INTRODUCTION
Endoscopic submucosal dissection (ESD) is widely recognized as an effective treatment strategy for early gastric cancer[1,2]. In recent years, the indications for ESD have been expanded to include lesions of the esophagus and the large intestine[3-5]. Although there are several reports of ESD performed for non-ampullary duodenal tumors[6-8], the indication of ESD for the treatment of these tumors remains controversial, because the procedure is technically difficult and associated with a high incidence rate of complications[1]. While ESD may be indicated for non-ampullary duodenal tumors, including adenomas, carcinomas, and neuroendocrine tumors (NET), there is the need to determine whether ESD or endoscopic mucosal resection (EMR) might be optimal. At present, the criteria for selection between ESD and EMR vary among institutions.
In order to determine whether ESD is indicated for duodenal tumors, examination of the site, size, and macroscopic and histological morphology of the tumors is necessary. Development of guidelines for ESD of duodenal lesions (duodenal ESD) is awaited.
DIFFICULTY IN DUODENAL ESD
The duodenum is curved in the shape of a letter C and divided into four portions. The first portion is covered by the peritoneum and is mobile, whereas the second and third portions are dorsally fixed by the peritoneum and located in the retroperitoneum. These portions are immobile. The duodenal wall is thin, which consists of the mucosal, submucosal, proper muscle, and subserosal layers, starting from the lumen inward. At the outermost layer, the anterior aspect of the duodenal wall (peritoneal cavity aspect) is covered by serosa (peritoneum), while the posterior aspect is connected with the retroperitoneum. There are numerous mucosal folds on the internal surface of the duodenum, except in the first portion. The surface of the folds carries many villi which function to absorb nutrients, etc. In the duodenal lumen from the second portion downward, a number of circular folds (Kerckring’s folds) composed of the mucosa and submucosa are arranged perpendicular to the long axis. Duodenal glands (Brunner’s glands), which produce alkaline fluid rich in mucus, are distributed in the submucosa.
In endoscopic therapies for lesions of the duodenum, the maneuverability of the endoscope is poor due to the anatomical features. Moreover, because of the presence of the folds and Brunner’s glands, it is more difficult to achieve sufficient bulging by local injection into the submucosa, as compared with the case in other parts of the gastrointestinal tract, and the duration of bulge of the submucosa is also short. Furthermore, because the duodenal wall is thin, the incidence rate of complications such as bleeding and perforation is high. Especially, duodenal ESD is technically difficult, often takes long time to perform, and is associated with a high risk of perforation[6]. Thus, it would seem that duodenal ESD should be performed by operators skilled in safe and reliable techniques for ESD of at least lesions of the stomach, esophagus, and large intestine.
SELECTION OF ENDOSCOPIC THERAPIES FOR LESIONS IN THE DUODENUM
The indication for ESD of duodenal tumors should be determined by assessment of the histopathology, macroscopic morphology, and diameter of the tumors. The three types of candidate lesions for endoscopic therapy are adenoma, carcinoma, and NET.
In the case of duodenal tumors, unlike tumors of the stomach and the large intestine, it is often difficult to differentiate between benign and malignant tumors on the basis of the macroscopic endoscopic findings alone. Thus, histopathological diagnosis is basically essential. However, the high risk of development of fibrosis in the submucosa occurring after biopsy reportedly makes endoscopic therapy difficult[9]. While magnifying endoscopy with narrow-band imaging has frequently been reported to be useful for qualitative diagnosis of early esophageal[10,11], gastric[12,13], and colorectal cancers[14], it is also useful for qualitative diagnosis of superficial non-ampullary duodenal epithelial tumors[15]. For depressed-type lesions, because fibrosis is likely to occur after biopsy, optical biopsy using magnifying endoscopy with narrow-band imaging has been reported to be more effective than tissue biopsy[16].
Endoscopic therapies for duodenal adenomas
Duodenal adenomas have the potential for malignant transformation[17,18]. Especially, those that are 2 cm or more in diameter and adenomas showing high-grade dysplasia on histopathology show a high likelihood of becoming malignant[19-21], and resection is preferable for such lesions. On the other hand, there is a report that low-grade adenomas measuring less than 1 cm in diameter remained low-grade lesions even at 2 years after the first diagnosis[22]. EMR of duodenal tumors has been reported to be safe and useful and to be associated with a favorable long-term prognosis[23-29]. In addition, piecemeal resection of adenomas is acceptable. Thus, EMR seems to be preferable for the treatment of duodenal tumors. However, a study showed that the preoperative pathological diagnosis was adenoma in 3 of 4 cancer patients who underwent EMR[30], and accurate preoperative diagnosis is necessary. At our institution, endoscopic therapy is not selected for patients with low-grade adenomas measuring less than 1 cm in diameter; instead, such patients are followed up with regular endoscopy. We select endoscopic therapies for adenomas that are at least 1 cm in diameter or show a tendency to grow, those that are histopathologically diagnosed as low-grade adenoma, but appear red and are macroscopically suspected as cancer, etc.
Endoscopic therapies for duodenal cancer
In a study of 128 lesions of early duodenal cancer for which surgery or endoscopic polypectomy was performed, it was reported that none of the cases of intramucosal carcinoma showed lymph node metastasis[31]. Thus, endoscopic therapies should be considered for well-differentiated noninvasive carcinomas not showing submucosal invasion. The complete remission rate after EMR for duodenal tumors ranges from 63% to 97%[24-39]. Lesions measuring 2 cm or more in diameter are likely to require piecemeal resection[23,29], and the persistence and recurrence rates are higher after piecemeal resection than after en bloc resection[16,29]. Complete (R0) resection is more frequently achieved by ESD than by EMR[16,30]. Furthermore, en bloc resection enables accurate histopathological assessment of deep and lateral surgical margins[33]. Thus, it seems preferable to perform EMR for lesions that can be resected en bloc by EMR and to perform ESD for lesions in which EMR is expected to result in piecemeal resection.
Endoscopic therapies for duodenal NET
The common sites of NET are the ileum, appendix, and rectum[34], and NET originating from the duodenum accounts for less than 5% of NET[35-38]. While according to one previously reported retrospective study, no recurrence was observed after local excision in any patients with tumors measuring less than 2 cm in diameter[39], another report indicated that lymph node metastasis was observed in 13% of patients with tumors measuring less than 1 cm in diameter[40]. No consensus has been reached on the association between tumor diameter and the likelihood of lymph node metastasis. Burke et al[41] reported the following three risk factors as being predictive of metastasis: tumor invasion to the muscle layer, tumor diameter 2 cm or more, and the presence of mitotic figures[41]. Zyromski et al[39] also reported that in cases of tumors measuring less than 2 cm in diameter, no metastasis was observed, regardless of the depth of invasion, recommending endoscopic therapies for tumors measuring less than 1 cm in diameter, and open transduodenal local excision for those measuring 1 to 2 cm in diameter[39]. There are reports that endoscopic resection is safe, minimally invasive, and effective for patients with tumors measuring less than 1 cm in diameter that are not identified by EUS as invading the muscle layer[42]. Although EMR may be well applicable in tumors measuring less than 1 cm in diameter invading the superficial layers of the submucosa, especially lesions with polypoid morphology, ESD may be useful for lesions that are difficult to resect en bloc by EMR. However, when the lower margin of a tumor lesion is widely attached to the muscle layer, ESD is associated with an extremely high risk of perforation, and the histopathological diagnosis of the deep surgical margin is also slightly uncertain; thus, surgical treatment should be considered for such cases[7].
TECHNICAL KNOW-HOW OF METHODS OF DUODENAL ESD
The most important technical issue in duodenal ESD is the submucosal dissection process, and it is common to encounter difficulties during submucosal dissection, such as when the tip of a knife is perpendicularly oriented to the dissection surface (Figure 1). In duodenal ESD, a short needle-type knife is suitable for the mucosal incision and submucosal dissection processes. The authors use the Dual knife (Olympus, Tokyo, Japan). Moreover, for the submucosal dissection process, the Small-caliber-tip Transparent (ST) hood (Fujifilm, Tokyo, Japan) is an important tool[6]. One of the important aspects of the procedure is to ensure a space for the ST hood to be placed directly under a lesion by incising the oral side of the lesion and slightly detaching it to form a mucosal flap in the early stage (Figure 2). This is the key for the success of the procedure. When a lesion is detached from the anterior wall, the tip of a knife is likely to be perpendicularly oriented to the dissection surface. Under such a situation, the authors make direct visualization of the submucosa easy using the ST hood and apply electrical current while keeping the knife slightly pressed on the lesion (Figure 3) or while the tissue to be detached is hooked and pulled toward the scope by the Hook knife (Olympus, Tokyo, Japan). When the submucosa is detached, it is important to leave as much submucosa on the dissection surface as possible in order not to expose the surface of the muscle layer. Moreover, because there is also a possibility of perforation due to an attachment of the knife such as ST hood, it seems preferable to slightly press the attachment on the dissection surface. The tips for ESD of lesions in the second portion of the duodenum are to push and pull the endoscope and control the intraduodenal air volume. In the third portion of the duodenum, the maneuverability of a scope is poor, and it is essential to check the maneuverability before the operation. If the maneuverability is poor, double-balloon enteroscopy may be useful[43].
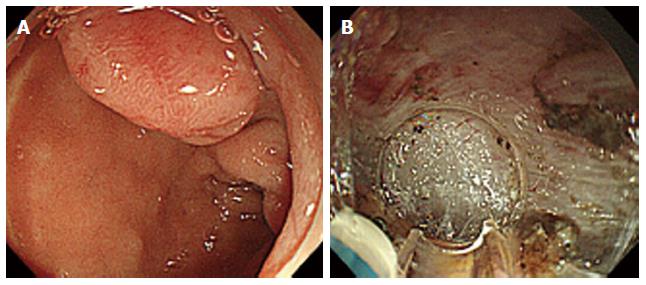
Figure 1 Endoscopic submucosal dissection of a neuroendocrine tumors in the superior duodenal bulb.
A: A protruded-type tumor 0.9 cm × 0.9 cm in size was identified; B: We performed a submucosal dissection. The tip of a knife is perpendicularly oriented to the dissection surface.
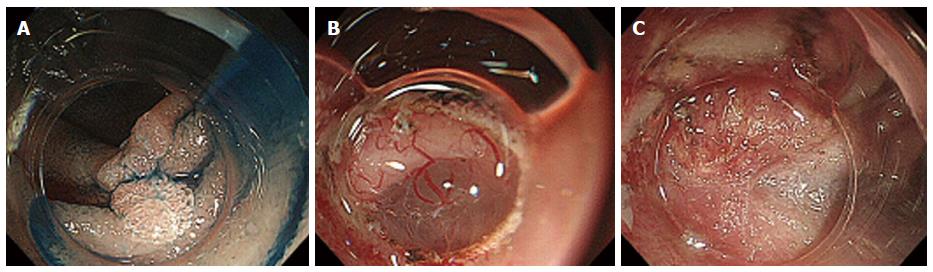
Figure 2 Endoscopic submucosal dissection of an adenoma in the descending part of the duodenum.
A: A depressed type tumor 1.2 cm × 1.2 cm in size was identified; B: After incising the oral side of the lesion, we slightly detached it to form a mucosal flap; C: A severe submucosal fibrosis was found.
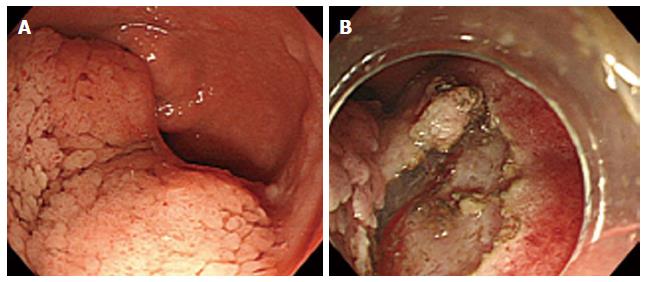
Figure 3 Endoscopic submucosal dissection of an adenoma in the anterior duodenal bulb.
A: A flat-elevated type tumor 6.0 cm × 5.0 cm in size was identified; B: We performed submucosal dissection using the ST hood.
Control of bleeding during the procedure is a key to the success of duodenal ESD. It is important to recognize the blood vessels and coagulate them before cutting. Hemostatic forceps should be slightly pulled away from the muscle layer before coagulation to prevent electrical injury of the thin muscle layer[43]. In addition, bipolar coagulation forceps are effective to prevent and restrain hemorrhage.
Because of the high incidence rate of complications caused by duodenal ESD, we have used carbon dioxide insufflation during the ESD. Carbon dioxide insufflation has been reported to be useful for early esophageal[44] and gastric ESD[45]. In addition, a system for ensuring backup by the surgical department may be essential when the procedure is performed. At our institution, in an effort to provide safer treatment, duodenal ESD has been performed under general anesthesia in the operating room since 2010.
Meanwhile, there are also difficult situations encountered during EMR of duodenal lesions. In EMR of lesions in the first portion of the duodenum, the pyloric ring may pose an obstacle to snaring. Moreover, because a lesion relatively often extends over several folds in the second portion, where the space between the folds is small, it may be difficult to ensure snaring in EMR lesions in the second portion of the duodenum.
AFTER DUODENAL ESD
At our institution, intravenous injection of a proton pump inhibitor is started on the day of the ESD, and intravenous cephem antibiotics are administered for approximately 3 d. A blood test is performed on the day after the ESD. If complications such as perforation do not occur, a rice gruel diet is started approximately 3 d after the ESD. Yamamoto suggests taking the fasting period a few days longer in duodenal ESD than other ESDs[43]. During the hospitalization, endoscopy is not performed to check for the formation of ulcers after ESD. If no complications occur, the patients are usually discharged within one week after the operation.
COMPLICATION OF DUODENAL ESD
The most common complication of endoscopic therapies for duodenal lesions is bleeding, which, in general, occurs within 24 h after the operation. The frequency of bleeding after EMR of adenomas ranges from 4% to 33%[23-28]. The frequency of bleeding after ESD ranges from 6.7% to 22.2%[6,30]. The incidence rate of perforation complicating duodenal ESD ranges from 21% to 35.7%[6,46,47], which is extremely high as compared to that of perforation complicating gastric ESD, which ranges from 1.2% to 3.6%[48-50]. Moreover, attention should be paid not only to intraoperative perforation, but also delayed perforation due to exposure to bile or pancreatic juice[6]. As compared to that in patients undergoing EMR, the incidence rate of perforation is significantly higher in those undergoing ESD, and the duration of postoperative hospital stay is also significantly longer[30]. If patients complain of abdominal pain or fever after procedure, they should be checked for their abdominal tenderness and free air in the abdomen by computerized tomography. Thus, the wound should be closed by clipping in order to prevent complications such as secondary hemorrhage and delayed perforation[46,51]. However, in some patients with lesions located in the first portion of the duodenum, closure of the wound by clipping may be difficult, and there is a report of patients in whom perforation occurred after closure of the wound by clipping[52]. In such patients, coverage of the wound with polyglycolic acid sheets (Neoveil; Gunze Ltd., Kyoto, Japan) and fibrin glue (Bolheal; Kaketsuden, Kumamoto, Japan) as a substitute to closure of wound by clipping may be effective for the prevention of delayed perforation[52].
SURGERY FOR NON-AMPULLARY DUODENAL TUMORS
At present, the frequency of complications of duodenal ESD is high, even in institutions with experts in endoscopic therapies. Unlike gastric ESD, it is more difficult to popularize the use of duodenal ESD around the world. Therefore, ESD for duodenal lesions should be performed at limited institutions with abundant experience in performing the procedure. There is also a report that surgery is preferable for lesions exceeding 20 mm in major axis[16]. It is necessary to always keep in mind surgery as one of the treatment options, and endoscopic therapies should not be insisted upon.
Recently, there have been an increasing number of institutions where endoscopists and surgeons cooperatively perform Laparoscopy and Endoscopy Cooperative Surgery (LECS). In a study conducted on 22 patients undergoing LECS for duodenal tumors, the mean tumor diameter was 13.3 mm; the mean diameter of the resected specimens was 28.9 mm; the mean operative time was 133 min; and the duration of postoperative hospital stay was 15.1 d. Complications were observed in 5 patients, 3 (13.6%) of whom had asymptomatic minor leakage. All patients recovered with conservative therapy, and no serious complications were encountered in this study[53].
LONG-TERM PROGNOSIS
In regard to the long-term prognosis, according to one study with a mean follow-up period of 10 mo, no recurrence was observed in any of the 16 patients treated by duodenal ESD, while recurrence was observed in one of the 31 patients undergoing duodenal EMR[30]. Another study also reported that no recurrence was observed with a mean follow-up period of 48 mo in any of the 37 patients treated by ESD for duodenal tumors measuring 20 mm in diameter[54]. Further accumulation of cases may be needed to clarify the long-term prognosis.
P- Reviewer: Albuquerque A, Cho JY, Richardson WS, Yamamoto S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL