Published online Apr 16, 2015. doi: 10.4253/wjge.v7.i4.308
Peer-review started: August 20, 2014
First decision: September 16, 2014
Revised: January 5, 2015
Accepted: January 18, 2015
Article in press: January 20, 2015
Published online: April 16, 2015
Processing time: 242 Days and 16.9 Hours
Biliary strictures present a diagnostic challenge and a conundrum, particularly when an initial work up including abdominal imaging and endoscopic retrograde cholangiopancreatography based sampling are non-diagnostic. Advances in endoscopic imaging have helped us diagnose these strictures better. However, even with modern technology, some strictures remain a diagnostic challenge. The proximity of bile fluid to the bile duct epithelia makes it an attractive option to investigate for bio-markers, which might be representative of the functions/abnormal changes taking place in the biliary system. A number of biomarkers in bile have been discovered recently in approaching biliary strictures with their potential future diagnostic utility, further supported by the immunohistochemical analysis of the resected tissue specimens. Novel biliary biomarkers especially carcinoembryonic cell adhesion molecule 6 and neutrophil gelatinase-associated lipocalin seem promising in differentiating malignant from benign biliary strictures. Recent developments in lipidomic profiling of bile are also very promising. Biliary biomarkers appear to complement endoscopic imaging in diagnosing malignant etiologies of biliary stricture. Future studies addressing these biomarkers need to be incorporated to the current endoscopic techniques to determine the best approach in determining the etiology of biliary strictures.
Core tip: Pancreato-biliary malignancies remain a diagnostic challenge despite advances in endoscopy and imaging. Serum carbohydrate antigen 19-9 which is the most commonly used tumor marker has not been able to complement the endoscopic techniques effectively. Bile fluid is a better representative of the pancreato-biliary malignancies and various tumor markers in bile have been described recently with advances in proteomics. Carcinoembryonic cell adhesion molecule 6, neutrophil gelatinase-associated lipocalin and other novel biliary markers seem promising with high sensitivities and specificities, little affected by the presence of inflammation or the degree of biliary obstruction. These are potential future tumor markers that can complement endoscopic techniques in diagnosing malignant biliary strictures.
- Citation: Lourdusamy V, Tharian B, Navaneethan U. Biomarkers in bile-complementing advanced endoscopic imaging in the diagnosis of indeterminate biliary strictures. World J Gastrointest Endosc 2015; 7(4): 308-317
- URL: https://www.wjgnet.com/1948-5190/full/v7/i4/308.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i4.308
Pancreato-biliary malignancies are often difficult to diagnose with the current diagnostics, and many are detected in their advanced stages with poor prognosis[1,2]. Endoscopic retrograde cholangiopancreatography (ERCP) with brushings is often the routine choice for the endoscopists to diagnose these malignancies, but is limited by its low to moderate sensitivities[3,4]. Also, the desmoplastic nature of cholangiocarcinoma (CCA) can make the histological diagnosis more complicated[5]. Fluoroscence in situ hybridization polysomy to increase the sensitivity of diagnosis has also not yielded very significant differences[6,7]. Imaging techniques like endoscopic ultrasound with needle aspirations have certain limitations. Though they offer better sensitivities for pancreatic malignancies[8], they have been found to increase the risk of peritoneal metastasis in hilar CCA and cannot be justified for routine use, particularly in hilar CCA[9]. Advanced endoscopic-imaging options such as use of cholangioscopes require expertise in the field and not much data is available on their use[10]. Peroral cholangioscopy can provide direct visualization of the bile ducts, and targeted biopsies obtained through spyglass cholangioscopy (single operator cholangioscopy) might help diagnose malignant lesions especially cholangiocarcinoma better than the conventional ERCP brushing/biopsy techniques[11,12]. But they are available only in a few centers, and more randomised trials comparing the effectiveness of spyglass biopsies with the routine ERCP brush cytology or forceps biopsies are necessary to justify their advantages in routine use. Clinical and/or radiological methods thus have not been successful in the early detection of the biliary tract malignancies. Surgery is the only cure for pancreato-biliary malignancies, and early detection of these lesions is necessary. With the limitations of the above diagnostics, several tumor markers have been analyzed to complement the endoscopic techniques. The relative rarity of these biliary tract neoplasms has been a hindrance for the progression in biomarker detection, though there have been recent advances in the techniques of biomarker analysis, especially the proteomics.
One of the most commonly employed diagnostic/prognostic markers in pancreato-biliary malignancies is serum carbohydrate antigen 19-9 (CA 19-9), which is also not without limitations. Firstly, in about 10% of the patients with a negative Lewis antigen, the test would prove futile[13]. Also there have been reports on the limitation of serum CA 19-9 with its values getting affected by the presence of biliary obstruction, which can be a confounding factor in differentiation of benign and malignant lesions[14,15]. Though it can be a reasonably good prognostic marker, its diagnostic utility is not very convincing. Hence the search for new markers continues.
Serum has been more easily the choice for many studies in identifying biomarkers, as it is easier to obtain unlike bile which requires ERCP. The proximity of bile to the bile duct epithelia makes it a harbor of various substances, which might be representative of the functions/abnormal changes taking place in the biliary system. Bile can be obtained during the routine diagnostic or therapeutic ERCPs performed in patients with indeterminate biliary strictures without imparting any additional risks apart from the baseline risks of the procedure. Novel methods have also been used for obtaining bile (BIDA-Bile Intraductal Aspiration)[16]. Here, the biliary catheter is connected to a central suction line through a specimen trap, and obtaining bile can be quick and simple. In one of the recent studies, it was found that a large proportion of the proteins detected in bile were cellular (“secreted’”from the surrounding biliary system), stressing the importance of bile fluid analysis[17]. The fact that after bile centrifugation, the supernatant analysis and not the cell debris (sediments) reveals the presence of these tumor markers could explain that it is mostly the secreted substances in bile that are analyzed[17]. Hence, paucity of shed cells in bile should not affect the bile analysis. The results of many of the recent studies identifying novel bile biomarkers have been encouraging with their potential future diagnostic utility, further supported by the immunohistochemical analysis of the resected tissue specimens. Table 1 summarizes the various bile bio-markers that have been studied in biliary strictures.
| Bile biomarkers | Cut off value | Identification of CCA/pancreatic cancer | Sensitivity | Specificity | Comments |
| VEGF[57] | 0.5 ng/mL | Pancreatic cancer (vs benign) | 93.3% | 72.7% | VEGF level in bile in CCA was not elevated. Another study[58] demonstrated increased serum VEGF in CCA-possible basolateral secretion of VEGF in bile duct epithelia in CCA? |
| 0.5 ng/mL | Pancreatic cancer (vs CCA) | 93.3% | 88.9% | ||
| IGF[58] | NA | CCA | NA | NA | ROC (area under the curve = 1); Serum IGF levels were similar among CCA, pancreatic cancer and benign groups |
| CEAM6[50] CEAM6 + Serum CA 19-9 | 67.9 ng/mL | Malignant (CCA + pancreatic cancer) | 93% | 83% | Biliary levels were not critically affected by bile duct obstruction; Serum CEAM6 levels were not significantly different between the malignant and benign groups |
| 97% | 83% | ||||
| 67.9 ng/mL, 157 kU/L | |||||
| NGAL[37] NGAL + Serum CA 19-9 | 459 ng/mL 459 ng/mL, 30.1 U/mL | Malignant (CCA + pancreatic cancer) | 77.3% 91% | 72.2% 66.7% | In both the studies, serum NGAL levels were not significantly different between benign and malignant groups; biliary levels were independent of serum bilirubin levels. Especially elevated in early well differentiated carcinomas in tissue immunohistochemistry-possible future application in PSC to R/O early malignant lesions/dysplasias |
| NGAL[38] NGAL + Serum CA 19-9 | 570 ng/mL 3000 ng /mL, 125 U/L | Malignant (CCA + Pancreatic cancer + GB carcinoma + metastasis) | 94% 85% | 55% 82% | |
| HSP[67] | |||||
| HSP 27 | 2.52 ng/mL | CCA | 90% | 90% | Serum levels of these markers were not significantly different between CCA and benign strictures |
| HSP 70 | 5.67 ng/mL | 80% | 80% | ||
| HSP 27 + HSP 70 | 10.2 ng/mL | 90% | 100% | ||
| Galectin Ligands | |||||
| Mac 2-BP[76] | 853 ng/mL | All malignant strictures | 69% | 67% | Serum levels were not elevated in malignancies |
| Fibronectin[77] | 40 ng/μmol | CCA | 57% | 79% | - |
| MCM 5[82] | 1000 (cells) | CCA + Pancreatic cancer | 66% | 94% | MCM 5 levels in bile were significantly more sensitive than brush cytology (66% vs 20%; P = 0.004) |
| Pancreatic Elastase/ Amylase[83] | 0.065 | CCA | 82% | 89% | mRNA of PE 3B was also up-regulated in CCA tissues |
| Lipids[84] | |||||
| ON-PC | 6020.1 nmol/L | CCA | 85.7% | 80.3% | - |
| S-PC | 12 nmol/L | CCA | 83.3% | 77.8% | |
| ON-PC + S-PC | 6032.2 nmol/L | CCA | 100% | 83.3% | |
| VOCs | |||||
| (TMA, acetone, isoprene, dimethyl sulfide, and acetaldehyde)[86] | Logarithmic model | Pancreatic cancer | 83.3% | 81.9% | - |
| (Acrylonitrile, methyl hexane and benzene)[87] | Logarithmic model | CCA in the setting of PSC | 90.5% | 72.7% | Biliary levels of VOCs in CCA (in the setting of PSC) were significantly lower than (benign) PSC |
Serum CA 19-9 and carcinoembryonic antigen (CEA) are the tumor markers routinely used in the diagnosis and prognosis of pancreato-biliary malignancies[18-20]. The utility of these glycoprotein tumor markers in bile has been studied too, and their diagnostic performance has not been consistent. In a large study involving 100 patients, reasonably high sensitivity of 84% and a specificity of 64% was obtained with biliary CEA (levels > 20 ng/mL), but there was a considerable overlap between the malignant and benign lesions. Moreover, in the multivariate analysis biliary CEA levels were not predictive of malignancy[21]. The low to moderate specificities for these markers suggest that they are increased in benign/inflammatory conditions too. Multiple studies have shown that biliary CA 19-9 and CEA did not add much to the diagnostic accuracy when compared to the serum levels, as they had high false positive results[22-25]. Further supporting this view, in an older study[26], a reasonably high specificity of 84% with CEA was obtained, when benign biliary diseases due to stones were excluded from the study. In another recent study of biliary strictures[27], CA19-9 levels in bile had a sensitivity of 74%, but a poor specificity of 34%, even after eliminating patients with cholangitis.
CA 125, a marker for ovarian cancer was found to be the most specific marker in bile for CCA (specificity-76%, sensitivity-59%) in a study, which could complement endoscopic methods either alone or in combination with CEA (specificity-88%) for diagnosing malignancy[22]. Summarizing, the available studies of these tumor markers in bile are limited. However these appear to have limited diagnostic utility.
The changes that occur at protein level when a normal cell undergoes malignant transformation form the basis of proteomics[28]. The analytical techniques in proteomics, which are used in quantifying the proteins, are the liquid chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy, apart from Western blot (Immuno blot) and ELISA. Bile serves as the direct media, which carries proteins from the local environment (liver, biliary tract and pancreas). This makes it a very valuable source of novel proteins for identifying biomarkers suggestive of biliary tract malignancy. But, one of the limitations of bile is its complex constitution with various components, and proteins accounting for a mere 7% of the total dry weight; and differential fractionation (centrifugation) could be used to reduce the complexity, concentrating the protein component as a preparatory for mass spectrometry[17]. Delipidation and desalination of bile to remove the abundant phospholipids and bile salts have also been proposed[29]. Protein biomarkers might be suggestive of the possible mechanisms of carcinogenesis, as they are reflective of the changes taking place in DNA, but more importantly in clinical context, might play a major role in improving the prognosis through early detection. Alterations of tissue proteins can occur during the early stages of carcinogenesis, and hence proteomics could detect cancers early[30].
Bile can be a host of various proteins, especially those secreted from the hepatocytes/biliary epithelia and the enzymes from the distally located pancreas. Presence of various classes of proteins such as the transport proteins (haptoglobulin, ceruloplasmin, albumin, and globulin), immune proteins (complements, immunoglobulins), and other liver and pancreatic enzymes (GGT, Adenosine deaminase, pancreatic lipase, carboxypeptidase) are expected to contribute to a large proportion of the proteins in bile[31]. Hence to identify the low abundance proteins that might play a role in tumorigenesis, albumin and immunoglobulins, were removed prior to separating the peptides with electrophoresis and subsequent analysis by mass spectrometry in a study[32]. Also, the presence of normally occurring proteins in elevated levels could be pathologic, suggestive of increased apoptosis/protein catabolism occurring in malignant conditions[33]. In this study, a model for identification of CCA was based on the differential levels of normally occurring proteins in bile. Hence it is not always the tumor-associated proteins that give clue regarding the possibility of malignancies.
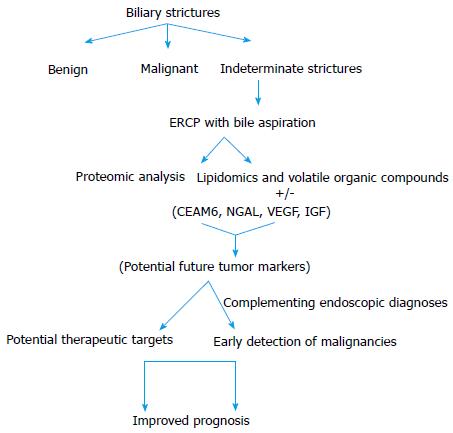
Novel biliary proteins that appear promising with supporting evidences from tissue immunochemistry are carcinoembryonic cell adhesion molecule 6 (CEAM6) and Neutrophil gelatinase-associated lipocalin (NGAL), though available literatures on their biliary levels are not many. Lipocalins are glycoproteins found to be associated with various inflammatory conditions and malignancies[34-36]. Table 1 describes the characteristics of the potential tumor markers in bile. Figure 1 shows the approach to the biliary strictures through bile biomarkers.
NGAL: The presence of NGAL in bile was first reported in a patient with CCA[31]. Two recent studies have found significantly elevated biliary levels of NGAL in pancreato-biliary malignancies[37,38]. In the most recent study, the sensitivities and specificities of NGAL in diagnosing malignant biliary strictures were 77% and 72% respectively when the cut off was taken as 459 ng/mL[37]. A higher sensitivity of 94% was achieved in the other study with the cutoff of 570 ng/mL, albeit with decreased specificity (55%)[38]. Addition of serum CA 19-9 to biliary NGAL had varying impacts on the sensitivities and specificities in both studies, but led to better results than obtained with biliary NGAL levels alone. Further encouraging was biliary NGAL’s low correlation to serum bilirubin levels in both the studies, indicating that NGAL’s elevation might be independent of the level of biliary obstruction. Significant NGAL elevation (tissue immunohistochemistry) in early dysplastic pancreatic lesions (including pancreatic intraepithelial neoplasia-1) in addition to well-differentiated adenocarcinoma was observed in a study[39]. Most studies report biliary/tissue NGAL rather than serum NGAL to be more representative of pancreato-biliary malignancies[37-40]. Prospective studies comparing both serum and biliary NGAL levels are much needed.
The role of NGAL in cancer progression, metastasis and potential therapy deserves mention[41,42]. Targeted silencing of NGAL gene in human CCA cell lines significantly decreased the in vitro cellular migration and invasion, suggestive of its role in cancer metastasis, and its potential for targeted anti-cancer therapy[41]. On the contrary, another study reported that NGAL as a potential suppressor of invasion and angiogenesis by suppressing vascular endothelial growth factor (VEGF) production in pancreatic cells[42]. Also in this study, tissue NGAL was expressed only by the well-differentiated cells and not by the poorly differentiated pancreatic adenocarcinoma cells. This suggests the possible diagnostic role of NGAL in early pancreato-biliary malignancies, such as in the setting of primary sclerosing cholangitis which is a risk factor for the development of CCA[43-45]. Also as most of these patients undergo repeated ERCP stenting for biliary drainage, obtaining bile would not be a major issue too. Future studies on bile levels of NGAL in primary sclerosing cholangitis (PSC) patients with suspicious strictures would be valuable and interesting.
CEAM6: Other biliary biomarker, which seems very promising with high diagnostic sensitivities and specificities, is CEAM6. It is a cell adhesion molecule belonging to the immunoglobulin super family, which plays an important role in cell adhesion, invasion and metastasis[46]. Increased tissue expression of CEAM6 on immunohistochemical analysis of tissues in 82/89 patients with pancreatic adenocarcinoma was reported initially[47]. In this study, it was also found that negative expression of CEAM6 was significantly associated with absent lymph node metastasis and increased postoperative survival. The same group had earlier demonstrated an increase in caspase mediated apoptotic response and inhibited in vivo metastatic potential of pancreatic adenocarcinoma cells with CEAM6 gene silencing. Thus this could be a possible therapeutic target for pancreatic adenocarcinoma[48]. Infact in a preclinical animal study, Strickland et al[49] targeted CEAM6 expressing pancreatic tumor cells using anti-CEAM6 monoclonal antibody, and observed marked inhibition of tumor growth. Its role in cancer progression, invasion and metastasis remains obvious.
Biliary CEAM6 levels were found to be elevated in malignant biliary lesions from a recent proteomic analysis of bile involving 41 patients, and the results appear promising[50]. With a cut off value of 67.9 ng/mL, the sensitivity and specificity of CEAM6 in diagnosing malignant strictures was 93% and 83% respectively, with area under the curve (AUC) of 0.92. The results were also not critically affected by biliary obstruction according to the authors when the correlation between the markers and bilirubin levels was analyzed. Addition of serum CA 19-9 further improved the diagnostic sensitivity, specificity and accuracy (sensitivity-97%, specificity-83%, AUC-0.96). The same group showed that CEAM6 was rather secreted into bile directly as it was found in the soluble form (supernatant) and not as a sediment along with the cellular debris, proving the role of bile analysis in identifying the marker.
VEGF: VEGF plays an important role in angiogenesis in cancer by stimulating the vascular endothelial proliferation, increasing vascular permeability and vasodilatation[51]. Expression of VEGF in pancreatic and cholangiocarcinoma has been described[52-54]. The role of VEGF in pancreatic cancers is especially significant as they are being used in clinical trials as therapeutic targets[55,56]. We recently analyzed the VEGF levels in bile from patients with biliary strictures; and with a cut off value of 0.5 ng/mL, we distinguished pancreatic cancer from CCA with a sensitivity of 93.3% and a specificity of 88.9%[57]. Using the same cut off value, pancreatic cancer could be differentiated from benign lesions with a sensitivity of 93.3% and a specificity of 72.7%. We also confirmed the pancreatic specificity of biliary VEGF through immunohistochemical analysis of the resected pancreatic specimens. An earlier study found increased levels of VEGF in serum of patients with CCA when compared to other groups, but the levels in bile did not differ significantly among the benign and malignant groups[58]. The insignificant levels of VEGF in bile in CCA patients could be linked to the baso-lateral secretion of VEGF from the bile duct epithelium, and not into the lumen. But in the Italian study, the levels of biliary VEGF were normal in the patients with pancreatic cancer, which contrasts with our observations. When compared to 84%, only 30% in the Italian study had histological confirmation. Future studies need to target the above mentioned issues.
Insulin like growth factor: In the same study as above, they also found biliary insulin like growth factor (IGF) to be diagnostic of extra-hepatic CCA, with the AUC = 1, when benign conditions or pancreatic cancer were taken as the control[58]. The levels of biliary IGF were also not correlating with the degree of cholestasis. IGF has been found to be associated with many cancers such as endometrial and other gynecological malignancies, lung cancers, and various other cancers including pancreatic cancers[59-62]. In a recently published study, silencing IGF 1 receptors in human pancreatic ductal adenocarcinoma cell lines inhibited pancreatic cell growth and metastasis by blocking many key signaling pathways[63]. IGF-1R antagonists have already entered clinical trials in patients with metastatic pancreatic cancer[64,65]. More studies on biliary levels of IGF to enhance its diagnostic significance in pancreato biliary malignancies are needed.
Heat shock proteins: Heat shock proteins (HSP) play an important role in protein folding and are anti-apoptotic and favors tumorigenesis[66]. A recent study showed that by combining the biliary values of HSP27 and HSP70, the sensitivity and specificity of diagnosing CCA was 90% and 100%, respectively[67]. However there was no significant increase of these proteins in serum of the patients with CCA when compared to benign lesions, though immunohistochemistry showed increased expression of these proteins in CCA and biliary intraepithelial neoplastic cells[67]. Plasma antibodies against HSP 70 were very recently described as one of the potential markers of CCA[68]. Expression of HSP 27 and HSP 70 has been found to modulate the response of pancreatic cells to chemotherapy and hence might be potential prognostic markers as well[69,70]. In a very recent study where CCA cell lines from 78 patients with intrahepatic CCA were treated with a combination of HSP 90 inhibitor and a PTEN related pathway inhibitor in vitro, antiproliferative and proapoptotic effects were observed in the cell lines, demonstrating their potential therapeutic use[71]. In another study HSPD1, a heat shock protein was overexpressed in bile in patients with CCA[72]. Here in this study, other markers such as SSP411 (spermatogenesis associated protein) and PGAM-1 (phosphoglycerate mutase) in bile were also significantly elevated in CCA. Its sensitivity and specificity for detecting CCA were 90% and 83% respectively in that study. The role of these proteins, although studied remains unclear because of low specificity.
Galectin ligands: Galectins mediate cell to cell, cell to matrix interactions, apoptosis and angiogenesis; Fibronectin, Mac 2-binding protein (Mac 2-BP) and laminin are some of the ligands[73-75]. Koopmann et al[76] found that biliary Mac 2-BP could differentiate benign and malignant biliary tract lesions with a sensitivity and specificity of 69% and 67% respectively, that was comparable to serum CA 19-9. Similarly fibronectin, another ligand for galectin, was found to be a biliary diagnostic marker for CCA with a sensitivity of 57% and a specificity of 79%, but it was also elevated in biliary inflammation[77]. Future studies to validate these observations are necessary.
Minichromosome maintenance proteins: These are involved in DNA replication and have been found to be associated with the carcinogenesis[78-81]. The role of minichromosome maintenance proteins (MCM) 2 and MCM 5 proteins was studied through immunohistochemistry prospectively on 102 consecutive patients undergoing ERCP for biliary strictures[82]. In this study, the levels of MCM 5 in bile were also determined by automated immunoflurometric assay and compared with brush cytology. An additional 45% of cases of pancreato-biliary malignancies were detected through MCM 5 analysis in bile. With a cutoff greater than 1000, the sensitivity and specificity were 66% and 94% respectively, with a good accuracy (AUC 0.8).
Elastase/amylase: Increased levels of pancreatic elastase and decreased amylase levels in bile were detected in patients with CCA compared to benign strictures in a study[83]. The elastase-amylase ratio could detect CCA with a sensitivity and specificity of 82% and 89% respectively, with AUC-0.877. They also detected increased pancreatic elastase 3B mRNA in the CCA tissues.
Lipidomic profiling: In a pilot study, we showed that lipidomic profiling of bile could help differentiating benign and malignant biliary strictures[84]. Oxidative stress in the setting of malignancy results in the expression of oxidized phospholipids on the cancer cells, which are recognized by the host defenses leading to apoptosis of cancer cells[85]. The oxidized phospholipids were analyzed using a specialized liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) assay. Two phosphatidylcholines {ON-PC [1-palmitoyl-2-(9-oxononanoyl)-sn -glycero-3-phosphatidylcholine], S-PC (1-palmitoyl-2-succinoyl-sn -glycero-3-phosphatidylcholine)} were elevated in CCA, with ON-PC being the most diagnostic with a sensitivity and specificity of 86% and 80% respectively (AUC-0.86). The combination of the two yielded even better results with a sensitivity of 100%, specificity of 83% and area under the curve of 0.91. The development of global lipidomics of bile could make this more interesting in the development of specific bio-markers for the diagnosis of CCA.
Volatile organic compounds: Our group has also shown, from our preliminary observation, that volatile organic compounds in bile in the headspaces (gas above the sample) may be useful for early diagnosis of CCA in the setting of PSC and in distinguishing malignant from benign strictures[86,87].
About 5 mL of bile collected at the time of ERCP is centrifuged for 8 min at 150 g and 4 °C and the sample heated to 40 °C to allow the volatile organic compounds (VOCs) in the headspace to equilibrate with the samples. Twenty milliliters of headspace gas was removed and analyzed with a selected ion flow tube mass spectrometry instrument. In a prospective cross sectional study, we showed that the concentrations of 6 compounds (acetaldehyde, acetone, benzene, carbon disulfide, pentane, and trimethylamine) were increased in patients with pancreatic cancer compared with controls (P < 0.05)[86]. In another study, we demonstrated that out of 22 analytes tested, a VOC signature consisting of acrylonitrile, methyl hexane and benzene, had a sensitivity and specificity of 90.5% and 72.7% respectively, with a significantly lower level in CCA in the setting of PSC, after accounting for all confounding variables[87]. By using receiver-operating characteristic curve analysis, we developed a model for the prediction and diagnosis of cholangio-pancreatic cancer based on the levels of signature VOC’s in these two settings[86,87]. This might need validation from our ongoing prospective study and results reproducible from other centers. The extension of this to develop biomarkers based on the concept of exhaled breath VOC print, which could be detected by a simple test, is intriguing as a potential non-invasive diagnostic marker for pancreato-biliary cancer.
To compare the biomarkers in bile and to identify the differentially expressed proteins between intra and extra hepatic CCA would be valuable, and might provide insight on their origin and pathogenesis. In a recent meta analysis, Wiggers and coworkers identified certain markers including VEGF-A, epidermal growth factor receptor, c-erbB-2 (HER-2/neu) through tissue immunohistochemistry that were significantly differing between the intra and extra hepatic CCA[88]. Based on the tumor markers, treatment strategies might also differ between the two. Future comparative studies on bile markers (Intrahepatic vs Extrahepatic CCA) would be worthful.
Novel biliary biomarkers especially CEAM6 and NGAL seem promising in differentiating malignant from benign biliary strictures. Also in malignant strictures, they appear to be elevated in bile rather than serum, which is interesting and must be, evaluated in future studies. Biliary VEGF, IGF, MCM’s, lipidomic profiles and VOC’s are new biomarkers in bile that might become available to clinicians in the near future when facing a challenging patient with biliary strictures. Analyses of biomarkers in bile have yielded encouraging results with supporting evidences from tissue immunohistochemistry in most of the studies. In addition, with their potential therapeutic implications, targeting the malignant cells/receptors with the antibodies/inhibitors remains plausible, and more future studies on establishing their therapeutic role are also necessary. Thus, biliary biomarkers complement endoscopic imaging in diagnosing malignant etiologies of biliary stricture. Future studies addressing these biomarkers need to incorporate endoscopic techniques to determine the best approach in determining the etiology of biliary strictures.
P- Reviewer: Makishima M, Sinagra E, Sirin G S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Friman S. Cholangiocarcinoma--current treatment options. Scand J Surg. 2011;100:30-34. [PubMed] |
| 3. | Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Smoczynski M, Jablonska A, Matyskiel A, Lakomy J, Dubowik M, Marek I, Biernat W, Limon J. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest Endosc. 2012;75:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:943-950.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Chin MW, Byrne MF. Update of cholangioscopy and biliary strictures. World J Gastroenterol. 2011;17:3864-3869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kalaitzakis E, Webster GJ. Endoscopic diagnosis of biliary tract disease. Curr Opin Gastroenterol. 2012;28:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Draganov PV, Chauhan S, Wagh MS, Gupte AR, Lin T, Hou W, Forsmark CE. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol. 1999;10 Suppl 4:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Lin MS, Huang JX, Yu H. Elevated serum level of carbohydrate antigen 19-9 in benign biliary stricture diseases can reduce its value as a tumor marker. Int J Clin Exp Med. 2014;7:744-750. [PubMed] |
| 15. | Ong SL, Sachdeva A, Garcea G, Gravante G, Metcalfe MS, Lloyd DM, Berry DP, Dennison AR. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci. 2008;53:3213-3217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Curcio G, Granata A, Barresi L, Tarantino I, Mocciaro F, Traina M. Bile intraductal aspiration (BIDA): a fast method for bile collection. Endoscopy. 2012;44 Suppl 2 UCTN:E230-E231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Farina A, Dumonceau JM, Delhaye M, Frossard JL, Hadengue A, Hochstrasser DF, Lescuyer P. A step further in the analysis of human bile proteome. J Proteome Res. 2011;10:2047-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB, Park JY. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J. 2013;54:643-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med. 2013;13:340-351. [PubMed] |
| 20. | Malaguarnera G, Paladina I, Giordano M, Malaguarnera M, Bertino G, Berretta M. Serum markers of intrahepatic cholangiocarcinoma. Dis Markers. 2013;34:219-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 21. | Buffet C, Fourré C, Altman C, Prat F, Fritsch J, Choury A, Briantais MJ, Desgrez A, Etienne JP. Bile levels of carcino-embryonic antigen in patients with hepatopancreatobiliary disease. Eur J Gastroenterol Hepatol. 1996;8:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Chen CY, Shiesh SC, Tsao HC, Lin XZ. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepatogastroenterology. 2002;49:616-620. [PubMed] |
| 23. | Lindberg B, Arnelo U, Bergquist A, Thörne A, Hjerpe A, Granqvist S, Hansson LO, Tribukait B, Persson B, Broomé U. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy. 2002;34:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Ker CG, Chen JS, Lee KT, Sheen PC, Wu CC. Assessment of serum and bile levels of CA19-9 and CA125 in cholangitis and bile duct carcinoma. J Gastroenterol Hepatol. 1991;6:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Ohshio G, Manabe T, Watanabe Y, Endo K, Kudo H, Suzuki T, Tobe T. Comparative studies of DU-PAN-2, carcinoembryonic antigen, and CA19-9 in the serum and bile of patients with pancreatic and biliary tract diseases: evaluation of the influence of obstructive jaundice. Am J Gastroenterol. 1990;85:1370-1376. [PubMed] |
| 26. | Nakeeb A, Lipsett PA, Lillemoe KD, Fox-Talbot MK, Coleman J, Cameron JL, Pitt HA. Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg. 1996;171:147-152; discussion 152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Ince AT, Yıldız K, Baysal B, Danalıoğlu A, Kocaman O, Tozlu M, Gangarapu V, Sarbay Kemik A, Uysal Ö, Şentürk H. Roles of serum and biliary CEA, CA19-9, VEGFR3, and TAC in differentiating between malignant and benign biliary obstructions. Turk J Gastroenterol. 2014;25:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Srinivas PR, Srivastava S, Hanash S, Wright GL. Proteomics in early detection of cancer. Clin Chem. 2001;47:1901-1911. [PubMed] |
| 29. | Bonney GK, Craven RA, Prasad R, Melcher AF, Selby PJ, Banks RE. Circulating markers of biliary malignancy: opportunities in proteomics? Lancet Oncol. 2008;9:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Wulfkuhle JD, Liotta LA, Petricoin EF. Proteomic applications for the early detection of cancer. Nat Rev Cancer. 2003;3:267-275. [PubMed] |
| 31. | Kristiansen TZ, Bunkenborg J, Gronborg M, Molina H, Thuluvath PJ, Argani P, Goggins MG, Maitra A, Pandey A. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3:715-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Farid SG, Craven RA, Peng J, Bonney GK, Perkins DN, Selby PJ, Rajendra Prasad K, Banks RE. Shotgun proteomics of human bile in hilar cholangiocarcinoma. Proteomics. 2011;11:2134-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Lankisch TO, Metzger J, Negm AA, Vosskuhl K, Schiffer E, Siwy J, Weismüller TJ, Schneider AS, Thedieck K, Baumeister R. Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology. 2011;53:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, Coppolino G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 36. | McLean MH, Thomson AJ, Murray GI, Fyfe N, Hold GL, El-Omar EM. Expression of neutrophil gelatinase-associated lipocalin in colorectal neoplastic progression: a marker of malignant potential? Br J Cancer. 2013;108:2537-2541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Budzynska A, Nowakowska-Dulawa E, Marek T, Boldys H, Nowak A, Hartleb M. Differentiation of pancreatobiliary cancer from benign biliary strictures using neutrophil gelatinase-associated lipocalin. J Physiol Pharmacol. 2013;64:109-114. [PubMed] |
| 38. | Zabron AA, Horneffer-van der Sluis VM, Wadsworth CA, Laird F, Gierula M, Thillainayagam AV, Vlavianos P, Westaby D, Taylor-Robinson SD, Edwards RJ. Elevated levels of neutrophil gelatinase-associated lipocalin in bile from patients with malignant pancreatobiliary disease. Am J Gastroenterol. 2011;106:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, Lele SM, Ouellette M, Pour PM, Sasson AR. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Leelawat K, Narong S, Wannaprasert J, Leelawat S. Serum NGAL to Clinically Distinguish Cholangiocarcinoma from Benign Biliary Tract Diseases. Int J Hepatol. 2011;2011:873548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Nuntagowat C, Leelawat K, Tohtong R. NGAL knockdown by siRNA in human cholangiocarcinoma cells suppressed invasion by reducing NGAL/MMP-9 complex formation. Clin Exp Metastasis. 2010;27:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, Ramachandran V, Sung B, Chakraborty A, Bresalier RS. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100-6108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 44. | Liu R, Cox K, Guthery SL, Book L, Witt B, Chadwick B, Adler DG. Cholangiocarcinoma and high-grade dysplasia in young patients with primary sclerosing cholangitis. Dig Dis Sci. 2014;59:2320-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 46. | Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007;7:2. [PubMed] |
| 47. | Duxbury MS, Matros E, Clancy T, Bailey G, Doff M, Zinner MJ, Ashley SW, Maitra A, Redston M, Whang EE. CEACAM6 is a novel biomarker in pancreatic adenocarcinoma and PanIN lesions. Ann Surg. 2005;241:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene. 2004;23:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Strickland LA, Ross J, Williams S, Ross S, Romero M, Spencer S, Erickson R, Sutcliffe J, Verbeke C, Polakis P. Preclinical evaluation of carcinoembryonic cell adhesion molecule (CEACAM) 6 as potential therapy target for pancreatic adenocarcinoma. J Pathol. 2009;218:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Farina A, Dumonceau JM, Antinori P, Annessi-Ramseyer I, Frossard JL, Hochstrasser DF, Delhaye M, Lescuyer P. Bile carcinoembryonic cell adhesion molecule 6 (CEAM6) as a biomarker of malignant biliary stenoses. Biochim Biophys Acta. 2014;1844:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Dimova I, Popivanov G, Djonov V. Angiogenesis in cancer - general pathways and their therapeutic implications. J BUON. 2014;19:15-21. [PubMed] |
| 52. | Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 237] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Park BK, Paik YH, Park JY, Park KH, Bang S, Park SW, Chung JB, Park YN, Song SY. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol. 2006;29:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Sahora K, Schindl M, Kuehrer I, Eisenhut A, Werba G, Brostjan C, Telek B, Ba’ssalamah A, Stift J, Schoppmann SF. A phase II trial of two durations of Bevacizumab added to neoadjuvant gemcitabine for borderline and locally advanced pancreatic cancer. Anticancer Res. 2014;34:2377-2384. [PubMed] |
| 56. | Watkins DJ, Starling N, Cunningham D, Thomas J, Webb J, Brown G, Barbachano Y, Oates J, Chau I. The combination of a chemotherapy doublet (gemcitabine and capecitabine) with a biological doublet (bevacizumab and erlotinib) in patients with advanced pancreatic adenocarcinoma. The results of a phase I/II study. Eur J Cancer. 2014;50:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Poptic E, Liu X, Sanaka MR, Jang S, Vargo JJ, Parsi MA. Vascular endothelial growth factor levels in bile distinguishes pancreatic cancer from other etiologies of biliary stricture: a pilot study. Dig Dis Sci. 2013;58:2986-2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Alvaro D, Macarri G, Mancino MG, Marzioni M, Bragazzi M, Onori P, Corradini SG, Invernizzi P, Franchitto A, Attili AF. Serum and biliary insulin-like growth factor I and vascular endothelial growth factor in determining the cause of obstructive cholestasis. Ann Intern Med. 2007;147:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Bruchim I, Sarfstein R, Werner H. The IGF Hormonal Network in Endometrial Cancer: Functions, Regulation, and Targeting Approaches. Front Endocrinol (Lausanne). 2014;5:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Zhang M, Li X, Zhang X, Yang Y, Feng Z, Liu X. Association of serum hemoglobin A1c, C-peptide and insulin-like growth factor-1 levels with the occurrence and development of lung cancer. Mol Clin Oncol. 2014;2:506-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Cao Y, Lindström S, Schumacher F, Stevens VL, Albanes D, Berndt S, Boeing H, Bueno-de-Mesquita HB, Canzian F, Chamosa S. Insulin-like growth factor pathway genetic polymorphisms, circulating IGF1 and IGFBP3, and prostate cancer survival. J Natl Cancer Inst. 2014;106:dju085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Lin Y, Tamakoshi A, Kikuchi S, Yagyu K, Obata Y, Ishibashi T, Kawamura T, Inaba Y, Kurosawa M, Motohashi Y. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the risk of pancreatic cancer death. Int J Cancer. 2004;110:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Subramani R, Lopez-Valdez R, Arumugam A, Nandy S, Boopalan T, Lakshmanaswamy R. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS One. 2014;9:e97016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | McCaffery I, Tudor Y, Deng H, Tang R, Suzuki S, Badola S, Kindler HL, Fuchs CS, Loh E, Patterson SD. Putative predictive biomarkers of survival in patients with metastatic pancreatic adenocarcinoma treated with gemcitabine and ganitumab, an IGF1R inhibitor. Clin Cancer Res. 2013;19:4282-4289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Rocha-Lima CM, Safran H, Chan D, Kocs DM, Galimi F. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834-2842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 67. | Sato Y, Harada K, Sasaki M, Yasaka T, Nakanuma Y. Heat shock proteins 27 and 70 are potential biliary markers for the detection of cholangiocarcinoma. Am J Pathol. 2012;180:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Rucksaken R, Pairojkul C, Pinlaor P, Khuntikeo N, Roytrakul S, Selmi C, Pinlaor S. Plasma autoantibodies against heat shock protein 70, enolase 1 and ribonuclease/angiogenin inhibitor 1 as potential biomarkers for cholangiocarcinoma. PLoS One. 2014;9:e103259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Tsiaousidou A, Lambropoulou M, Chatzitheoklitos E, Tripsianis G, Tsompanidou C, Simopoulos C, Tsaroucha AK. B7H4, HSP27 and DJ-1 molecular markers as prognostic factors in pancreatic cancer. Pancreatology. 2013;13:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Hyun JJ, Lee HS, Keum B, Seo YS, Jeen YT, Chun HJ, Um SH, Kim CD. Expression of heat shock protein 70 modulates the chemoresponsiveness of pancreatic cancer. Gut Liver. 2013;7:739-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Chen MH, Chiang KC, Cheng CT, Huang SC, Chen YY, Chen TW, Yeh TS, Jan YY, Wang HM, Weng JJ. Antitumor activity of the combination of an HSP90 inhibitor and a PI3K/mTOR dual inhibitor against cholangiocarcinoma. Oncotarget. 2014;5:2372-2389. [PubMed] |
| 72. | Shen J, Wang W, Wu J, Feng B, Chen W, Wang M, Tang J, Wang F, Cheng F, Pu L. Comparative proteomic profiling of human bile reveals SSP411 as a novel biomarker of cholangiocarcinoma. PLoS One. 2012;7:e47476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 73. | Lee JH, Zhang X, Shin BK, Lee ES, Kim I. Mac-2 binding protein and galectin-3 expression in mucinous tumours of the ovary: an annealing control primer system and immunohistochemical study. Pathology. 2009;41:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Compagno D, Gentilini LD, Jaworski FM, Pérez IG, Contrufo G, Laderach DJ. Glycans and galectins in prostate cancer biology, angiogenesis and metastasis. Glycobiology. 2014;24:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Fortuna-Costa A, Gomes AM, Kozlowski EO, Stelling MP, Pavão MS. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol. 2014;4:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 76. | Koopmann J, Thuluvath PJ, Zahurak ML, Kristiansen TZ, Pandey A, Schulick R, Argani P, Hidalgo M, Iacobelli S, Goggins M. Mac-2-binding protein is a diagnostic marker for biliary tract carcinoma. Cancer. 2004;101:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Chen CY, Lin XZ, Tsao HC, Shiesh SC. The value of biliary fibronectin for diagnosis of cholangiocarcinoma. Hepatogastroenterology. 2003;50:924-927. [PubMed] |
| 78. | You Z, De Falco M, Kamada K, Pisani FM, Masai H. The mini-chromosome maintenance (Mcm) complexes interact with DNA polymerase α-primase and stimulate its ability to synthesize RNA primers. PLoS One. 2013;8:e72408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Das M, Prasad SB, Yadav SS, Govardhan HB, Pandey LK, Singh S, Pradhan S, Narayan G. Over expression of minichromosome maintenance genes is clinically correlated to cervical carcinogenesis. PLoS One. 2013;8:e69607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Williams GH, Swinn R, Prevost AT, De Clive-Lowe P, Halsall I, Going JJ, Hales CN, Stoeber K, Middleton SJ. Diagnosis of oesophageal cancer by detection of minichromosome maintenance 5 protein in gastric aspirates. Br J Cancer. 2004;91:714-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, Marr J, Turner WH, Bullock N, Doble A. Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst. 2002;94:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Ayaru L, Stoeber K, Webster GJ, Hatfield AR, Wollenschlaeger A, Okoturo O, Rashid M, Williams G, Pereira SP. Diagnosis of pancreaticobiliary malignancy by detection of minichromosome maintenance protein 5 in bile aspirates. Br J Cancer. 2008;98:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Chen CY, Tsai WL, Wu HC, Syu MJ, Wu CC, Shiesh SC. Diagnostic role of biliary pancreatic elastase for cholangiocarcinoma in patients with cholestasis. Clin Chim Acta. 2008;390:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Navaneethan U, Gutierrez NG, Venkatesh PG, Jegadeesan R, Zhang R, Jang S, Sanaka MR, Vargo JJ, Parsi MA, Feldstein AE. Lipidomic profiling of bile in distinguishing benign from malignant biliary strictures: a single-blinded pilot study. Am J Gastroenterol. 2014;109:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Volinsky R, Kinnunen PK. Oxidized phosphatidylcholines in membrane-level cellular signaling: from biophysics to physiology and molecular pathology. FEBS J. 2013;280:2806-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 86. | Navaneethan U, Parsi MA, Gutierrez NG, Bhatt A, Venkatesh PG, Lourdusamy D, Grove D, Hammel JP, Jang S, Sanaka MR. Volatile organic compounds in bile can diagnose malignant biliary strictures in the setting of pancreatic cancer: a preliminary observation. Gastrointest Endosc. 2014;80:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Navaneethan U, Parsi , MA , Lourdusamy D, Bhatt A, Gutierrez NG, Grove D, Venkatesh PGK, Sanaka MR, Hammel JP. Volatile organic compounds in Bile for Early Diagnosis of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis: A Pilot Study. Gastrointest Endosc. 2014;In press. |
| 88. | Wiggers JK, Ruys AT, Groot Koerkamp B, Beuers U, ten Kate FJ, van Gulik TM. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:1582-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |