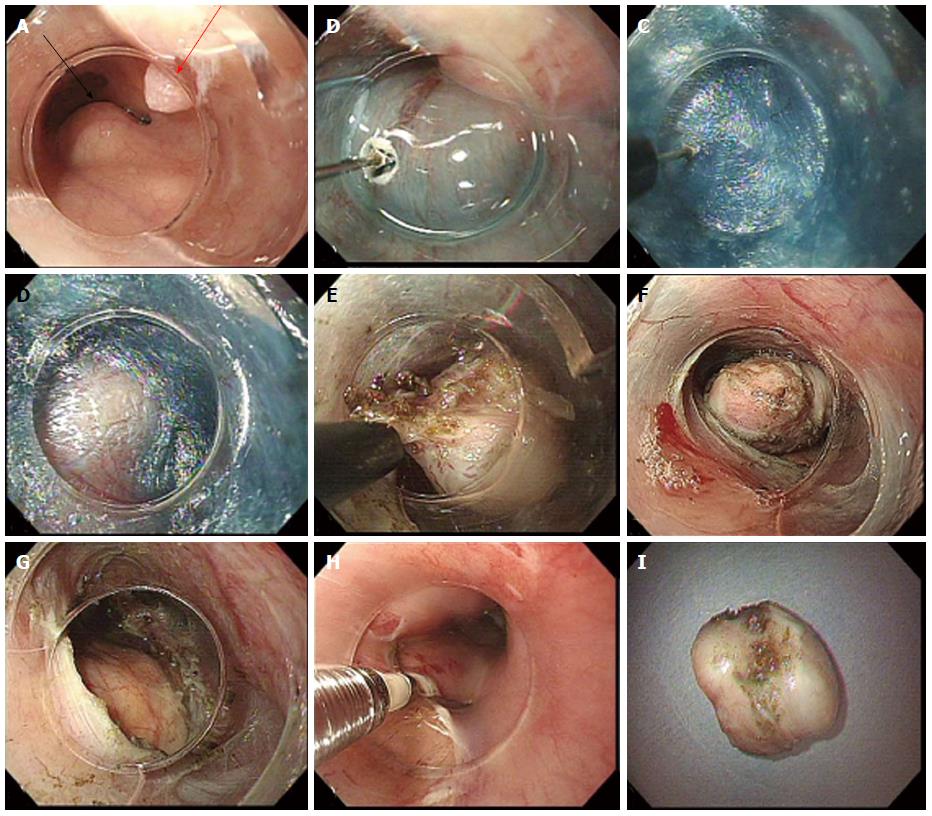
An essential part of POET (and POEM) is use of low flow carbon dioxide insufflation to prevent complication from barotrauma as noted by Wang et al[98], where air insufflation was used in the first half of their series, which resulted in high rates subcutaneous emphysema, pneumothorax and pneumomediastinum. Subsequently, they used carbon dioxide insufflation for the remaining cases and did not have further adverse events related to insufflation[98]. The POET technique can be summarized as follows with the various steps shown in Figure 4: Step 1: Submucosal Injection and Incision. The area of mucosal incision is generally 5 cm proximal to the tumor and is made as described for POEM; Step 2: Creation of the submucosal tunnel. The submucosal tunnel is extended 1-2 cm distal to the tumor to ensure sufficient working space for the dissection of the tumour; Step 3: Tumour Resection. Once the mass is identified and the tunnel is sufficient, resection of the tumor can proceed. Careful dissection of the mass from the muscular layer should be performed to prevent rupture of capsule or perforation of the overlying mucosa. Tumors that extend to the deep muscular layer can be removed with a full thickness resection of the circular and longitudinal muscles. The free tumor can be withdrawn through the mucosal incision using a snare, grasping forceps or suctioning into the transparent hood; and Step 4: Closure of Mucosal entry. The tunnel is re-examined to confirm adequate hemostasis and the mucosal incision is closed with endoscopic clips. There are also reports of using endoscopic staples, OTSCs, as well as covered metal stents to seal the mucosal incision site[99-102].
Patients are managed analogous to post-POEM patients. Patients are kept nil per os for 24 h. Day 1 post-procedure the patient has an endoscopy as well as a contrast study to check for leak. Some centers perform routine post-procedure CT scan to check for insufflation related complications and perforation[103]. The patient’s diet is advanced to clear liquids day-1 post-procedure, and advanced to regular diet by day 4 if asymptomatic. Endoscopy and endoscopic ultrasound are generally performed for follow-up on patients that underwent POET resection. If the lesion removed is malignant or with malignant potential, closer follow-up is performed and includes a CT scan to assess for tumor recurrence and the occurrence of distant metastasis[98,104].
Almost all of the reported complications have been insufflation related (subcutaneous emphysema, pneumoperitoneum and pneumomediastinum). All were managed with decompression or conservatively without sequelae. Analogous to POEM series, there is variability in reporting and classification of complications.
Nearly all series report 100% successful resection (refer to Table 2). With almost all being en bloc with intact capsule. A complete resection refers to an en bloc resection of the tumor with intact capsule. This factor is important to prevent seeding especially if the pre-procedure diagnosis is suggestive of a malignant or pre-malignant lesion. The limiting factor for resection of SETs via POET is size. The largest SET removed to date was 60 mm × 28 mm × 22 mm[100]. The tumor (known to be a leiomyoma) required fragmentation to be extracted. In addition, the mucosal incision could not be closed and necessitated placement of fully covered SEMS. Anecdotally, it appears that the upper limit for a complete resection is 4-5 cm depending if the shape of the tumor allows for extraction through the mucosal incision site. The efficacy data is summarized in Table 2[97-99,105-111] below.
| Ref. | Country | No. of patients | Mean tumor size (mm) | Complete resectionb (%) | Piecemeal or disrupted capsule (%) | Complications(%) |
| Inoue et al[97] 2011a | Japan | 9 | 29.4 | 100 (7/9) | 0 | 0 |
| Cai et al[105] 2012 | China | 1 | 20 | 100 | NS | 100 |
| Gong et al[106] 2012 | China | 12 | 19.5 | 83.3 (10/12) | 16.7 (2/12) | 16.7 |
| Xu et al[107] 2012 | China | 15 | 19 | 100 | 0 | 13.3 |
| Liu et al[103] 2013 | China | 12 | 18.5 | 100 | 0 | 66.7 |
| Xu et al[108] 2013 | China | 23 | 21 | 100 | 0 | 39 |
| Wang et al[99] 2013 | China | 18 | 33 | NS | NS | 16.7 |
| Chen et al[109] 2014 | China | 1 | #1 = 25 #2 = 30 | 100 | 0 | 0 |
| Kumbhari et al[100] 2014 | United States | 1 | 60 | 0 | 100 | NS |
| Lu et al[110] 2014 | China | 42 | 12.1 | 97.7 (44/45) | 2.3 (1/45) | 15.6 |
| Ye et al[104] 2014 | China | 85 | 19.2 | 100 | 0 | 9.4 |
| Wang et al[98] 2014 | China | 57 | 21.5 | 100 | 0 | 21 |
| Lu et al[111] 2014c | China | 18 | 21 | 100 | 0 | 11.1 |
Subepithelial tumors of the esophagus and cardia are usually incidental findings on endoscopic or radiologic examinations for unrelated symptoms, with the majority being benign. With the moderate yield of EUS, morbidity and costs and surgical resection, a minimally invasive diagnostic and therapeutic procedure is required for the management of SETs. The careful performance of POET is effective and safe, and with continued supportive evidence will likely be performed with increased frequency for resection of most esophageal and gastric cardia SETs, with surgical resection reserved for very large or malignant SETs.
When first introduced in 1959, esophageal stents were placed intra-operatively and were indicated only for palliation of dysphagia for non-operable malignant strictures[112]. Endoscopic stents were subsequently introduced in 1977, but were plagued with high complication rates[113]. Since then, SEMS have become widely used for palliation of dysphagia for non-operable malignant esophageal strictures with good safety, efficacy, and cost effectiveness data[114,115]. With the success of SEMS for malignant esophageal disease, there was an effort to expand the use of uncovered/partially covered SEMSS for the use of benign esophageal disease. However, it was found early on that SEMS resulted in increased complications when used for benign disease. Such complications included migration, tissue ingrowth, stent induced stenosis, development of tracheoesogeal fistula, and hemorrhage[116-118].
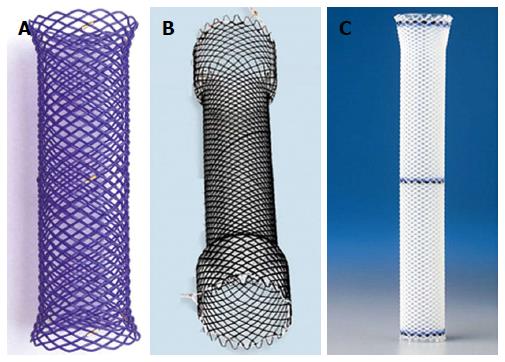
With the hope to ameliorate the serious issues encountered with SEMS when used for benign disease, manufacturers introduced the fully covered self-expandable metal stents (FCSEMS), fully covered self-expandable plastic stents (SEPS) and biodegradable stents (BDS).
For patients with iatrogenic perforations, tracheoesophgeal fistula, and/or surgical interventions complicated by anastomotic leaks, the treatment has traditionally been surgical. However, with the advent of FCSEMS and fully covered SEPS, these have been increasingly used as means to prevent reoperation and to allow healing to take place. Another emerging use is for refractory benign esophageal strictures in which traditional management with dilation has failed.
There are currently a variety of stents available depending on the country. Below is a brief summary (Table 3) of the general differences between the FCSEMS, SEPS and BDS with focus on benign esophageal strictures. Examples of each group are shown in Figure 5.
| Stent type | Advantages | Disadvantages |
| FCSEMS | No requirement for pre-dilation Recapture is possible | Expensive High migration risk Increased tissue hyperplasia |
| SEPS | Cheaper than other covered stents Decreased tissue hyperplasia | High migration risk (potentially more than FCSEMS) Require manual loading Require pre-dilation |
| BDS | No need to remove Less migration risk | Expensive Increased risk of post-procedure pain Require manual loading Require pre-dilation |
Once the stricture has been deemed refractory and stenting is considered, or a defect requires closure, then the choice of stent depends on the position and length of stricture/defect and preference of the endoscopist. The length of the stent should be at least about 3-4 cm longer the stricture/defect. The endoscopist should carefully assess the stricture/defect noting the proximal and distal margins, the distance from the upper esophageal sphincter and LESs. The stricture/defect should be greater than 2 cm distal from the upper esophageal sphincter, as if this distance is less it increase the risk of of pain, globus sensation, aspiration pneumonia or development of tracheoesophageal fistula. If the stent is to be deployed across the LES, a stent with an antireflux valve can be considered if available. Once the location of stent placement is chosen, the proximal and distal margins can be marked endoscopically (submucosal injection of radiopaque substance or placement of clips), by specific anatomic landmarks under X-ray or placement of radiopaque markers on the patient. If simultaneous endoscopic visualization is desired, an ultra-slim scope can be used transnasally. Under fluoroscopic control, the stent is deployed keeping adequate margins on both sides. Endoscopic clips, OTSCs or an endoscopic suturing device can used to decrease the risk of stent migration[34,119-122]. After deployment, the stents will radially expand and shorten reaching their final form.
Efficacy is defined as technical and clinical success. Technical success is defined as successful deployment of the stent and clinical success is the achievement of the intended clinical outcome (improvement in dysphagia, closure and healing of defect). FCSEMS and fully covered SEPS show excellent technical and good clinical efficacy for the closure of benign gastrointestinal disruptions with a technical success of 91% and clinical success of 81%[123]. In the cases where only partial closure fully covered achieved, surgical reinvention is still often avoided[123].
Unfortunately, for benign strictures, the clinical efficacy of FCSEMS and fully covered SEPS is less promising than for benign disruptions with a range of clinical success of 40%-50%[124,125]. Biodegradable stents were introduced with the hopes of improving the shortcomings of modest clinical efficacy of FCSEMS and fully covered SEPS. Unfortunately, the clinical efficacy of BDS has not differed significantly compared to its predecessors, with a mean clinical success rate of 47%[126]. However, in the pediatric population, with the use of custom made plastic stents higher efficacy has been demonstrated. Also, with the stents fastened to a nasogastric tube with an external silicon bar at the naris to avoid distal migration, much lower migration rates have been observed[127,128].
FCSEMS and fully covered SEPS have a modest complication rate, with the most common being stent migration at about 25%-30% with some evidence that the risk of migration is higher with SEPS[129,130]. The risk of migration may also be higher for proximal and anastomotic strictures[131]. Other rare complications of FCSEMS and fully covered SEPS include perforation, tissue hyperplasia, stent induced strictures, hemorrhage, and post-procedure pain. A very rare but dreaded complication is the development of an aortoenteric fistula, which is usually fatal[132-134]. BDS have a lower risk of migration of about 20% and fewer complications overall, but may have increased post-procedure pain[126,135,136].
There is mounting evidence for the efficacy of FCSEMS and fully covered SEPS for closure benign gastrointestinal disruptions with a moderate risk of stent migration. For refractory strictures, the efficacy is less promising likely owing to varying techniques, heterogeneity of patients and the severity of stricture pathology being treated. Depending on the individual case and the experience of the endoscopist, FCSEMS, fully covered SEPS, and BDS are potential options for select patients with refractory strictures. The particular choice of stent depends on the endoscopists preference and experience, perceived risk or migration, tissue hyperplasia and other complications. Hopefully with improvement in stent design, refinement in technique and patient selection, there will be improved clinical efficacy and safety for stents used for benign esophageal strictures.
Endoscopy has drastically advanced from being primarily a diagnostic tool to becoming the favored modality for treatment of benign disease of the esophagus. Promising efficacy and safety data of POEM and POET is accumulating, and with careful application, these procedures may soon be heralded as the standard of care for various diseases. Despite being a novel procedure, there is extensive experience with the technique used in ARMs in the setting of EMR/ESD. With the early promising results of ARMs, it has the potential to become a prominent treatment of GERD if efficacy confirmed by larger randomized control trials. OTSC usage is becoming widespread and has a remarkably low complication rate with good efficacy in facilitating the closure of esophageal perforations, fistula, and leaks. At present, the evidence for treatment of benign esophageal disruptions is promising and FCSEMS and SEPS should be considered in their treatment. However, for benign esophageal strictures the evidence for the use of FCSEMS, fully covered SEPS and BDS has been conflicting, but with further improvement in stent design and refinement of technique, there is potential for improved clinical efficacy.
With the ongoing introduction of novel procedures and equipment, it is critical that patient safety remain the top priority. International collaboration in the form of large multi-centered trials provide the opportunity to optimally study safety and clinical efficacy of newly introduced equipment and techniques.
P- Reviewer: Aly EH, Chung JW, Kamberoglou D, Stephenne X, Syam AF S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1263] [Article Influence: 114.8] [Reference Citation Analysis (2)] |
| 3. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1037] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 4. | Telem DA, Altieri M, Gracia G, Pryor AD. Perioperative outcome of esophageal fundoplication for gastroesophageal reflux disease in obese and morbidly obese patients. Am J Surg. 2014;208:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Attwood SE, Lundell L, Ell C, Galmiche JP, Hatlebakk J, Fiocca R, Lind T, Eklund S, Junghard O. Standardization of surgical technique in antireflux surgery: the LOTUS Trial experience. World J Surg. 2008;32:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Långström G, Lind T, Lundell L. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 7. | Kellokumpu I, Voutilainen M, Haglund C, Färkkilä M, Roberts PJ, Kautiainen H. Quality of life following laparoscopic Nissen fundoplication: assessing short-term and long-term outcomes. World J Gastroenterol. 2013;19:3810-3818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (5)] |
| 8. | Lundell L, Miettinen P, Myrvold HE, Hatlebakk JG, Wallin L, Engström C, Julkunen R, Montgomery M, Malm A, Lind T. Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clin Gastroenterol Hepatol. 2009;7:1292-1298; quiz 1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Makdisi G, Nichols FC, Cassivi SD, Wigle DA, Shen KR, Allen MS, Deschamps C. Laparoscopic repair for failed antireflux procedures. Ann Thorac Surg. 2014;98:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Wakeam E, Wee J, Lebenthal A, Ali SO, Gilbert RJ, Bueno R. Does BMI predict recurrence or complications after reoperative reflux surgery? Review of a single center’s experience and a comparison of outcomes. J Gastrointest Surg. 2014;18:1965-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Auyang ED, Carter P, Rauth T, Fanelli RD. SAGES clinical spotlight review: endoluminal treatments for gastroesophageal reflux disease (GERD). Surg Endosc. 2013;27:2658-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Wong RF, Davis TV, Peterson KA. Complications involving the mediastinum after injection of Enteryx for GERD. Gastrointest Endosc. 2005;61:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Cicala M, Gabbrielli A, Emerenziani S, Guarino MP, Ribolsi M, Caviglia R, Costamagna G. Effect of endoscopic augmentation of the lower oesophageal sphincter (Gatekeeper reflux repair system) on intraoesophageal dynamic characteristics of acid reflux. Gut. 2005;54:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Alzahrani A, Anvari M, Dallemagne B, Mutter D, Marescaux J. Surgical approach after failed enteryx injection for GERD. JSLS. 2007;11:97-100. [PubMed] |
| 15. | Perry KA, Banerjee A, Melvin WS. Radiofrequency energy delivery to the lower esophageal sphincter reduces esophageal acid exposure and improves GERD symptoms: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Dundon JM, Davis SS, Hazey JW, Narula V, Muscarella P, Melvin WS. Radiofrequency energy delivery to the lower esophageal sphincter (Stretta procedure) does not provide long-term symptom control. Surg Innov. 2008;15:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Aziz AM, El-Khayat HR, Sadek A, Mattar SG, McNulty G, Kongkam P, Guda MF, Lehman GA. A prospective randomized trial of sham, single-dose Stretta, and double-dose Stretta for the treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Arts J, Bisschops R, Blondeau K, Farré R, Vos R, Holvoet L, Caenepeel P, Lerut A, Tack J. A double-blind sham-controlled study of the effect of radiofrequency energy on symptoms and distensibility of the gastro-esophageal junction in GERD. Am J Gastroenterol. 2012;107:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Dughera L, Rotondano G, De Cento M, Cassolino P, Cisarò F. Durability of Stretta Radiofrequency Treatment for GERD: Results of an 8-Year Follow-Up. Gastroenterol Res Pract. 2014;2014:531907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Tada MSM, Murakami F, Shimada M, Mizumachi MAT, Yanai H, Oka S, Shigeeda M, Ogino M, Aibe TOY, Takemoto T. Development of the strip-off biopsy. Gastroenterol Endosc. 1984;26:833-839. [DOI] [Full Text] |
| 21. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 265] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Inoue H, Endo M. Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc. 1990;4:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Repici A, Hassan C, De Paula Pessoa D, Pagano N, Arezzo A, Zullo A, Lorenzetti R, Marmo R. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012;44:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014;59:1862-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Qumseya B, Panossian AM, Rizk C, Cangemi D, Wolfsen C, Raimondo M, Woodward T, Wallace MB, Wolfsen H. Predictors of esophageal stricture formation post endoscopic mucosal resection. Clin Endosc. 2014;47:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EG, Hayee B, Igarashi K, Yoshida A, Ikeda H, Onimaru M. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, Sasaki R, Namiki H, Okano T, Yamamoto M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582-588.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 30. | Honda M, Nakamura T, Hori Y, Shionoya Y, Nakada A, Sato T, Yamamoto K, Kobayashi T, Shimada H, Kida N. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy. 2010;42:1092-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Satodate H, Inoue H, Yoshida T, Usui S, Iwashita M, Fukami N, Shiokawa A, Kudo SE. Circumferential EMR of carcinoma arising in Barrett’s esophagus: case report. Gastrointest Endosc. 2003;58:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Inoue H, Ito H, Ikeda H, Sato C, Sato H, Phalanusitthepha C, Hayee B, Eleftheriadis N, Kudo SE. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol. 2014;27:346-351. [PubMed] |
| 33. | Kobara H, Mori H, Rafiq K, Fujihara S, Nishiyama N, Kato K, Oryu M, Tani J, Miyoshi H, Masaki T. Successful endoscopic treatment of Boerhaave syndrome using an over-the-scope clip. Endoscopy. 2014;46 Suppl 1 UCTN:E82-E83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Irani S, Baron TH, Gluck M, Gan I, Ross AS, Kozarek RA. Preventing migration of fully covered esophageal stents with an over-the-scope clip device (with videos). Gastrointest Endosc. 2014;79:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Zhang J, Samarasena JB, Milliken J, Lee JG. Large esophageal fistula closure using an over-the-scope clip: two unique cases. Ann Thorac Surg. 2013;96:2214-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Law R, Wong Kee Song LM, Irani S, Baron TH. Immediate technical and delayed clinical outcome of fistula closure using an over-the-scope clip device. Surg Endosc. 2014;Oct 3; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Pohl J, Borgulya M, Lorenz D, Ell C. Endoscopic closure of postoperative esophageal leaks with a novel over-the-scope clip system. Endoscopy. 2010;42:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Seebach L, Bauerfeind P, Gubler C. “Sparing the surgeon”: clinical experience with over-the-scope clips for gastrointestinal perforation. Endoscopy. 2010;42:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Zolotarevsky E, Kwon Y, Bains M, Schattner M. Esophagobronchial fistula closure using a novel endoscopic over-the-scope-clip. Ann Thorac Surg. 2012;94:e69-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Traina M, Curcio G, Tarantino I, Soresi S, Barresi L, Vitulo P, Gridelli B. New endoscopic over-the-scope clip system for closure of a chronic tracheoesophageal fistula. Endoscopy. 2010;42 Suppl 2:E54-E55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest Endosc. 2010;72:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc. 2012;76:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 45. | Conio M, Blanchi S, Repici A, Bastardini R, Marinari GM. Use of an over-the-scope clip for endoscopic sealing of a gastric fistula after sleeve gastrectomy. Endoscopy. 2010;42 Suppl 2:E71-E72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Iacopini F, Di Lorenzo N, Altorio F, Schurr MO, Scozzarro A. Over-the-scope clip closure of two chronic fistulas after gastric band penetration. World J Gastroenterol. 2010;16:1665-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Manta R, Galloro G, Mangiavillano B, Conigliaro R, Pasquale L, Arezzo A, Masci E, Bassotti G, Frazzoni M. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endosc. 2013;27:3162-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752-2760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Parodi A, Repici A, Pedroni A, Blanchi S, Conio M. Endoscopic management of GI perforations with a new over-the-scope clip device (with videos). Gastrointest Endosc. 2010;72:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Repici A, Arezzo A, De Caro G, Morino M, Pagano N, Rando G, Romeo F, Del Conte G, Danese S, Malesci A. Clinical experience with a new endoscopic over-the-scope clip system for use in the GI tract. Dig Liver Dis. 2009;41:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 53. | Mangiavillano B, Morandi E, Masci E. Accidental endoscopic piercing of the tongue with an Ovesco clip. Endoscopy. 2012;44 Suppl 2 UCTN:E221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Coriat R, Leblanc S, Pommaret E, Berretta O, Prat F, Chaussade S. Endoscopic management of endoscopic submucosal dissection perforations: a new over-the-scope clip device. Gastrointest Endosc. 2011;73:1067-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Sarker S, Gutierrez JP, Council L, Brazelton JD, Kyanam Kabir Baig KR, Mönkemüller K. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy. 2014;46:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of gastrointestinal fistulae--a meta-analysis. Minim Invasive Ther Allied Technol. 2012;21:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 57. | Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, Vezakis A, Gonzalez JM, Turino SY, Tsiamoulos ZP. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 58. | Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-1249; quiz 1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 59. | Gockel I, Bohl JR, Eckardt VF, Junginger T. Reduction of interstitial cells of Cajal (ICC) associated with neuronal nitric oxide synthase (n-NOS) in patients with achalasia. Am J Gastroenterol. 2008;103:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Boeckxstaens GE. Achalasia: virus-induced euthanasia of neurons? Am J Gastroenterol. 2008;103:1610-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc. 1980;26:8-10. [PubMed] |
| 62. | Pasricha PJ, Hawari R, Ahmed I, Chen J, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Gostout CJ. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 360] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 63. | Minami H, Isomoto H, Yamaguchi N, Matsushima K, Akazawa Y, Ohnita K, Takeshima F, Inoue H, Nakao K. Peroral endoscopic myotomy for esophageal achalasia: clinical impact of 28 cases. Dig Endosc. 2014;26:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Von Renteln D, Fuchs KH, Fockens P, Bauerfeind P, Vassiliou MC, Werner YB, Fried G, Breithaupt W, Heinrich H, Bredenoord AJ. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309-311.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 65. | Costamagna G, Marchese M, Familiari P, Tringali A, Inoue H, Perri V. Peroral endoscopic myotomy (POEM) for oesophageal achalasia: preliminary results in humans. Dig Liver Dis. 2012;44:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 66. | Lee BH, Shim KY, Hong SJ, Bok GH, Cho JH, Lee TH, Cho JY. Peroral endoscopic myotomy for treatment of achalasia: initial results of a korean study. Clin Endosc. 2013;46:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Swanstrom LL, Kurian A, Dunst CM, Sharata A, Bhayani N, Rieder E. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg. 2012;256:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 68. | Friedel D, Modayil R, Iqbal S, Grendell JH, Stavropoulos SN. Per-oral endoscopic myotomy for achalasia: An American perspective. World J Gastrointest Endosc. 2013;5:420-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Swanström LL, Rieder E, Dunst CM. A stepwise approach and early clinical experience in peroral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg. 2011;213:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Yoshida A, Inoue H, Ikeda H, Hosoya T, Onimaru M, Sudo K, Eleftheriadis N, Maselli R, Kudo S. Clinical Results of POEM (Per-Oral Endoscopic Myotomy) for Esophageal Achalasia in 161 Consecutive Cases. Gastrointest Endosco. 2012;75:AB212. [DOI] [Full Text] |
| 71. | Stavropoulos SN, Modayil R, Brathwaite CE, Halwan B, Ghevariya V, Korrapati V, Dejesus D, Iqbal S, Friedel D, Grendell JH. POEM (PerOral Endoscopic Myotomy): 3 Year Experience by a Gastroenterologist At a US Center. Still Safe and Effective Even in Patients With Advanced Age, Severe Achalasia and Severe Comorbidities. Gastrointest Endosco. 2013;77:AB459. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Zhou P, Yao L, Zhang Y, Cai M, Zhong Y, Ren Z, Xu M, Chen WF, Li QL, Qin XY. PerOral Endoscopic Myotomy (POEM) for Esophageal Achalasia: 205 Cases Report. Gastrointest Endosco. 2012;75:AB132-133. [DOI] [Full Text] |
| 73. | Sharata AM, Dunst CM, Pescarus R, Shlomovitz E, Wille AJ, Reavis KM, Swanström LL. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg. 2015;19:161-170; discussion 170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 74. | Stavropoulos SN, Modayil RJ, Friedel D, Savides T. The International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc. 2013;27:3322-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 75. | Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 76. | Hungness ES, Teitelbaum EN, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, Soper NJ. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 77. | Teitelbaum EN, Rajeswaran S, Zhang R, Sieberg RT, Miller FH, Soper NJ, Hungness ES. Peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy produce a similar short-term anatomic and functional effect. Surgery. 2013;154:885-891; discussion 891-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 78. | Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg. 2014;259:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 79. | von Renteln D, Inoue H, Minami H, Werner YB, Pace A, Kersten JF, Much CC, Schachschal G, Mann O, Keller J. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol. 2012;107:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 80. | Inoue H, Ikeda H, Onimaru M, Yoshida A, Sato H, Santi EG, Maselli R, Eleftheriadis N, Kudo S. Clinical results in 300 cases of POEM for esophageal achalasia: a single institute registered prospective study. Gastrointest Endosco. 2013;77:ab121-122. [DOI] [Full Text] |
| 81. | Onimaru M, Inoue H, Ikeda H, Yoshida A, Santi EG, Sato H, Ito H, Maselli R, Kudo SE. Peroral endoscopic myotomy is a viable option for failed surgical esophagocardiomyotomy instead of redo surgical Heller myotomy: a single center prospective study. J Am Coll Surg. 2013;217:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 82. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1231] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 83. | Zhou PH, Li QL, Yao LQ, Xu MD, Chen WF, Cai MY, Hu JW, Li L, Zhang YQ, Zhong YS. Peroral endoscopic remyotomy for failed Heller myotomy: a prospective single-center study. Endoscopy. 2013;45:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 84. | Sharata A, Kurian AA, Dunst CM, Bhayani NH, Reavis KM, Swanström LL. Peroral endoscopic myotomy (POEM) is safe and effective in the setting of prior endoscopic intervention. J Gastrointest Surg. 2013;17:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 85. | Ren Z, Zhong Y, Zhou P, Xu M, Cai M, Li L, Shi Q, Yao L. Perioperative management and treatment for complications during and after peroral endoscopic myotomy (POEM) for esophageal achalasia (EA) (data from 119 cases). Surg Endosc. 2012;26:3267-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 86. | Kandulski A, Fuchs KH, Weigt J, Malfertheiner P. Jackhammer esophagus: high-resolution manometry and therapeutic approach using peroral endoscopic myotomy (POEM). Dis Esophagus. 2014;Jan 27; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Khashab MA, Saxena P, Kumbhari V, Nandwani M, Roland BC, Stein E, Clarke JO, Stavropoulos S, Inoue H, Pasricha PJ. Peroral endoscopic myotomy as a platform for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc. 2014;79:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Li QL, Chen WF, Zhou PH, Yao LQ, Xu MD, Hu JW, Cai MY, Zhang YQ, Qin WZ, Ren Z. Peroral endoscopic myotomy for the treatment of achalasia: a clinical comparative study of endoscopic full-thickness and circular muscle myotomy. J Am Coll Surg. 2013;217:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 89. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [PubMed] |
| 90. | Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54:3-24. [PubMed] |
| 91. | Winant AJ, Gollub MJ, Shia J, Antonescu C, Bains MS, Levine MS. Imaging and clinicopathologic features of esophageal gastrointestinal stromal tumors. AJR Am J Roentgenol. 2014;203:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Mutrie CJ, Donahue DM, Wain JC, Wright CD, Gaissert HA, Grillo HC, Mathisen DJ, Allan JS. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg. 2005;79:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 93. | Punpale A, Rangole A, Bhambhani N, Karimundackal G, Desai N, de Souza A, Pramesh CS, Jambhekar N, Mistry RC. Leiomyoma of esophagus. Ann Thorac Cardiovasc Surg. 2007;13:78-81. [PubMed] |
| 94. | Robb WB, Bruyere E, Amielh D, Vinatier E, Mabrut JY, Perniceni T, Piessen G, Mariette C. Esophageal gastrointestinal stromal tumor: is tumoral enucleation a viable therapeutic option? Ann Surg. 2015;261:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (33)] |
| 95. | Jiang G, Zhao H, Yang F, Li J, Li Y, Liu Y, Liu J, Wang J. Thoracoscopic enucleation of esophageal leiomyoma: a retrospective study on 40 cases. Dis Esophagus. 2009;22:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Shin S, Choi YS, Shim YM, Kim HK, Kim K, Kim J. Enucleation of esophageal submucosal tumors: a single institution’s experience. Ann Thorac Surg. 2014;97:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 98. | Wang XY, Xu MD, Yao LQ, Zhou PH, Pleskow D, Li QL, Zhang YQ, Chen WF, Zhong YS. Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc. 2014;28:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Wang L, Ren W, Zhang Z, Yu J, Li Y, Song Y. Retrospective study of endoscopic submucosal tunnel dissection (ESTD) for surgical resection of esophageal leiomyoma. Surg Endosc. 2013;27:4259-4266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Kumbhari V, Saxena P, Azola A, Messallam AA, El Zein MH, Khashab MA. Submucosal tunneling endoscopic resection of a giant esophageal leiomyoma. Gastrointest Endosc. 2015;81:219-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Saxena P, Chavez YH, Kord Valeshabad A, Kalloo AN, Khashab MA. An alternative method for mucosal flap closure during peroral endoscopic myotomy using an over-the-scope clipping device. Endoscopy. 2013;45:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Yang D, Draganov PV. Closing the gap in POEM. Endoscopy. 2013;45:677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 103. | Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27:4354-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 104. | Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 105. | Cai M, Chen J, Zhou P, Yao L. The rise of tunnel endoscopic surgery: a case report and literature review. Case Rep Gastrointest Med. 2012;2012:847640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 107. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 108. | Xu MD, Lu W, Li QL, Zhou PH, Zhong YS, Chen WF, Zhang YQ, Yao LQ. Application and evaluation of submucosal tunneling endoscopic resection of gastric submucosal tumors originating from the muscularis propria layer. Zhonghua Weichang Waike Zazhi. 2012;15:671-674. [PubMed] |
| 109. | Chen H, Xu Z, Huo J, Liu D. Submucosal tunneling endoscopic resection for simultaneous esophageal and cardia submucosal tumors originating from the muscularis propria layer (with video). Dig Endosc. 2015;27:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 110. | Lu J, Jiao T, Zheng M, Lu X. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc. 2014;28:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 111. | Lu J, Zheng M, Jiao T, Wang Y, Lu X. Transcardiac tunneling technique for endoscopic submucosal dissection of gastric fundus tumors arising from the muscularis propria. Endoscopy. 2014;46:888-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 112. | Celestin LR. Permanent intubation in inoperable cancer of the oesophagus and cardia: a new tube. Ann R Coll Surg Engl. 1959;25:165-170. [PubMed] |
| 113. | Atkinson M, Ferguson R. Fibreoptic endoscopic palliative intubation of inoperable oesophagogastric neoplasms. Br Med J. 1977;1:266-267. [PubMed] |
| 114. | Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329:1302-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 499] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 115. | van Heel NC, Haringsma J, Boot H, Cats A, Vanhoutvin SA, Kuipers EJ. Comparison of 2 expandable stents for malignant esophageal disease: a randomized controlled trial. Gastrointest Endosc. 2012;76:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 116. | Sandha GS, Marcon NE. Expandable metal stents for benign esophageal obstruction. Gastrointest Endosc Clin N Am. 1999;9:437-446. [PubMed] |
| 117. | Hramiec JE, O’Shea MA, Quinlan RM. Expandable metallic esophageal stents in benign disease: a cause for concern. Surg Laparosc Endosc. 1998;8:40-43. [PubMed] |
| 118. | Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Expandable metallic stents should not be used in the treatment of benign esophageal strictures. J Gastroenterol Hepatol. 2001;16:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Martins BC, Retes FA, Medrado BF, de Lima MS, Pennacchi CM, Kawaguti FS, Safatle-Ribeiro AV, Uemura RS, Maluf-Filho F. Endoscopic management and prevention of migrated esophageal stents. World J Gastrointest Endosc. 2014;6:49-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Vanbiervliet G, Filippi J, Karimdjee BS, Venissac N, Iannelli A, Rahili A, Benizri E, Pop D, Staccini P, Tran A. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc. 2012;26:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Fujii LL, Bonin EA, Baron TH, Gostout CJ, Wong Kee Song LM. Utility of an endoscopic suturing system for prevention of covered luminal stent migration in the upper GI tract. Gastrointest Endosc. 2013;78:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 122. | Mudumbi S, Velazquez-Aviña J, Neumann H, Kyanam Kabir Baig KR, Mönkemüller K. Anchoring of self-expanding metal stents using the over-the-scope clip, and a technique for subsequent removal. Endoscopy. 2014;46:1106-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 124. | Thomas T, Abrams KR, Subramanian V, Mannath J, Ragunath K. Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy. 2011;43:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Repici A, Hassan C, Sharma P, Conio M, Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Aliment Pharmacol Ther. 2010;31:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Ham YH, Kim GH. Plastic and biodegradable stents for complex and refractory benign esophageal strictures. Clin Endosc. 2014;47:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 127. | Foschia F, De Angelis P, Torroni F, Romeo E, Caldaro T, di Abriola GF, Pane A, Fiorenza MS, De Peppo F, Dall’Oglio L. Custom dynamic stent for esophageal strictures in children. J Pediatr Surg. 2011;46:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | De Peppo F, Zaccara A, Dall’Oglio L, Federici di Abriola G, Ponticelli A, Marchetti P, Lucchetti MC, Rivosecchi M. Stenting for caustic strictures: esophageal replacement replaced. J Pediatr Surg. 1998;33:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Eloubeidi MA, Talreja JP, Lopes TL, Al-Awabdy BS, Shami VM, Kahaleh M. Success and complications associated with placement of fully covered removable self-expandable metal stents for benign esophageal diseases (with videos). Gastrointest Endosc. 2011;73:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 130. | van Boeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, Vleggaar FP, Siersema PD. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 131. | Bakken JC, Wong Kee Song LM, de Groen PC, Baron TH. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc. 2010;72:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 132. | Schweigert M, Dubecz A, Stadlhuber RJ, Muschweck H, Stein HJ. Risk of stent-related aortic erosion after endoscopic stent insertion for intrathoracic anastomotic leaks after esophagectomy. Ann Thorac Surg. 2011;92:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 133. | Rogart J, Greenwald A, Rossi F, Barrett P, Aslanian H. Aortoesophageal fistula following Polyflex stent placement for refractory benign esophageal stricture. Endoscopy. 2007;39 Suppl 1:E321-E322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | van Geenen EJ, Visser NC, Bonenkamp JJ. Fatal aortogastric fistula following fully covered metal stent placement for refractory esophageal stricture. Endoscopy. 2014;46 Suppl 1 UCTN:E16-E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Hirdes MM, Siersema PD, van Boeckel PG, Vleggaar FP. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: a prospective follow-up study. Endoscopy. 2012;44:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 136. | Repici A, Vleggaar FP, Hassan C, van Boeckel PG, Romeo F, Pagano N, Malesci A, Siersema PD. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |