Published online Mar 16, 2015. doi: 10.4253/wjge.v7.i3.253
Peer-review started: September 18, 2014
First decision: December 1, 2014
Revised: November 9, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: March 16, 2015
Processing time: 183 Days and 5.2 Hours
Traditionally, treatment of renal lesions is indicated based only on imaging features. Although controversy exists about tissue sampling from small renal masses, renal biopsy is indicated in some cases. In this review, we discuss the rationale for endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and summarize the recent advances in this field, providing recommendations for the practicing clinician. The use of EUS-FNA appears to be a safe and feasible means of confirming or excluding malignancy. EUS allows assessment and biopsy of masses or lesions within both kidneys and related complications are rare. The main advantages of EUS-FNA are that it can be done as an outpatient procedure, with good results, minimal morbidity and a short hospital stay. Nevertheless, EUS-FNA of renal masses should be indicated only in selected cases, in which there is potential to decrease unnecessary treatment of small renal masses and to best select tumors for active surveillance and minimally invasive ablative therapies. Additionally, some renal lesions may be ineligible for EUS-guided biopsy because of anatomical limitations. EUS-FNA renal biopsy will probably be best applied to central anterior renal masses, while tumors on the posterior aspect of the kidney, percutaneous access will probably be superior.
Core tip: Although controversy exists on the need of renal biopsy, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) can be used in selected cases. In this review we discuss the rationale for EUS-FNA kidney and summarize the recent advances in this field, providing recommendations for the practicing clinician.
- Citation: Lopes RI, Moura RN, Artifon E. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of kidney lesions: A review. World J Gastrointest Endosc 2015; 7(3): 253-257
- URL: https://www.wjgnet.com/1948-5190/full/v7/i3/253.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i3.253
Improvements on imaging technology and widespread use of imaging studies have not only increased the detection, but also allowed better characterization of incidental renal masses, which resulted in smaller lesions being depicted on such studies[1]. Up to 80% of renal cell carcinomas (RCC) are incidentally detected during radiological work-up, usually for non-urological indications. At time of nephrectomy, 70%-90% of solid renal lesions prove to be RCC[2,3], accounting for 2% of all cancers and being the leading kidney malignancy[2,4,5]. Therefore, an enhancing renal neoplasm on computed tomography (CT) or magnetic resonance imaging (MRI) has been considered by most urologists to be a sufficient indication for surgery because about 80% of such lesions prove to be RCC.
Some recent studies demonstrated that up to 30% of detected renal lesions are benign at surgery, depending on renal lesion size[6,7]. Furthermore, current management of small renal tumors involves from surveillance strategies to alternative minimally invasive and nephron-sparing options, such as laparoscopic/robotic partial nephrectomy, cryotherapy and radiofrequency ablation. In this scenario, pre-therapeutic guided biopsy might be helpful to avoid unnecessary surgery and to choose the most appropriate management strategy. In almost 30% of selected patients, a surgical procedure became non-mandatory after renal biopsy results were obtained[8]. Therefore, if a renal biopsy might impact treatment decisions, the use of core biopsy and fine needle aspiration (FNA) for better characterization of suspicious renal masses preoperatively should be considered.
In most patients, treatment of renal lesions is indicated based on imaging features alone. Although controversy exists about tissue sampling from small renal masses (tumors with less than 4 cm, since they have up to 30% chance of being benign), renal biopsy is indicated to: (1) characterize radiographically indeterminate lesions; (2) confirm malignancy in patients, who either are not surgical candidates or plan primary treatment with minimally invasive ablative therapy; and (3) rule out non-renal cell primary tumors (metastasis and lymphoma) or benign conditions (abscess), which may not require surgery[9-11].
Biopsy has also been used to confirm the diagnosis and the histological subtype of a renal primary lesion in patients with disseminated metastasis or unresectable retroperitoneal mass. In metastatic RCC, patients with clear cell subtype histology are most likely to benefit from adjuvant immunotherapy following cytoreductive nephrectomy. Additionally, new target therapies demonstrate variant response rates with distinctive RCC subtypes[2,8].
Tissue sampling of renal lesions is traditionally performed by using percutaneous sonographic or CT guidance. The use of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is infrequently performed for the evaluation for RCC and there are few reported studies addressing the safety and feasibility of this technique[2,8,11-14], as shown in Table 1.
| Ref. | Design | Location | Mean size | Approach | No. of EUS-FNA | Technical success | Complications |
| Farrell et al[2] | Case report | Right kidney | 9 cm | Duodenum 22 G needle 2 passes | 1 | 100% | No |
| Eloubeidi et al[13] | Prospective study | N/A | N/A | N/A 22 G needle up to 5 passes | 1 | N/A | N/A |
| Artifon et al[12] | Case report | Left kidney | 1.3 cm | Gastric body 22 G needle 3 passes | 1 | 100% | No |
| DeWittet al[11] | Case series | Right kidney (n = 5)Left kidney (n = 10) | 3.2 cm (1.1-6 cm) | Duodenum for right kidney and gastric body for left kidney 22 G needle 2 - 4 passes | 15 | 80% (12/15) | No |
| Lakhtakia et al[14] | Case report | Right kidney | 1.5 cm | Duodenum 22 G needle N/A passes | 1 | 100 | Transient hematuria |
| Moura et al[8] | Case series | Right kidney (n = 4)Left kidney (n = 4)Bilateral (n = 1) | 6 cm (1.3-16 cm) | Duodenum for right kidney and gastric body for left kidney 22 G needle 3 passes | 10 | 90% (9/10) | No |
The objective of this review is to: (1) outline the rationale for EUS-FNA kidney; (2) detail the procedural technique; (3) evaluate the clinical outcomes and limitations of the method; and (4) provide recommendations for the practicing clinician.
Since EUS initial report in the 1980s, it rapidly crawled from a pure imaging modality used mainly for diagnostic purposes, especially for lesions of digestive tract, to a more interventional and therapeutic application[15]. With the subsequent advent of FNA, this technique has became the gold-standard procedure for the assessment of benign and malignant diseases of the gastrointestinal tract and of adjacent organs[16,17]. EUS-FNA is highly accurate, sensitive and specific with estimates reaching 80%, 90% and 100%, respectively for cytological diagnosis[18-20].
As discussed above, percutaneous renal mass biopsy must not be performed for renal lesions less than 40 mm but it should be indicated for incompletely accurate renal imaging diagnosis after a full imaging evaluation. As well, EUS-FNA cannot currently be recommended as routine for cytologic diagnosis of renal masses, however, it might be useful in the aforementioned clinical situations when a renal biopsy should have an impact on clinical decision, especially for central and anterior renal masses. The advantages of a EUS-FNA in these cases is the potential to decrease unnecessary treatment of small renal masses and to best select renal tumors for active surveillance and minimally invasive ablative therapies[12,21]. EUS-FNA appears to be a safe and cost-effective way of confirming or excluding malignancy and may hinder the need for CT-guided exams[2].
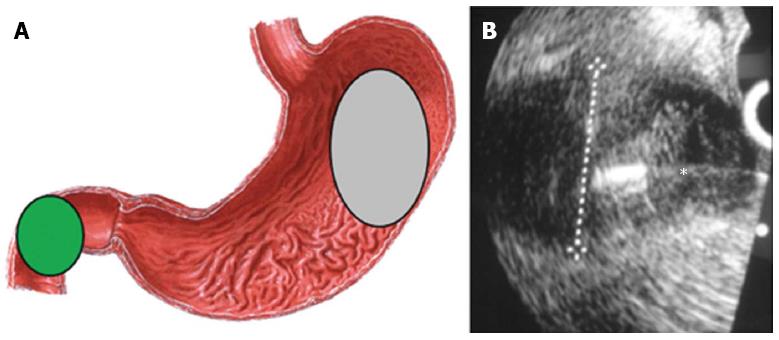
Anatomic approximation to both kidneys allows access for tissue sampling with the echoendoscope positioned in the upper gastrointestinal (GI) tract. Translating the probe within the duodenum or stomach, with the extension of 12.5 cm for 7.5 MHz probe, is sufficient to visualize both kidneys. The right kidney can be readily imaged by locating the transducer in the second portion of the duodenum (green area Figure 1) and rotating laterally, and the left kidney can be visualized when the transducer is facing posterolaterally into the body of the stomach (grey area Figure 1A)[12]. Color doppler ultrasound can verify the presence of major trespassing vascular structures, which should be identified and avoided during FNA.
EUS-FNA is performed (Figure 1B) using curvilinear array echoendoscopes that are produced by three leading manufacturers: Olympus (Olympus Medical Systems Inc., Tokyo, Japan), Pentax (Pentax, Tokyo, Japan) and Fujinon (Fujifilm Corp., Tokyo, Japan). The working channel must be at least of 2.8 mm to accept the FNA needle and the echoendoscopes present at an elevator located on the side of the scope at the tip portion, that is able to make changes in the exit angle of the FNA needle to facilitate the targeting process[15].
Needles for renal EUS-FNA are currently available in 3 sizes (19, 22 and 25 gauge). Thinner needles are used to gather cytological specimens, while thicker needle are better applied for acquisition of a tissue specimen for histological examination, that can be more useful to reach the definitive diagnosis. The choice of the needle depends on the type and site of the lesion to be sampled. In all the studies listed in Table 1, the kidney was punctured using a 22-gauge needle. More data is probably needed to characterize the correct needle size depending to the type and location of the lesion.
Whenever possible, EUS-FNA should be done under deep sedation with the assistance of an anesthesiologist. The main advantages of EUS-FNA are that it can be done as an outpatient procedure, and it appears to be safe with good results, minimal morbidity and a short hospital stay, as demonstrated in Table 1.
Some renal masses may be ineligible for EUS-guided biopsy because of anatomical limitations. EUS-FNA renal biopsy will probably be best applied to central anterior renal masses, while tumors on the posterior aspect of the kidney, percutaneous access will probably be superior. Among other reasons, these limitations are likely to restrict widespread application of EUS for this indication[11].
EUS-FNA related complications of kidney masses sampling are similar to those for aspiration of GI masses and include localized bleeding, infection, hematoma, hematuria, pneumothorax, and needle tract seeding[14]. The risk of complications associated with EUS-FNA spans from less than 1% to 6%. Tracheal suction (5%), vomiting (0.3%), aspiration (0.3%), esophageal perforation and death (less than 0.06%) are reported complications of EUS. In a relatively small group of patients, the frequency of bleeding as a result of fine-needle aspiration of the kidney was 0.5%, whereas that associated with fine-needle aspiration of GI lesions was 1.3%[2].
Since the EUS needle has to transverse fewer tissue layers, the risk of needle seeding may be lower, with few cases reported. Overall, the prospect of needle track seeding is minor and it should be balanced against the benefit of a tissue diagnosis[12]. In a retrospective review of patients submitted to pancreatic mass FNA, either by EUS-FNA or percutaneous access, the incidence of peritoneal carcinomatosis was lower in the EUS-FNA group, which might suggest a lower risk of needle seeding[22].
Higher accuracy rates are achieved with on-site cytopathology examination to assess specimen adequacy that, however, is not available in all centers and may increase the cost of the procedure[15].
EUS-FNA is not done in situations when it is unlikely to alter the management of a cancer. In addition to the usual contraindications for any endoscopic procedure, including severe bleeding diathesis and thrombocytopenia, EUS-FNA is not advocated when good views of the lesion are not obtained or when a major vascular structure is present on the way to the target[15].
New techniques in EUS are emerging and will likely have a niche in aiding the diagnosis of undeterminate lesions. EUS allows visualization and sampling renal masses. This technique is evolving and will possibly have a role in diagnostic EUS in the future, as it appears to be a safe and feasible procedure with good results, minimal morbidity and a short hospital stay in the cases reported on the literature[2,8,11-13].
We recommend that EUS-FNA of renal masses should be indicated only in selected cases, in which the procedure may alter clinical management by avoiding unnecessary treatment and helping to select patients for active surveillance and minimally invasive ablative therapies. Further research should evaluate the benefits of preoperative renal biopsy use and randomization of percutaneous, laparoscopic and echoendoscopic approach should be compared.
P- Reviewer: Hu H, Sofi A, Tepes B S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
| 1. | Kutikov A, Fossett LK, Ramchandani P, Tomaszewski JE, Siegelman ES, Banner MP, Van Arsdalen KN, Wein AJ, Malkowicz SB. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68:737-740. |
| 2. | Farrell JJ, Brugge WR. EUS-guided fine-needle aspiration of a renal mass: an alternative method for diagnosis of malignancy. Gastrointest Endosc. 2002;56:450-452. |
| 3. | Davis CJ. Pathology of renal neoplasms. Semin Roentgenol. 1987;22:233-240. |
| 4. | Sweeney JP, Thornhill JA, Graiger R, McDermott TE, Butler MR. Incidentally detected renal cell carcinoma: pathological features, survival trends and implications for treatment. Br J Urol. 1996;78:351-353. |
| 5. | Konnak JW, Grossman HB. Renal cell carcinoma as an incidental finding. J Urol. 1985;134:1094-1096. |
| 6. | Glassman D, Chawla SN, Waldman I, Johannes J, Byrne DS, Trabulsi EJ, Gomella LG. Correlation of pathology with tumor size of renal masses. Can J Urol. 2007;14:3616-3620. |
| 7. | Ozen H, Colowick A, Freiha FS. Incidentally discovered solid renal masses: what are they? Br J Urol. 1993;72:274-276. |
| 8. | Moura RN, Lopes RI, Srougi M, Dall’oglio MF, Sakai P, Artifon EL. Initial experience with endoscopic ultrasound-guided fine needle aspiration of renal masses: indications, applications and limitations. Arq Gastroenterol. 2014;51:337-340. |
| 9. | Renshaw AA, Granter SR, Cibas ES. Fine-needle aspiration of the adult kidney. Cancer. 1997;81:71-88. |
| 10. | Khan AA, Shergill IS, Gujral SS, Timoney AG. Management of small indeterminate renal tumours: is there a case for needle biopsy? BJU Int. 2007;100:1-3. |
| 11. | DeWitt J, Gress FG, Levy MJ, Hernandez LV, Eloubeidi MA, Mishra G, Sherman S, Al-Haddad MA, LeBlanc JK. EUS-guided FNA aspiration of kidney masses: a multicenter U.S. experience. Gastrointest Endosc. 2009;70:573-578. |
| 12. | Artifon EL, Lopes RI, Kumar A, Lucon AM, Dall’oglio M, Hawan B, Sakai P, Srougi M. Endoscopic ultrasound facilitates histological diagnosis of renal cell cancer. J Endourol. 2008;22:2447-2450. |
| 13. | Eloubeidi MA, Tamhane A, Jhala N, Chhieng D, Jhala D, Crowe DR, Eltoum IA. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841-2847. |
| 14. | Lakhtakia S, Wee E, Gupta R, Anuradha S, Kalpala R, Monga A, Arjunan S, Reddy DN. Hematuria after endoscopic ultrasound-guided fine needle aspiration of a renal tumor in von Hippel-Lindau disease. Endoscopy. 2012;44 Suppl 2 UCTN:E133. |
| 15. | Trindade AJ, Berzin TM. Clinical controversies in endoscopic ultrasound. Gastroenterol Rep (Oxf). 2013;1:33-41. |
| 16. | Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267-279. |
| 17. | Tharian B, Tsiopoulos F, George N, Pietro SD, Attili F, Larghi A. Endoscopic ultrasound fine needle aspiration: Technique and applications in clinical practice. World J Gastrointest Endosc. 2012;4:532-544. |
| 18. | Chang KJ, Katz KD, Durbin TE, Erickson RA, Butler JA, Lin F, Wuerker RB. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694-699. |
| 19. | Vilmann P, Hancke S, Henriksen FW, Jacobsen GK. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lesions in the upper gastrointestinal tract. Gastrointest Endosc. 1995;41:230-235. |
| 20. | Karadsheh Z, Al-Haddad M. Endoscopic ultrasound guided fine needle tissue acquisition: where we stand in 2013? World J Gastroenterol. 2014;20:2176-2185. |
| 21. | Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, Saravanan A, Jewett MA. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol. 2007;178:379-386. |
| 22. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. |