Published online Nov 10, 2015. doi: 10.4253/wjge.v7.i16.1222
Peer-review started: March 2, 2015
First decision: June 18, 2015
Revised: July 21, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: November 10, 2015
Processing time: 257 Days and 14.2 Hours
AIM: To determine the optimal generator settings for endobiliary radiofrequency ablation.
METHODS: Endobiliary radiofrequency ablation was performed in live swine on the ampulla of Vater, the common bile duct and in the hepatic parenchyma. Radiofrequency ablation time, “effect”, and power were allowed to vary. The animals were sacrificed two hours after the procedure. Histopathological assessment of the depth of the thermal lesions was performed.
RESULTS: Twenty-five radiofrequency bursts were applied in three swine. In the ampulla of Vater (n = 3), necrosis of the duodenal wall was observed starting with an effect set at 8, power output set at 10 W, and a 30 s shot duration, whereas superficial mucosal damage of up to 350 μm in depth was recorded for an effect set at 8, power output set at 6 W and a 30 s shot duration. In the common bile duct (n = 4), a 1070 μm, safe and efficient ablation was obtained for an effect set at 8, a power output of 8 W, and an ablation time of 30 s. Within the hepatic parenchyma (n = 18), the depth of tissue damage varied from 1620 μm (effect = 8, power = 10 W, ablation time = 15 s) to 4480 μm (effect = 8, power = 8 W, ablation time = 90 s).
CONCLUSION: The duration of the catheter application appeared to be the most important parameter influencing the depth of the thermal injury during endobiliary radiofrequency ablation. In healthy swine, the currently recommended settings of the generator may induce severe, supratherapeutic tissue damage in the biliary tree, especially in the high-risk area of the ampulla of Vater.
Core tip: The use of endoscopic retrograde cholangio-pancreatography-guided endobiliary radiofrequency ablation is expanding quickly, from the clearing of obstructed biliary stents in malignant biliary stenoses, to the treatment of benign biliary strictures. However, the morbidity associated with this procedure remains high, of course because of the severity of the disease treated, but also possibly due to suboptimal generator settings. Therefore, we conducted an animal study in live pigs. We report novel data, highlighting the importance of the effect setting on the generator, and suggesting specific settings for radiofrequency ablation in the ampulla of Vater.
- Citation: Barret M, Leblanc S, Vienne A, Rouquette A, Beuvon F, Chaussade S, Prat F. Optimization of the generator settings for endobiliary radiofrequency ablation. World J Gastrointest Endosc 2015; 7(16): 1222-1229
- URL: https://www.wjgnet.com/1948-5190/full/v7/i16/1222.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i16.1222
Radiofrequency (RF) ablation is currently used for the destruction of a wide range of liver neoplasms, including hepatocarcinoma, intrahepatic cholangiocarcinoma, and colorectal metastases[1]. RF waves can be applied either intraoperatively or percutaneously. The recent development of endobiliary RF ablation devices has been justified by two situations in which pre-existing methods were non or partially satisfactory: (1) unresectable extrahepatic cholangiocarcinoma[2], in which chemotherapy is poorly efficient and tumor response is difficult to assess[1]; and (2) obstruction of biliary stents by tumor ingrowth in unresectable pancreatic head cancers, which requires endoscopic desobstruction. Endobiliary RF ablation is currently performed using a device which can be used percutaneously[3,4] or through retrograde endoscopic cannulation of the main bile duct[5]. After preclinical studies in ex vivo[6] and in vivo pig livers[7], early endobiliary RF experience has been reported in patients. Because of the lack of control group in these retrospective or pilot prospective studies, the clinical efficacy of the method cannot be assessed with a high degree of confidence. However, endobiliary RF ablation in the setting of malignant biliary strictures of the common bile duct was technically feasible in a small series of patients presenting one of the above-mentioned indications. The method seems capable of clearing occluded metal stents[4] and may have the potential to improve medium to long-term biliary stent patency[5,8]. Other indications of choice could be foreseen, such as the destruction of benign biliary lesions or the ablation of shallow intraductal or intra-ampullary neoplasia. However, in view of the limited preclinical data and the need for an accurate assessment of tolerable electrosurgical settings before using endobiliary RF in such indications, additional animal data is needed. We therefore conducted a preclinical study on live pig liver to assess the tissular effect of endobiliary RF ablation in the intrahepatic, common, and periampullary bile ducts with various generator settings.
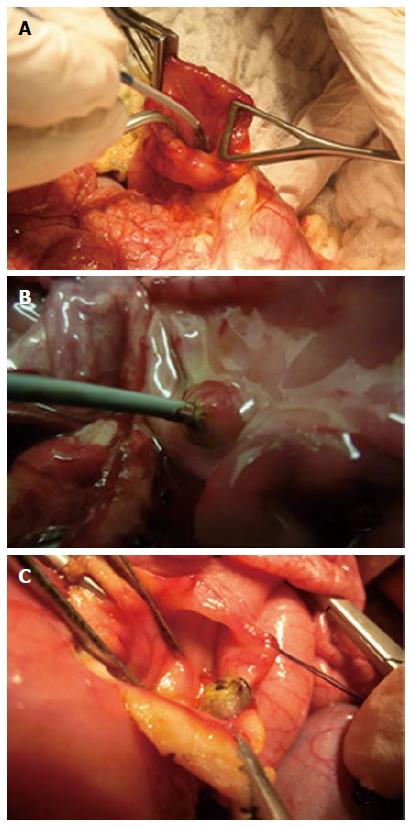
Landrace pigs weighing 30-35 kg and stemming from the same farm were used for the study. The pigs were accommodated at our facility for 48 h prior to the procedure. Procedures were performed under general anesthesia. The animal protocol was designed to minimize pain or discomfort to the animals. All animals were prepared for anesthesia with a 12-h diet, and administered an intramuscular injection of 10 mg/kg ketamine and 2 mg/kg azaperone 30 min before induction. After induction with 8 mg/kg intravenous 1% propofol and endotracheal intubation, anesthesia was maintained through inhalation of 1% to 2% isoflurane. All animals received an intravenous infusion of 10 mg/kg per hour crystalloid solution. Median laparotomy was performed, and anterior duodenotomy at the level of the first portion of the duodenum was made in order to expose the duodenal papilla, which is located 15 mm distal to the pylorus. The common bile duct was cannulated using a 0.035 Inch guidewire (Jagwire, Boston Scientific, Natick, MA, United States) and its actual position was ascertained by direct visualization of the hepatic pedicle. A Habib EndoHPBTM (EMcision UK, London, United Kingdom) probe was used for RF ablation. It is a bipolar device, 8 F (2.6 mm) in diameter, 1.8 m long, that passes over 0.035-inch guidewires, has 2 ring electrodes disposed on the catheter 8 mm from one another, with the distal electrode 5 mm from the leading edge; bipolar current activation allows for tissue ablation by creating coagulation necrosis over a 2.5 cm length[9]. The endoHPB™ probe was connected to a VIO300D electrosurgical generator (Erbe, Tübingen, Germany) in bipolar mode, delivering a high-frequency (450 kHz) electrical effect. The catheter was introduced manually inside the common bile duct and RF ablation was performed: (1) in the ampulla of Vater by positioning the space between both electrodes, which is the zone of highest energy deposition, exactly within the ampullary area, meaning that the most distal electrode was inside the bile duct and the most proximal one mostly within the duodenum; and (2) in the common bile duct. One or two separate thermal lesions could be performed along the bile duct, and only one in the ampullary region. The RF procedures on the ampulla of Vater are shown on Figure 1. Because of the limited space in the biliary tract and the ampullary region, separate tests were performed on the hepatic parenchyma prior to those biliary lesions, in order to predetermine a range of parameters most likely to provide the best safety-to-effectiveness balance for biliary lesions. Liver thermal injuries were performed by inserting the RF probe into the liver after a small incision in the Glisson’s capsule. Different ablation parameters, such as effect and power, but also duration of RF shots, were allowed to vary. Two hours after the procedure, with the animals still under general anesthesia, the pigs were euthanized with 100 mg/kg intravenous injection pentobarbital. The liver, the common bile duct, and the duodenum were collected en-bloc with the pancreatic head and fixed for 24 h in 10% buffered formalin. After macroscopic examination, ablated segments were embedded in paraffin, processed into 3 μm-thick sections, and stained with hematoxylin, eosin and saffron. Histological assessment was performed by a pathologist experienced in digestive pathology (AR), using an ocular micrometer to measure the maximal extent of tissue necrosis in depth from the epithelial surface. Tissue presenting disorganization of the layers, absence of normally shaped native cells, edema and/or inflammatory cell infiltrate was considered necrotic. Statistical analysis was performed using Graphpad Software (GraphPad Software Inc., San Diego, CA). Results are expressed as mean values ± SD.
The experimental protocol was approved by the scientific committee of the Surgery School of Paris (Ecole de Chirurgie de l’Assistance Publique-Hopitaux de Paris, France) and the experiments were performed according to the standard animal research guidelines established by the French Ministry of Agriculture.
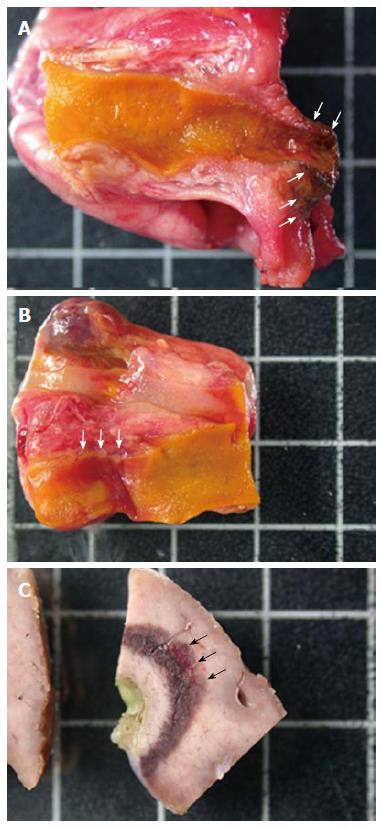
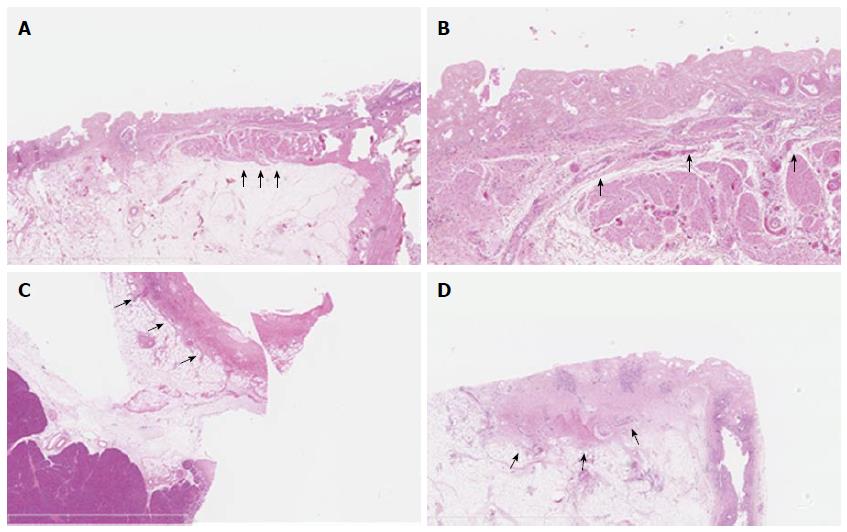
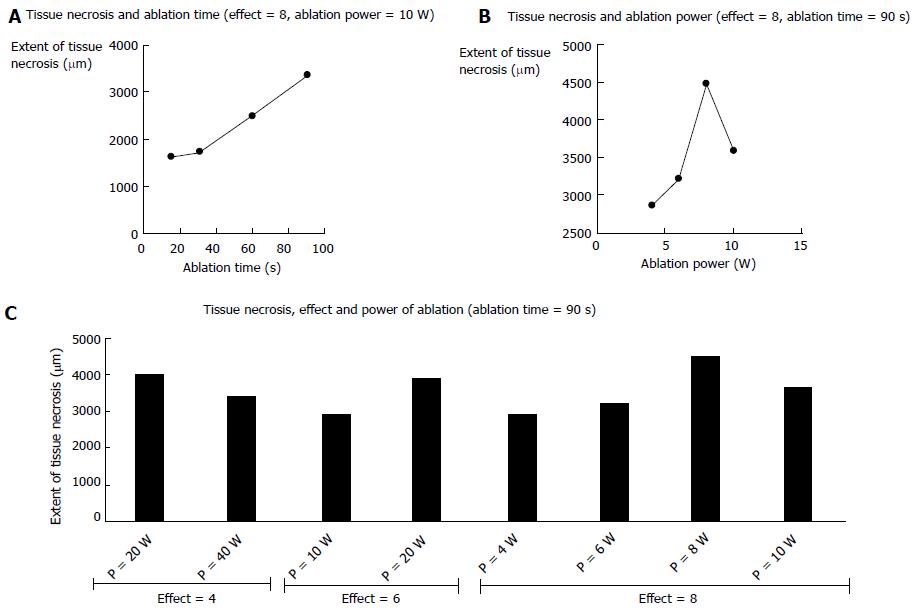
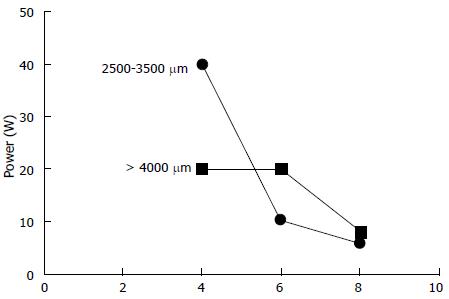
Three pigs, on which 25 RF ablations were conducted, were included in the study. Eighteen RF ablations were conducted in the hepatic parenchyma allowing the testing 11 ablation conditions. The depth of tissue necrosis ranged from 1620 μm for an effect of 8, a power of 10 W, and a 15 s ablation time, up to 4480 μm for an effect of 8, a power of 8 W, and a 90 s ablation. The full set of corresponding figures is displayed in Table 1. Based on those results, RF shots conducted in the common bile duct allowed the testing of three ablation settings. With an effect varying from 4 to 8, a power output from 6 to 20 W, and shot duration from 30 to 90 s, a depth of tissue injury allowing for deep, yet non transmural tissue necrosis up to 1075 μm was obtained with an effect set at 8, a power of 8 W, and a short ablation time of 30 s; longer or more powerful or intensive shots led to transmural tissue necrosis up to 2700 μm away from the epithelium, with destruction of the peribiliary adipose tissue. Finally, three RF ablations were conducted in the ampullary region under three different ablating conditions. Effect was set at 8, power varied from 6 to 10 W, and the duration of ablation shots from 30 to 90 s. The depth of the tissue injury ranged from 350 μm, with ablation limited to the mucosa, for a power output of 6 W and an ablation time of 30 s, up to 2810 μm, with extensive tissue necrosis involving the adipose tissue and the pancreas, for a power of 10 W and a 90 s long shot. The results of RF in the bile duct and the ampulla of Vater are presented in Table 2. Macroscopic assessment of the ablated tissues is presented in Figure 2, and histological views of ablated ampullae are shown in Figure 3. Figure 4 presents the respective impact of power and ablation time variation on tissue damage after RF. Whereas power and ablation time appeared to be linearly correlated to the depth of tissue damage, variations in effect settings only had a modest influence on tissue damage, although our results showed an inverse relationship between effect and power output (Figure 5).
| Effectsetting | Power output (W) | Shot duration (s) | Shot location | Depth of tissue injury (μm) | Observations |
| 4 | 20 | 90 | Bile duct | 2700 | Transmural necrosis |
| 6 | 10 | 90 | Bile duct | 2600 | Transmural necrosis |
| 8 | 10 | 90 | Ampulla | 2810 | Extensive necrosis of the papilla, involving pancreas and adipose tissue |
| 8 | 10 | 30 | Ampulla | 1530 | Deep coagulation, necrosis of the duodenal wall. No significant pancreatic injury |
| 8 | 6 | 60 | Bile duct | 1420 | Deep transmural coagulation, involving peribiliary adipose tissue |
| 8 | 8 | 30 | Bile duct | 1075 ± 4171 | Coagulation involving almost the entire duct wall |
| 8 | 6 | 30 | Ampulla | 350 | Superficial coagulation, moderate duodenal lesion |
Clinical studies of RF endobiliary ablation with the EndoHPB™ probe have not been undertaken without previous animal studies designed to assess the depth of tissue damage[6,7,10]. It is noteworthy that EndoHPB probes are designed to be used only with VIO200-300, which is one of the most popular HF units in endoscopy wards. This should make comparisons between studies relatively easy and reliable, since the parameters used are strictly comparable. Itoi et al[6] assessed thermal injury in the hepatic parenchyma in three ex vivo pig livers, and tested 12 ablation settings, with power varying from 5 to 20 W and ablation times ranging from 60 to 120 s. Their observations were mainly macroscopical, and the authors reported tissue necrosis extending from 4.3 ± 0.6 mm to 11.3 ± 1.2 mm in depth. However, these numbers are hardly comparable with ours, as the authors measured the entire thickness of the damaged tissue, including the proper width of the catheter. They concluded that: (1) for the 5 W power value, 60 and 90 s ablation times were insufficient to achieve tissue necrosis; (2) the 5-10 W power output range was a zone of continuously increasing tissue damage, as opposed to power values of 15 W and over, for which tissue necrosis seemed to be stable. However, this study was performed ex vivo, lacked thorough histological analysis, was limited to the liver parenchyma, and did not take into account the effect parameter, which was set a priori at 8. In a study closer to clinical conditions, Zacharoulis et al[7] assessed the effects of endobiliary RF ablation on the bile ducts of 20 live pigs. The authors tested 10 different conditions of power output ranging from 1 to 10 W, left a plastic stent in the common bile duct, and performed histological assessment of the common bile duct 24 h after the procedure. They concluded that power as well as ablation time had an impact on the depth of tissue damage, and found the optimal settings to be a power of 6-7 W and an ablation time of 60 s[7]. In this study however, the variation of the effect was also not taken into account, histological assessment was semi-quantitative, and only the common bile duct was studied.
Based on the results of those preclinical studies, it has been suggested that the Habib EndoHPBTM probe be set at a power of 10 W, an effect of 8, and a shot duration of 90-120 s[9]. In published clinical reports, endobiliary RF has been associated with a 10%-20% morbidity rate[5,8,11,12], including cases of cholangiosepsis, liver infarction, hepatic coma, cholecystitis, and pancreatitis, all of which can be explained at least in part by excessive tissue damage to the distal bile duct or to the hepatic parenchyma. Moreover, generator settings reported in clinical studies have been heterogeneous, ranging from 5 W and 120 s[13] to 10 W and 120 s[4], and frequent use of intermediate power values or ablation times[5,8,11,12]. Only one publication reported the use of endobiliary RF ablation on a periampullary tumor, but RF was actually used to treat a malignant stricture at the proximal end of a biliary stent, thus at a distance from the ampulla[14]. For these reasons, we chose to re-assess the effects of RF on living pig liver before undertaking evaluations on the healthy bile duct and the ampullary region.
In its step-by-step guide to endobiliary RF procedures, the manufacturer suggests that for periampullary procedures, the power should be lowered to 7 W, and the procedure should be stopped as soon as the duodenal mucosa started blanching[9]. We first confirmed that the standard settings of the RF generator (effect 8, power 10 W) resulted in excessive damage to the ampulla of Vater, even with very short RF ablation times such as 30 s. An effect of 8, a low power of 6 W, and a short ablation time of 30 s seemed to provide optimal tissue ablation of the epithelium and the lamina propria in the ampulla. In the common bile duct, we observed a deep tissue necrosis reaching the entire bile duct wall and the peribiliary adipose tissue with settings as low as effect 8, power 6 W, and a 60 s ablation. This is consistent with the observations of Zacharoulis et al[7] who observed damage to adjacent organs or full thickness tissue necrosis (leading to bile duct perforation in three cases out of four at 24 h) in all animals treated with a power > 6 W. We can also conclude from these data that the effect setting has a major importance in the RF energy delivered: indeed, for a 90 s RF shot, a four point variation (from 4 to 8) of the effect causes the same tissue damage as a 30 W variation of the power (from 10 to 40 W). In the hepatic parenchyma, a 3 mm tissue damage around the probe could be achieved equally with either high power values or a 2 point increase of the effect setting. Power values, as reported by others, seemed to clearly influence the depth of tissue damage up to 10 W[6,7]: The impact of raising the power beyond 10 W did not appear to be significant. By contrast, the time of RF ablation was linearly correlated to the extent of tissue necrosis.
To our knowledge, this is the first study that considers the impact of effect variation in endobiliary RF. Furthermore, based on consistent data obtained in the hepatic parenchyma, we tested RF ablation in the whole biliary tree, including the ampulla of Vater.
However, our study presents some limitations; first, given the small number of animals, we only tested a limited number of generator settings, and did not duplicate the measurements for most values obtained in the common bile duct, although the figures found are in keeping with those reported by other authors[6,7]. Second, the short time span between RF and the sacrifice of animals could have led us to overlook the actual extent of the lesions. However, we waited for two hours before the animals were euthanized in order for the tissue damage to appear, which was sufficient to make coagulation necrosis clearly visible with standard staining. Third, although the in vivo pig liver is currently the cheapest and most easily available, it may not be a perfect model for thermal ablation, since it lacks tumoral or inflammatory thickening of the biliary wall. This could account for the supratherapeutic effects of the recommended RF generator settings in swine. Other explanations have been suggested, such as different blood supplies between the swine and human common bile duct, or major heat sink phenomenon in humans compared with swine due to higher blood flow in the hepatic pedicle[7]. Furthermore, the dramatic RF impact in other swine epithelia, such as esophageal has also been reported to be more severe than in humans[15].
In conclusion, our work demonstrates that the currently recommended settings for endobiliary radiofrequency ablation may result in supratherapeutic effects in the pig liver, bile duct and periampullary region. Our data also underlined the critical importance of the effect setting on the VIO™ RF generator for this application. These points should be kept in mind when designing RF ablation clinical trials, especially since clinical indications of endobiliary RF ablation tend to spread outside the field of malignant stricture management, such as benign biliary stricture[16] or possibly residual neoplastic tissue after endoscopic papillectomy.
The authors wish to thank Mrs Alice Bertucat for her help in the animal experiments, as well as the animal attendants from the Ecole de Chirurgie de l’Assistance Publique-Hopitaux de Paris.
Endoscopic endobiliary radiofrequency (RF) ablation has been developped for the treatment of malignant biliary obstructions. It currently allows to clear occluded metal stents and may have the potential to improve medium to long-term biliary stent patency.
Endobiliary RF ablation has mainly been studied for lesions of the common bile duct. The optimal generator settings for the treatment of ampullary or intrahepatic lesions is uncertain. Moreover, the consequences of the variations of « effect » parameter of the generator have not been studied.
This is the first study that considers the impact of effect variation in endobiliary RF. Furthermore, based on consistent data obtained in the hepatic parenchyma, the authors tested RF ablation in the whole biliary tree, including the ampulla of Vater.
Results of this study should be kept in mind when performing endobiliary radiofrequency ablation for intrahepatic or ampullary lesions, or for non-neoplastic, shallow lesions of the common bile duct.
Endobiliary radiofrequency ablation consists in the application of radiofrequency energy using a bipolar probe inserted into the biliary tree, either percutaneously, or by retrograde cannulation via endoscopic retrograde cholangiopancreatography.
This study gave a good result for using endobliary radiofrequency ablation or not in clinic, and did have clinical value to guide the treatment procedure.
P- Reviewer: Misra SP, Xu Z S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Wadsworth CA, Westaby D, Khan SA. Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 590] [Article Influence: 45.4] [Reference Citation Analysis (1)] |
| 3. | Mizandari M, Pai M, Xi F, Valek V, Tomas A, Quaretti P, Golfieri R, Mosconi C, Guokun A, Kyriakides C. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2013;36:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Pai M, Valek V, Tomas A, Doros A, Quaretti P, Golfieri R, Mosconi C, Habib N. Percutaneous intraductal radiofrequency ablation for clearance of occluded metal stent in malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2014;37:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Itoi T, Isayama H, Sofuni A, Itokawa F, Tamura M, Watanabe Y, Moriyasu F, Kahaleh M, Habib N, Nagao T. Evaluation of effects of a novel endoscopically applied radiofrequency ablation biliary catheter using an ex-vivo pig liver. J Hepatobiliary Pancreat Sci. 2012;19:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Zacharoulis D, Lazoura O, Sioka E, Potamianos S, Tzovaras G, Nicholls J, Koukoulis G, Habib N. Habib EndoHPB: a novel endobiliary radiofrequency ablation device. An experimental study. J Invest Surg. 2013;26:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Dolak W; Emcision. Habib EndoHPB. Available from: http://www.emcision.com/products/habib-endohpb/. |
| 10. | Khorsandi S. In vivo experiments for the development of a novel bipolar radiofrequency probe (EndoHPB) for the palliation of malignant biliary obstruction. EASL Monothematic Conference Liver Cancer: From Molecular Pathogenesis to New Therapies (P97) 2008; . |
| 11. | Alis H, Sengoz C, Gonenc M, Kalayci MU, Kocatas A. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int. 2013;12:423-427. [PubMed] |
| 12. | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc. 2011;74:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Mao EJ, Watson JB, Soares G, Habr FG. Successful percutaneous endobiliary radiofrequency ablation for unresectable malignant biliary obstruction: a case report and review of the literature. J Gastrointest Cancer. 2014;45 Suppl 1:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Schölvinck DW, Alvarez Herrero L, Visser M, Bergman JJ, Weusten BL. Effects of Lugol staining on stenosis formation induced by radiofrequency ablation of esophageal squamous epithelium: a study in a porcine model. Dis Esophagus. 2015;28:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Hu B, Gao DJ, Wu J, Wang TT, Yang XM, Ye X. Intraductal radiofrequency ablation for refractory benign biliary stricture: pilot feasibility study. Dig Endosc. 2014;26:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |