Published online Apr 16, 2015. doi: 10.4253/wjge.v7.i4.370
Peer-review started: August 8, 2014
First decision: November 28, 2014
Revised: December 20, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: April 16, 2015
Processing time: 254 Days and 8.8 Hours
Endoscopic resection (ER) is at present an accepted treatment for superficial gastrointestinal neoplasia. ER provides similar efficacy to surgery; however, it is minimally invasive and less expensive. Endoscopic mucosal resection (EMR) is superior to biopsy for diagnosing advanced dysplasia and can change the diagnostic grade and the management. Several EMR techniques have been described that are alternatively used dependent upon the endoscopist personal experience, the anatomic conditions and the endoscopic appearance of the lesion to be resected. The literature suggests that EMR offers comparable outcomes to surgery for selected indications. EMR techniques using a cap fitted endoscope and EMR using a ligation device [multiband mucosectomy (MBM)] are the most frequently use. MBM technique does not require submucosal injection as with the endoscopic resection-cap technique, multiple resections can be performed with the same snare, pre-looping the endoscopic resection-snare in the ridge of the cap is not necessary, MBM does not require withdrawal of the endoscope between resections and up to six consecutive resections can be performed. This reduces the time and cost required for the procedure, while also reducing patient discomfort. Despite the increasing popularity of MBM, data on the safety and efficacy of this technique in upper gastrointestinal lesions with advanced dysplasia, defined as those lesions that have high-grade dysplasia or early cancer, is limited.
Core tip: Early detection of upper gastrointestinal lesions with advanced dysplasia is especially important in the management of the patients. These changes may indicate an increased risk of cancer or may detect cancer at an earlier stage, when it can be more effectively treated. Multiband mucosectomy (MBM) is an easy endoscopic mucosal resection technique allowing a definitive histologic diagnosis and potentially being curative. The available evidence suggests that MBM for these conditions, has an initial success rate comparable to surgical treatment, but with fewer complications.
- Citation: Espinel J, Pinedo E, Ojeda V, Rio MGD. Multiband mucosectomy for advanced dysplastic lesions in the upper digestive tract. World J Gastrointest Endosc 2015; 7(4): 370-380
- URL: https://www.wjgnet.com/1948-5190/full/v7/i4/370.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i4.370
Most commonly, the treatment of high-grade dysplasia (HGD) and mucosal cancer has been surgical. However, it does carry procedure-related morbidity and mortality[1-4]. In addition, a notable proportion of these patients have significant comorbidities, which medically preclude them from undergoing surgery. These high rates of morbidity and mortality have filed attention in other types of less invasive treatment. Endoscopic mucosal resection (EMR) is an endoscopic therapeutic proposal in which the dysplastic epithelium is removed, thus making it possible for a definitive histologic diagnosis and treatment[5-9]. EMR is possible due to the existence of a loose adhesion between the submucosa and the muscular layer in the gastrointestinal tract’s wall because of a different embryologic origin. This anatomic characteristic allows, for example, the saline injection between the two layers, thus transforming a flat or depressed lesion into an elevated one. This permits the safe resection of mucosal lesions without causing damage of the deeper muscle layer, and reduces the risk of perforation. EMR has been used not only for Barrett’s esophagus with HGD but also for early cancer in which the risk of hematogenous dissemination or lymph node involvement is low[10-12]. EMR is effective and safe for total resection of superficial lesions. Furthermore, EMR does not compromise subsequent ablative therapy. Ablative techniques do not supply specimen for histopathologic evaluation and are mainly use as an adjunct therapy to EMR[13]. Several different EMR techniques have been described[14]: (1) strip biopsy; (2) endoscopic double snare polypectomy; (3) EMR using a transparent cap fitted endoscope; and (4) EMR using a ligation device [multiband mucosectomy (MBM)]. EMR is a technique that requires skill, both to resect lesions in a safe and effective manner and to manage complications. EMR should only be carried out by experienced endoscopists in advanced therapeutic endoscopy. Despite the increasing popularity of MBM, limited data on the safety and efficacy of this technique in lesions with advanced dysplasia (LAD), are available.
This article reviews the current evidence and gaps in knowledge in the understanding of management of LAD of the upper gastrointestinal tract with MBM. “Advanced dysplasia” was defined as those lesions that have HGD or early cancer (EC).
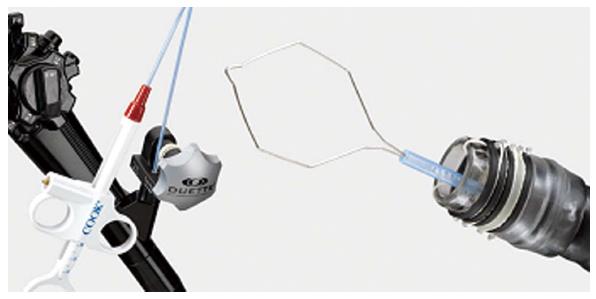
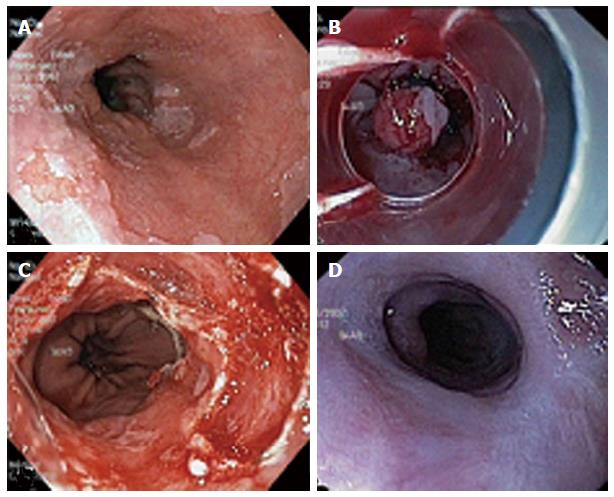
MBM (Duette; Cook Medical) uses a modified variceal band ligator that includes a transparent cap with 6 bands and a handle that allows the passage of a snare through the accessory channel (Figure 1). The target mucosa is sucked into the cap and a pseudopolyp is created. The pseudopolyp can then be removed (Figure 2). MBM has several advantages: (1) no lifting is need because the esophageal muscle layer will immediately retract when captured within a band; (2) several resections can be performed by repetitive suck-band-snare sequences; (3) pre-looping the endoscopic resection-snare in the ridge of the cap is not required; (4) MBM does not need withdrawal of the endoscope between resections, and sequential 6 bands resections can be carried out; (5) MBM yields tissue specimen for hystology and staging[7]; (6) MBM is minimally invasive and carries lower morbidity and mortality compared to surgical treatment; and (7) surgery can be performed if advanced neoplasia is confirmed on histologic evaluation of the MBM specimen. By contrast, MBM has some disadvantages: (1) MBM demands advanced endoscopic skills; (2) larger lesions can only be resected by piecemeal technique which might preclude complete histological evaluation; and (3) there are no randomized trials directly comparing MBM with surgery.
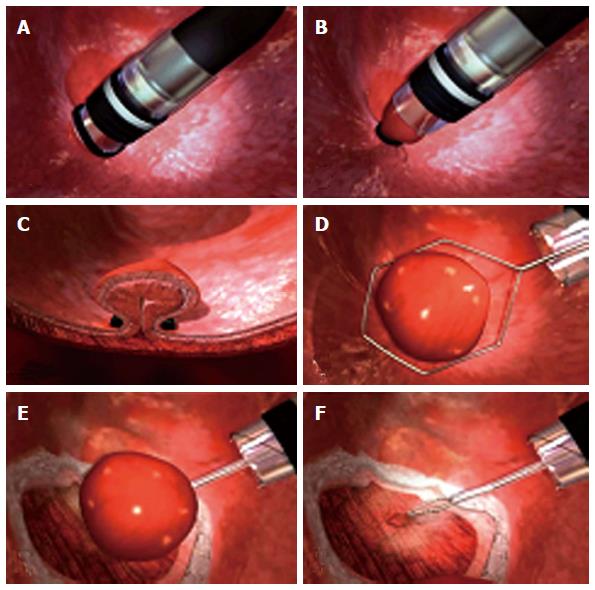
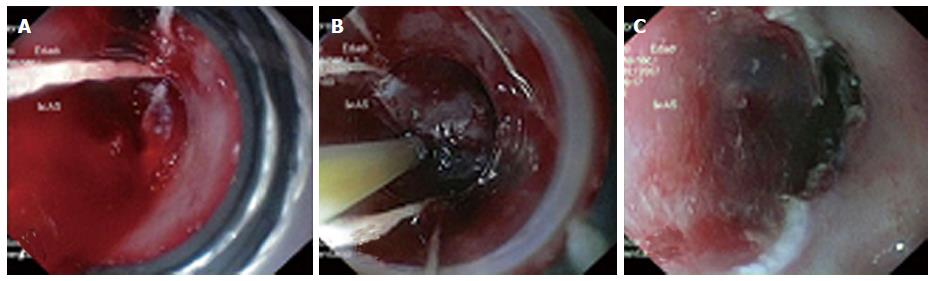
MBM is generally performed with the patient under unconscious sedation with titrated intravenous propofol. After, the endoscope is introduced without the ligator and the lesion for resection is recognized. The lesion is outlined by using argon plasma coagulation. Marks are placed 2-5 mm outside the margins of the lesion (Figure 3). Then, the endoscope is withdrawn and the ligator assembled on the endoscope. The wires are placed in line with the working channel to provide the best endoscopic view (Figure 4). The endoscope is then reintroduced with the ligator, the dysplastic mucosa is sucked into the cap, and a rubber band is deployed. The rubber band forms a pseudopolyp which is then immediately resected by using pure coagulating current (Figure 5). It does not matter whether the snare is placed above or below the band. In most of the cases, however, the snare will lie below the rubber band. The second ligation is performed by suctioning the adjacent mucosa with a small overlap to ensure that no dysplastic mucosa remnant remains[15-18]. After each resection, the specimen is pushed into the stomach by using the tip of the snare’s catheter. Resected specimens are retrieved from the stomach with a polypectomy snare or retrieval net. If cancer diagnosis is made, the histological report should include these characteristics: tumor infiltration depth, tumor differentiation grade, existence of lymphatic or vascular infiltration and the radicality of the lateral margins. After MBM, patients are put on a proton pump inhibitor and sucralfate suspension. A pureed diet is recommended. In patients without comorbidities, MBM can be performed on an outpatient basis. However, we prefer that patients are discharged after 24 h of observation. Primary endoscopic follow-up is performed 4 wk later on an outpatient basis.
The most common indication for EMR in the upper gastrointestinal tract is the staging and treatment of early neoplasia in Barrett’s esophagus (BE). MBM has been applied not only to mucosal lesions with HGD but also to early cancer in which the risk of lymph node involvement or hematogenous dissemination is low enough to justify a relatively conservative approach compared with surgery[15-31].
At present, there are no randomized controlled trials reviewing the role of endoscopic treatment compared with surveillance alone in nondyspastic BE. Probably, the number needed to treat to prevent one cancer is high and the risk of endoscopic treatment outweighs the benefits of this procedure. Thus, the current American Gastroenterological Association (AGA) guidelines do not recomend endoscopic eradication therapy (EET) in patients with nondysplastic BE[32].
The natural history of low-grade dysplasia (LGD) in BE is unclear with variability in the rates of development to esophageal adenocarcinoma (EAC), poor interobserver concordance, unclear risk stratification, and lack of established benefit of eradication[33-35]. Therefore, systematic EET of patients with LGD is not currently advised. Now, AGA guidelines suggest the use of RFA as an alternative for the treatment of verified LGD but, this decision should be individualize with agreement between the patient and the physician[32].
At the present, AGA guidelines recommend EET in the management of patients with HGD[32]. Current evidence suggests that EMR of HGD and early cancer EC has similar success rates as surgical treatment[6,36]. The indications for EMR in the setting of Barrett’s neoplasia include the following: flat mucosal lesions, tumor size between 20-30 mm, and good to moderate differentiation on histology[6]. Furthermore, EMR has better diagnostic reproducibility compared to mucosal biopsies alone, suggesting a possible role in BE surveillance[37].
Usually, EMR is indicated for superficial well- or moderately differentiated squamous cell carcinoma without venous or lymphatic involvement that is limited to the lamina propria[38].
Candidates for MBM must meet the following criteria: well- or moderately differentiated adenocarcinoma, confined to the mucosa, < 20 mm for elevated lesions, < 10 mm for flat or depressed lesions, with no evidence of ulceration, lymphatic or venous involvement[39].
Accurate T-staging is critical in making therapeutic decisions in patients with dysplastic Barrett’s esophagus. The distinction between different categories of dysplastic lesions can be difficult since it depends in part upon the size, location, depth, and number of biopsies. The Seattle biopsy protocol is recommended for mapping Barrett’s esophagus with HGD[40]. Targeted biopsies are acquired from all visible abnormalities and random four-quadrant biopsies are taken every 1 cm starting from the top of the gastric folds up to the most proximal extent of the BE (squamocolumnar junction). Another concern with the diagnosis of dysplastic lesions is the interobserver reliability among pathologists. Therefore, it is recommended that a second, experienced pathologist should confirm the diagnosis of HGD. Studies comparing routine biopsies of visible lesions with EMR report a 30% to 48% rate in change in diagnosis after obtaining an EMR[26,28]. Furthermore, in a study comparing preoperative EMR with histologic examination on esophagectomy specimens, there was perfect agreement between the two[41]. We consider MBM may represent not only a reasonable treatment option but also the final step of the diagnostic work-up for patients with dysplastic lesions[37]. Assessment of the depth of infiltration and estimation of local nodal metastasis can be achieved by endoscopic resection of these areas within a lesion which look suspicious[42,43]. Among patients diagnosed with dysplastic lesions, other imaging techniques could be taken into account to evaluate tumor infiltration depth, local lymph node status and metastatic spread. Endoscopic ultrasonography (EUS) and computerized tomography (CT) scan are the most widely used techniques. Although the role of EUS has been established in the accurate T and N staging of invasive EAC, recent studies have shown only a modest accuracy in delineating T-staging in patients with HGD and intramucosal EAC[44-47]. Recent studies report that the overall accuracy of EUS in establishing T-stage (depth of invasion), using EMR/surgical pathology as the gold standard, was 65%-72%. Based on this information, EUS has a limited role in the evaluation of patients with early neoplasia[44,48]. Other techniques, such as magnetic resonance imaging and positron emission tomography scanning, do not have a role in the evaluation of patients with these lesions.
The first objective of endoscopic therapy is to prevent the development of invasive EAC by treating the dysplastic lesion. The available evidence suggests that endoscopic resection (ER) for these conditions has an initial success rate comparable to surgical treatment, but with fewer complications[6,8,26,28,36]. The rate of complete remission ranges from 59% to 99% in different studies[6,8,26,28,36,49,50]. Higher degrees of success are seen in patients with lower risk lesions. In a systematic review, complete eradication of HGD or EC was achieved in 95% of patients, and complete eradication of all Barrett's mucosa was achieved in 89%[51]. ER is best performed on patients with small (< 20 mm diameter), solitary, flat type lesion that is limited to the mucosa. Histopathologic differentiation is less important, since the great majority of these early lesions will be classified as HGD or well differentiated cancers[7]. However, patients who develop dysplasia are at higher risk of recurrence of neoplasia and metachronous lesions from the remaining segment of BE, which occurs in up to 30% of patients undergoing EET[6,8,28,36,52-54]. Factors associated with recurrence in BE are larger diameter, long segment, piecemeal resection, lack of adjunctive ablative therapy, presence of multifocal neoplasia, an elapsed time of more than 10 mo prior to achieving complete remission and the presence of residual dysplasia[8,36]. In most patients, recurrences can be successfully treated endoscopically[54]. Recurrence is a possible limitation after EMR. Patients therefore require regular follow up with endoscopy (every three months during the first year and annually thereafter) and treatment of any residual Barrett’s mucosa. Endoscopic ablative therapy with radiofrequency ablation or photodynamic therapy allows treatment of the whole Barrett’s segment in a few sessions. Complete ER of the whole Barrett’s segment may also be used as endoscopic treatment [stepwise radical endoscopic resection (SRER)][21-23,49] (Figure 5). Most experts believe that EMR resection of the entire Barrett segment can be performed in patients with Barrett segment length of less than or equal to 5 cm. This technique has several advantages over ablative therapy: it allows complete removal of the whole mucosa at risk for malignant progression and provides tissue samples for histological diagnosis. Furthermore, the feasibility and safety of ER of the entire Barrett’s segment has been demonstrated on several series[21-23,49]. However, the role of the stepwise radical endoscopic resection technique seems restricted to selected patients in the treatment of HGD or EC in Barrett’s esophagus. Although the SRER technique is equally effective and has several advantages over ablative treatment, it is related to a much higher rate of strictures than ER plus RFA. Currently, it is advised for complete eradication of intestinal metaplasia, that patients with HGD and early esophageal adenocarcinoma(EAC) undergo EMR of a visible lesion followed by RFA to the remaining Barrett segment, or to use the SRER procedure only for patients with more extensive lesions in BE up to 5 cm[30].
The three major EMR-complications include: (1) bleeding; (2) perforation; and (3) strictures[20,29,55-58]. Bleeding is apparent in 0% to 46% of cases and can be managed with endoscopic treatment. Immediate bleeding can be considered as a complication if there are clinical signs. Perforation has been described in less than 5%. The risk is higher in piecemeal resection. Strictures have been described in 2% to 88% of patients undergoing EMR for dysplastic Barrett’s esophagus. The size/length of the mucosal defect and the circumferential involvement by the BE predicts stenosis formation. Stenosis are more frequent if the BE involves more than 75% of the esophageal circumference. Stenosis can be successfully treated with endoscopic dilation. Chest pain occurs in about 30% of patients undergoing EMR.
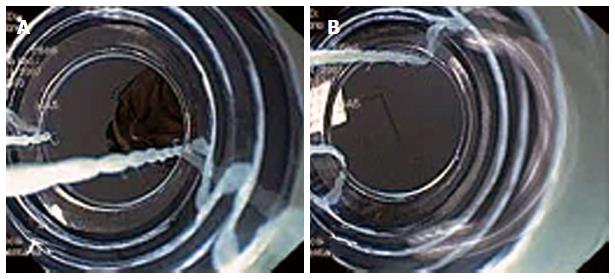
Several studies demonstrated that the MBM is safe and effective[15,17,18,29] (Table 1). In these studies, acute complications were observed in 3% and no perforations occurred[15,17,26]. MBM does not appear to be associated with more complications than endoscopic resection-cap, despite lack of submucosal lifting. Perforations occur in approximately 1% of the endoscopic resections performed with the widely used cap technique in Barrett’s esophagus[59,60], compared to MBM where the probability of perforation seems to be very low, with perforation rates reported in the range of 0% to 1.2%[16-31]. Most acute bleedings with MBM resolve spontaneously or can effectively be treated by adrenaline injection or coagulation techniques (Figure 6). Several studies have reported stenosis rates of 26%-70% after radical resection with MBM of the whole Barrett’s segment[16,23,25,26]. A larger study evidenced stricture requiring dilatation in 48% of the patients who underwent the MBM procedure as part of the (stepwise) radical resection protocol. Stenosis rates increase with the extent of the resected area in the esophagus, especially if the resection is more than 3 cm in length and comprises more than 75% of the circumference[61]. Suitable data comparing stenosis rate with MBM and cap technique, is not available.
| Ref. | Numberandprocedures | Complete eradication | Recurrence rate | Complications | Follow-up(mo) |
| Soehendra et al[16] | 10 MBM | 90% | N/A | Stricture (SRER 70%) | N/A |
| Ell et al[62] | 100 MBM (%N/A) Cap | 99% | 11% | 0% | 33 |
| Peters et al[31] | 40 MBM | N/A | N/A | Bleeding (6%) | N/A |
| Chennat et al[26] | 49 MBM (4%) Cap FH | 65% | 2.50% | Stricture (SRER 36.7%) | 23 |
| Espinel et al[15] | 8 MBM | 100% | 0% | Stricture (SRER 25%) | 32 |
| Moss et al[28] | 75 MBM (%N/A) Cap | 94% | 0% | Stricture (SRER 8%) | 31 |
| Pouw et al[27] | 169 MBM (%N/A) Cap FH | 95.30% | 1.80% | Bleeding (1.8%) Perforation (2.4%) Stricture (SRER 50%) | 32 |
| Brahmania et al[63] | 22 MBM | 82% | 18% | Stricture (SRER 13%) | 24 |
| Pouw et al[18] | 42 MBM | 100% | N/A | Perforation (2%) | N/A |
| Alvarez Herrero et al[17] | 243 MBM | 91% | 0% | Bleeding (3%) Stricture (SRER 48%) | 3 |
| Van Vilsteren et al[30] | 25 MBM (48%) Cap FH | 100% | 4% | Perforation (4%) Stricture (SRER 88%) | 25 |
| Gerke et al[29] | 41 MBM (76%) Cap | 78% | 9% | Perforation (4.9%) Stricture (SRER 44%) | 25 |
| Tomizawa et al[56] | 681 MBM (18%) Cap | N/A | N/A | Bleeding (1.2%) Stricture (1%) | 63 |
Multiband mucosectomy and cap-assisted EMR are new minimally invasive therapies alternatives for LAD. A randomized controlled trial comparing these two techniques demonstrated that there is no difference in the thickness of the specimen and submucosal resection; however, the multiband mucosectomy had a shorter procedure time and produced smaller EMR specimens. The clinical relevance of these findings may be questioned, since there was no significant difference in the depth of resection between the two techniques[18]. In addition, costs for disposables were significantly lower for MBM procedures. Rates of complete endoscopic resection were similar for MBM (91% of delineated focal lesions, 86% of delineated areas in Barrett’s esophagus, and 100% of the escape treatments) and the cap technique (88% success rate for complete endoscopic resection)[60]. Both techniques are very effective in this respect[18,60,62,63]. MBM can fail if there is significant fibrosis which impeded suctioning of the mucosa into the cap and subsequent rubber band ligation[17]. Similarly, both techniques seem equally safe and the lack of submucosal lifting with MBM does not increase the risk of perforation compared with that of the cap technique. A disadvantage for MBM may be decreased visibility due to the effect of the black rubber bands. Therefore, it is desirable to have previously correctly delineated the target area by placement of markers, in order to maximize complete endoscopic resection. The learning curve for MBM is shorter compared with that of cap-assisted EMR, because it combines the techniques of variceal band ligation and polypectomy.
Endoscopic submucosal dissection (ESD) was initially introduced for the endoscopic treatment of early gastric cancer in Japan[64,65]. It was developed for the en-bloc resection of large lesions and enables precise histological assessment of specimens. The comparison between ESD and EMR in the treatment of early esophageal carcinoma is debatable. EMR and ESD have been suggested as alternatives to esophagectomy in the treatment of these lesions, without lymph node metastasis. A meta-analysis has compared the efficacy and safety of EMR and ESD for the treatment of early esophageal carcinoma[66]. Five retrospective trials were identified and a total of 710 patients and 795 lesions were included. The results confirmed substantial advantages of ESD over EMR for early esophageal carcinoma regarding en bloc resection rate, histologically complete resection rate and local recurrence even for small lesions, without increasing the complication rate. A previous meta-analysis by Cao et al[67] compared clinical outcomes of ESD with EMR in the treatment of tumors of the gastrointestinal tract, and they found that ESD showed better en bloc and curative resection rates and local recurrence, but was more time-consuming and had higher rates of bleeding and perforation complications.
A recent review on the safety and efficacy of MBM compared with ESD for the treatment of early neoplasia in Barrett’s or neoplasias at the esophagogastric junction (EGJ), showed that the recurrence rate was slightly higher in the EMR group (2.8%) compared with the ESD group (0.3%), but the difference did not reach statistical significance (P = 0.06)[68]. All recurrences in the EMR group were managed by additional endoscopic resections. Complete eradication rate in the EMR group was 95.5%. Curative resection rate in the ESD group was 75.5%. The risk of delayed bleeding and perforation rates in both groups was similar (EMR group 1.2%; ESD group 2.1%, P = 0.26). The perforation rate in the EMR group (1.2%) was similar to that in the ESD group (1.5%), and the difference was not statistically significant. The stricture rate was similar in both groups when comparing resection of the neoplastic lesion alone. Stricture rates increased rapidly in the SRER group when the complete Barrett’s mucosa was resected. The procedure time was less time-consuming in the EMR group (mean time: 36.7 min, 95%CI: 34.5-38.9) compared with the ESD group (mean time: 83.3 min, 95%CI: 57.4-109.2). The authors concluded that the MBM technique appears as effective as ESD when comparing important outcome parameters on the eradication of early Barrett’s or EGJ neoplasia. There are no differences in the outcome when comparing strictures, bleedings and perforation rates for both EMR and ESD in experienced hands. The MBM technique has considerable advantages in being both easier to master and less time-consuming.
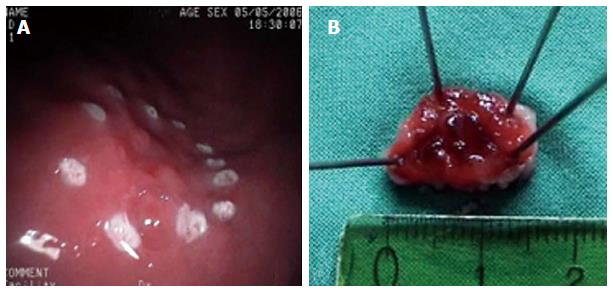
The endoscopic treatment of early gastric cancer (EGC) with mucosectomy has increasingly proven to be an effective modality for local treatment, especially if the tumor is limited to the mucosa, of a size no greater than 2 cm, with neither histologic ulceration nor lymphatic vessel invasion and a cancer-negative resection line. Mucosectomy has also demonstrated to be useful in the resection of precancerous lesions such as adenomas[69-71]. European experience in EMR for early gastric cancer is still relatively low, since early stomach cancer is diagnosed at a much lower rate in Europe than in Japan and generally, operable patients are referred to surgery for radical resection. With EMR, complete resection rates have been reported in 74%-97% and survival rates between 95%-100%[14,72]. The most frequent complication is bleeding (1%-20%)[73] and recurrence rates were observed to be between 2%-13%[74]. EMR appears to have a better post-procedure quality of life compared with surgical gastrectomy[75]. Data on the use of MBM in the management of patients with EGC is small. Our experience is very limited but, highly positive, in selected patients[15]. Three patients diagnosed by biopsy of EGC (type IIa) and 1 patient with HGD were treated by MBM (Figure 3). The length of lesions ranged between 10 mm and 20 mm. MBM was accomplished in 1 session in each patient. The histological analysis of MBM specimens confirmed mucinous adenocarcinoma with submucosal infiltration (1 patient who was referred for surgery), EGC (2 patients), and HGD (1 patient). Minor bleeding without clinical consequences occurred in 1 patient and was controlled by local adrenaline injection. Endoscopic surveillance was recommended for all our patients and Helicobacter pylori was eradicated. Regular follow-up did not detect any recurrent lesions. MBM in EGC may have also diagnostic and therapeutic implications. Further studies are needed in this field to determine the clinical impact of this therapeutic approach.
MBM is an exciting EMR technique that provides heightened levels of diagnostic accuracy and minimally invasive therapy for the management of upper gastrointestinal tract lesions with advanced dysplasia. This minimally invasive technique is safe and effective for complete resection of superficial lesions with high-grade dyspasia or early cancer.
P- Reviewer: Noguera J, Vynios D S- Editor: Tian YL L-Editor: A E- Editor: Wu HL
| 1. | van Lanschot JJ, Hulscher JB, Buskens CJ, Tilanus HW, ten Kate FJ, Obertop H. Hospital volume and hospital mortality for esophagectomy. Cancer. 2001;91:1574-1578. [PubMed] |
| 2. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2215] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 3. | Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2455] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 4. | Swisher SG, Deford L, Merriman KW, Walsh GL, Smythe R, Vaporicyan A, Ajani JA, Brown T, Komaki R, Roth JA. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 254] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc. 2000;52:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Ell C, May A, Gossner L, Pech O, Günter E, Mayer G, Henrich R, Vieth M, Müller H, Seitz G. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 430] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Vieth M, Ell C, Gossner L, May A, Stolte M. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy. 2004;36:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 454] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 9. | Ahmadi A, Draganov P. Endoscopic mucosal resection in the upper gastrointestinal tract. World J Gastroenterol. 2008;14:1984-1989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Nigro JJ, Hagen JA, DeMeester TR, DeMeester SR, Theisen J, Peters JH, Kiyabu M. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg. 1999;230:433-448; discussion 438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Stein HJ, Feith M, Mueller J, Werner M, Siewert JR. Limited resection for early adenocarcinoma in Barrett’s esophagus. Ann Surg. 2000;232:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Crumley AB, Going JJ, McEwan K, McKernan M, Abela JE, Shearer CJ, Stanley AJ, Stuart RC. Endoscopic mucosal resection for gastroesophageal cancer in a U.K. population. Long-term follow-up of a consecutive series. Surg Endosc. 2011;25:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M, Sanders S, Gay L, Pech O, Longcroft-Wheaton G. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Marc G, Lopes CV. Endoscopic resection of superficial gastrointestinal tumors. World J Gastroenterol. 2008;14:4600-4606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Espinel J, Pinedo E, Rascarachi G. Endoscopic mucosal resection with a multiband ligator for the treatment of Barrett s high-grade dysplasia and early gastric cancer. Rev Esp Enferm Dig. 2009;101:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Soehendra N, Seewald S, Groth S, Omar S, Seitz U, Zhong Y, de Weerth A, Thonke F, Schroeder S. Use of modified multiband ligator facilitates circumferential EMR in Barrett’s esophagus (with video). Gastrointest Endosc. 2006;63:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Alvarez Herrero L, Pouw RE, van Vilsteren FG, ten Kate FJ, Visser M, Seldenrijk CA, van Berge Henegouwen MI, Weusten BL, Bergman JJ. Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett’s esophagus. Endoscopy. 2011;43:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Pouw RE, van Vilsteren FG, Peters FP, Alvarez Herrero L, Ten Kate FJ, Visser M, Schenk BE, Schoon EJ, Peters FT, Houben M. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc. 2011;74:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Gerke H, Siddiqui J, Parekh KR, Vanderheyden AD, Mitros FA. Esophageal perforation complicating band ligator-assisted mucosal resection. Gastrointest Endosc. 2009;69:153-154; discussion 154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | van Vilsteren FG, Pouw RE, Herrero LA, Peters FP, Bisschops R, Houben M, Peters FT, Schenk BE, Weusten BL, Visser M. Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program. Endoscopy. 2012;44:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Seewald S, Akaraviputh T, Seitz U, Brand B, Groth S, Mendoza G, He X, Thonke F, Stolte M, Schroeder S. Circumferential EMR and complete removal of Barrett’s epithelium: a new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Giovannini M, Bories E, Pesenti C, Moutardier V, Monges G, Danisi C, Lelong B, Delpero JR. Circumferential endoscopic mucosal resection in Barrett’s esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy. 2004;36:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Peters FP, Kara MA, Rosmolen WD, ten Kate FJ, Krishnadath KK, van Lanschot JJ, Fockens P, Bergman JJ. Stepwise radical endoscopic resection is effective for complete removal of Barrett’s esophagus with early neoplasia: a prospective study. Am J Gastroenterol. 2006;101:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Larghi A, Lightdale CJ, Ross AS, Fedi P, Hart J, Rotterdam H, Noffsinger A, Memeo L, Bhagat G, Waxman I. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Pouw RE, Peters FP, Sempoux C, Piessevaux H, Deprez PH. Stepwise radical endoscopic resection for Barrett’s esophagus with early neoplasia: report on a Brussels’ cohort. Endoscopy. 2008;40:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, Lin S, Ferguson MK, Posner MC, Waxman I. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Pouw RE, Seewald S, Gondrie JJ, Deprez PH, Piessevaux H, Pohl H, Rösch T, Soehendra N, Bergman JJ. Stepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Gerke H, Siddiqui J, Nasr I, Van Handel DM, Jensen CS. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc. 2011;74:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | van Vilsteren FG, Pouw RE, Seewald S, Alvarez Herrero L, Sondermeijer CM, Visser M, Ten Kate FJ, Yu Kim Teng KC, Soehendra N, Rösch T. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 31. | Peters FP, Kara MA, Curvers WL, Rosmolen WD, Fockens P, Krishnadath KK, Ten Kate FJ, Bergman JJ. Multiband mucosectomy for endoscopic resection of Barrett’s esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol. 2007;19:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 33. | Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, Gupta N, Gaddam S, Singh M, Singh V. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179-1186, 1186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Wani S. Management of low-grade dysplasia in Barrett’s esophagus. Curr Opin Gastroenterol. 2012;28:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, Bohmer C, Mallant-Hent RC, van Oijen A, Naber AH. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 36. | Esaki M, Matsumoto T, Hirakawa K, Nakamura S, Umeno J, Koga H, Yao T, Iida M. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy. 2007;39:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Mino-Kenudson M, Hull MJ, Brown I, Muzikansky A, Srivastava A, Glickman J, Park DY, Zuckerberg L, Misdraji J, Odze RD. EMR for Barrett’s esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660-676; quiz 767, 769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Seewald S, Ang TL, Omar S, Groth S, Dy F, Zhong Y, Seitz U, Thonke F, Yekebas E, Izbicki J. Endoscopic mucosal resection of early esophageal squamous cell cancer using the Duette mucosectomy kit. Endoscopy. 2006;38:1029-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Tsujitani S, Oka S, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery. 1999;125:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Reid BJ, Blount PL, Feng Z, Levine DS. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:3089-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Prasad GA, Buttar NS, Wongkeesong LM, Lewis JT, Sanderson SO, Lutzke LS, Borkenhagen LS, Wang KK. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett’s esophagus. Am J Gastroenterol. 2007;102:2380-2386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Peters FP, Brakenhoff KP, Curvers WL, Rosmolen WD, Fockens P, ten Kate FJ, Krishnadath KK, Bergman JJ. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc. 2008;67:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Young PE, Gentry AB, Acosta RD, Greenwald BD, Riddle M. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Pouw RE, Heldoorn N, Alvarez Herrero L, ten Kate FJ, Visser M, Busch OR, van Berge Henegouwen MI, Krishnadath KK, Weusten BL, Fockens P. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc. 2011;73:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Pech O, Günter E, Dusemund F, Origer J, Lorenz D, Ell C. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | May A, Günter E, Roth F, Gossner L, Stolte M, Vieth M, Ell C. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004;53:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 48. | Wani SB, Edmundowicz SA, Abrams JA, Gupta N, Green D, Hovis CE, Gaddam S, Higbee AD, Bansal A, Early DS. Accuracy of Endoscopic Ultrasonography (EUS) in Staging Early Neoplasia in Barrett’s Esophagus (BE): Results From a Large Multicenter Cohort Study. Gastrointest Endosc. 2011;73 Suppl 4:AB166–AB167. [DOI] [Full Text] |
| 49. | Chung A, Bourke MJ, Hourigan LF, Lim G, Moss A, Williams SJ, McLeod D, Fanning S, Kariyawasam V, Byth K. Complete Barrett’s excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy. 2011;43:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Wu J, Pan YM, Wang TT, Gao DJ, Hu B. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc. 2014;79:233-241.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Chadwick G, Groene O, Markar SR, Hoare J, Cromwell D, Hanna GB. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc. 2014;79:718-731.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg. 2011;254:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 53. | Yamada M, Oda I, Nonaka S, Suzuki H, Yoshinaga S, Taniguchi H, Sekine S, Kushima R, Saito Y, Gotoda T. Long-term outcome of endoscopic resection of superficial adenocarcinoma of the esophagogastric junction. Endoscopy. 2013;45:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, Hartmann U, Manner N, Huijsmans J, Gossner L. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652-660.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 55. | Qumseya B, Panossian AM, Rizk C, Cangemi D, Wolfsen C, Raimondo M, Woodward T, Wallace MB, Wolfsen H. Predictors of esophageal stricture formation post endoscopic mucosal resection. Clin Endosc. 2014;47:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Tomizawa Y, Iyer PG, Wong Kee Song LM, Buttar NS, Lutzke LS, Wang KK. Safety of endoscopic mucosal resection for Barrett’s esophagus. Am J Gastroenterol. 2013;108:1440-1447; quiz 1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Konda VJ, Gonzalez Haba Ruiz M, Koons A, Hart J, Xiao SY, Siddiqui UD, Ferguson MK, Posner M, Patti MG, Waxman I. Complete endoscopic mucosal resection is effective and durable treatment for Barrett’s-associated neoplasia. Clin Gastroenterol Hepatol. 2014;12:2002-10.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Masci E, Viale E, Notaristefano C, Mangiavillano B, Fiori G, Crosta C, Dinelli M, Maino M, Viaggi P, Della Giustina F. Endoscopic mucosal resection in high- and low-volume centers: a prospective multicentric study. Surg Endosc. 2013;27:3799-3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Peters FP, Brakenhoff KP, Curvers WL, Rosmolen WD, ten Kate FJ, Krishnadath KK, Fockens P, Bergman JJ. Endoscopic cap resection for treatment of early Barrett’s neoplasia is safe: a prospective analysis of acute and early complications in 216 procedures. Dis Esophagus. 2007;20:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | May A, Gossner L, Behrens A, Kohnen R, Vieth M, Stolte M, Ell C. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 62. | Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc. 2007;65:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 341] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 63. | Brahmania M, Lam E, Telford J, Enns R. Endoscopic mucosal resection: early experience in British Columbia. Can J Gastroenterol. 2010;24:239-244. [PubMed] |
| 64. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 264] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Kodashima S, Fujishiro M, Yahagi N, Kakushima N, Omata M. Endoscopic submucosal dissection using flexknife. J Clin Gastroenterol. 2006;40:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Wang J, Ge J, Zhang XH, Liu JY, Yang CM, Zhao SL. Endoscopic submucosal dissection versus endoscopic mucosal resection for the treatment of early esophageal carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:1803-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 67. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 68. | Komeda Y, Bruno M, Koch A. EMR is not inferior to ESD for early Barrett’s and EGJ neoplasia: An extensive review on outcome, recurrence and complication rates. Endoscopy International Open. 2014;2:E58-E64. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Barreda B F, Sánchez L J. Endoscopic treatment of early gastric cancer andprecancerous gastric lesions with mucosectomy. Rev Gastroenterol Peru. 1998;18:214-226. [PubMed] |
| 70. | Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, Kida M, Oida M, Saigenji K. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Tada M, Tanaka Y, Matsuo N, Shimamura T, Yamaguchi K. Mucosectomy for gastric cancer: current status in Japan. J Gastroenterol Hepatol. 2000;15 Suppl:D98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Park JC, Lee SK, Seo JH, Kim YJ, Chung H, Shin SK, Lee YC. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24:2842-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009;41:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Ono H, Hasuike N, Inui T, Takizawa K, Ikehara H, Yamaguchi Y, Otake Y, Matsubayashi H. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2008;11:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 75. | Ohyama T, Kobayashi Y, Mori K, Kano K, Sakurai Y, Sato Y. Factors affecting complete resection of gastric tumors by the endoscopic mucosal resection procedure. J Gastroenterol Hepatol. 2002;17:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |