Published online Aug 10, 2015. doi: 10.4253/wjge.v7.i10.987
Peer-review started: October 29, 2014
First decision: November 27, 2014
Revised: June 9, 2015
Accepted: July 16, 2015
Article in press: July 17, 2015
Published online: August 10, 2015
Processing time: 292 Days and 10.7 Hours
In the majority of cases, duodenal papillary tumors are adenomas or adenocarcinomas, but the endoscopy biopsy shows low accuracy to make the correct differentiation. Endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography are important tools for the diagnosis, staging and management of ampullary lesions. Although the endoscopic papillectomy (EP) represent higher risk endoscopic interventions, it has successfully replaced surgical treatment for benign or malignant papillary tumors. The authors review the epidemiology and discuss the current evidence for the use of endoscopic procedures for resection, the selection of the patient and the preventive maneuvers that can minimize the probability of persistent or recurrent lesions and to avoid complications after the procedure. The accurate staging of ampullary tumors is important for selecting patients to EP or surgical treatment. Compared to surgery, EP is associated with lower morbidity and mortality, and seems to be a preferable modality of treatment for small benign ampullary tumors with no intraductal extension. The EP procedure, when performed by an experienced endoscopist, leads to successful eradication in up to 85% of patients with ampullary adenomas. EP is a safe and effective therapy and should be established as the first-line therapy for ampullary adenomas.
Core tip: Although the endoscopic papillectomy (EP) represent higher risk endoscopic interventions, it has successfully replaced surgical treatment for benign or malignant papillary tumors. The accurate staging of ampullary tumors is important for selecting patients to EP or surgical treatment. Compared to surgery, EP is associated with lower morbidity and mortality, and seems to be a preferable modality of treatment for small benign ampullary tumors with no intraductal extension. The EP procedure, when performed by an experienced endoscopist, leads to successful eradication in up to 85% of patients with ampullary adenomas. EP is a safe and effective therapy and should be established as the first-line therapy for ampullary adenomas.
- Citation: Ardengh JC, Kemp R, Lima-Filho &R, Santos JSD. Endoscopic papillectomy: The limits of the indication, technique and results. World J Gastrointest Endosc 2015; 7(10): 987-994
- URL: https://www.wjgnet.com/1948-5190/full/v7/i10/987.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i10.987
Ampullomas represent an uncommon group of gastrointestinal malignancies. Advances in endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) have significantly impacted the clinical approach to patients with suspected premalignant or malignant lesions of the duodenal papilla[1]. The present review leads us to the discussion of numerous current issues related to the epidemiology of ampullary tumors, the role of the endoscopy biopsy, EUS, and ERCP, as well as indications, optimal technique, complications and outcomes in patients with benign or malignant tumor.
The term “endoscopic papillectomy” refers to the duodenal mucosa and submucosa resection, including all the anatomic attachments of the ampulla of Vater, and the tissues around the bile and pancreatic ducts. In turn, the term ampullectomy should be used to define this surgical procedure, which consists in the resection of the ampulla of Vater, through a duodenotomy including the cephalic pancreatic tissue resection, followed by reinsertion of common bile duct (CBD) and main pancreatic duct (MPD) in the duodenal wall[2].
The endoscopic papillectomy (EP) was first reported as a route of access to the biliary tract[3]. Years later, it was used as a treatment modality for two cases of duodenal papilla cancer[4], and today it is accepted as a viable alternative therapy to surgery in patients with sporadic adenoma of the major or minor duodenal papilla due to its high success rate and low recurrence[2].
Tumors of the duodenal papilla may be classified as benign, premalignant, and malignant[5]. The annual incidence of ampullary lesions in the United States is 3000, with reported prevalence rates of 0.04%-0.12% in autopsy series[6,7]. Ampullary adenomas may occur sporadically or in the setting of hereditary polyposis syndromes, including familial adenomatous polyposis (FAP) with adenomatous polyposis coli gene mutations. In patients with FAP, ampullary adenomas occur in up to 80% of individuals during their lifetime and progress to malignancy in 4%[8]. Ampullary adenomas are likely to follow an adenoma-to-carcinoma sequence similar to colorectal adenocarcinoma[9]. These lesions are considered premalignant, with an incidence of transformation to carcinoma ranging from 25%-85% for sporadic adenomas. As with all neoplasms, tumor stage dictates the appropriate therapy[10].
It seems that the knowledge of the histological and immunohistochemical characteristics is useful for precisely indicate an EP. In this context, the study of such characteristics is useful for selecting the appropriate surgical or endoscopic procedure. To corroborate this fact, japanese authors reported the results of this analysis in 56 noninvasive ampullary tumors. They demonstrated that the intestinal type cancer of intra-ampullary location shows lower CK20 expression than tumors of the periampullary location, and besides that, the intestinal type tumors without CDX2 expression, that included extended and intra-ampullary location types, tend to show a compromised vertical margin after EP. This suggests that periampullary tumors, intestinal histology and high CK20-positive rate can be regarded as good indications for the EP procedure. On the other hand, this study shows that tumors that are either pancreatobiliary or intestinal type without CDX2 expression have a higher chance of involvement of the common channel inside duodenal papilla, CBD and MPD[11].
The indications for EP are based on features that can predict a complete tumor removal, while minimizing complications related to the procedure[1]. Currently the indications are not fully established and are far from a consensus.
The main criteria for EP include the lesion size (up to 5 cm), no evidence of intraductal tumor growth or malignancy in endoscopic findings, such as ulceration, spontaneous bleeding and friability[1,12-18]. However, the indications for EP are expanding[10,19-24]. For example, the endoscopic piecemeal resection technique, is used to removing tumors that can’t be removed “en bloc”, and provided increasing resections, when properly performed[25]. The clinical results of this technique are very good, but the chance of recurrence is higher.
The ductal invasion in an extension less than 1 cm does not seem to be an absolute contraindication for EP, because the tumor can be exposed by endoscopic maneuvers, such as the use of an extractor balloon into the lumen, and thus it can be completely resected with a polypectomy snare[26-28].
The cancer arising within an adenoma without invasion of duodenal muscularis propria and pancreas, or CBD and MPD, are liable to resection by EP[29-33]. However, in some situations, EP can be used as a macrobiopsy procedure for a simple local tumoral staging, if the resection margins are compromised[34].
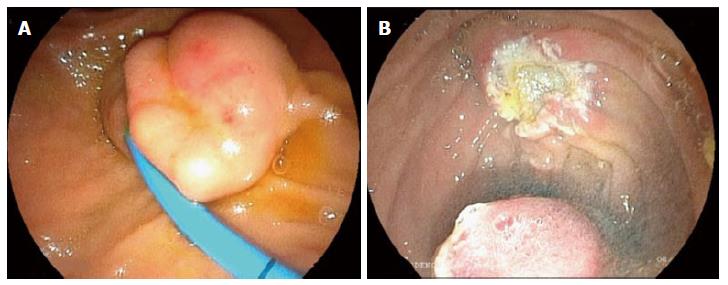
The most common preoperative concern is to define if a papillary tumor is benign or malignant. The endoscopic aspect alone cannot always distinguish adenomas from carcinomas and even from adenomatous polyps, carcinoids, gangliocytic paraganglioma, and other tumors that may occur in this region[35,36]. Some endoscopic aspects like ulceration, friability, spontaneous bleeding are usually relate to malignant lesions. The use of endoscopic tools such as NBI, FICE and magnifying endoscopy are useful to select patients for EP (Figure 1)[37].
A definitive histological diagnosis is a basic pre-requisite for adequate management of these patients, but we must remember that endoscopic biopsy of the duodenal papilla misses 30% of malignant tumors[38]. Moreover, the coexistence of carcinoma and adenoma cannot be excluded by endoscopic biopsy. Some authors advocate deep biopsy after sphincterotomy, to increase diagnostic accuracy of endoscopic biopsy[39]. We do not recommend this procedure, because endoscopic sphincterotomy eliminates the possibility of endoscopic en bloc resection of ampullary tumors, impeding a possible curative resection.
Favoring our impression, a prospective study showed that endoscopic biopsy is not reliable for preoperative diagnosis of tumors of the duodenal papilla (sensitivity of 21% before and 37% after sphincterotomy)[40]. Thus, in some cases, EP can be recommended as a technique for preoperative diagnosis because a high false negative rate of endoscopic biopsy[34].
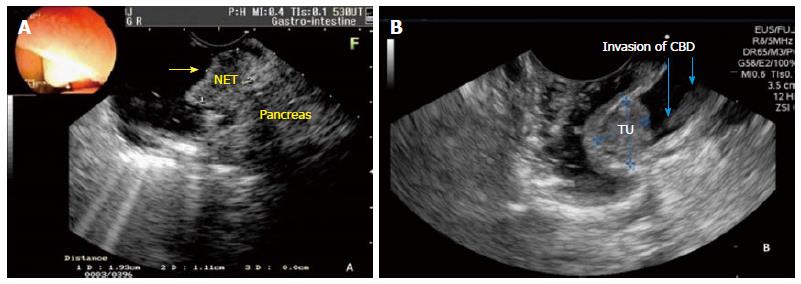
EUS is the imaging modality of choice for local staging (T). EUS is superior to helical computed tomography (CT) for preoperative evaluation of tumor size, detection of regional lymph node metastasis, vascular invasion in patients with periampullary neoplasms and also to detect tumor infiltration of biliary and pancreatic ducts (Figure 2A)[40].
Many experts believe that EUS is not useful in lesions less than 1 cm in diameter, with no suspicious signs of malignancy (ulceration, induration, bleeding and/or biopsies with high-grade dysplasia or carcinoma)[12]. Our experience shows that, when EUS is performed for staging ampullary tumor prior to EP, it allows deciding for EP, because it shows the relationship between CBD and MPD, as well their diameter. EUS allows the verification of the relationship of the borders of the tumor in the duodenal wall, CBD and MPD, regardless of the size of the tumor. However, prospective studies are needed to evaluate the accuracy of these findings.
The use of intraductal ultrasound (IDUS), with a 20 MHz probe can be more accurate in visualizing mucosa layers compared to conventional EUS[41]. According to literature, EUS and IDUS accuracy before surgical resection or diagnostic EP was 97% and 94% for pTis, 73% and 73% for pT1, 50% and 50% for pT2 and 50% and 100% for pT3-4 respectively. The overall EUS and IDUS accuracy was 85% and 80% for T stage[42]. In our experience with this type of technology, the interpretation is more difficult, especially when the mini-probe is placed within the biliary or pancreatic ducts. If this is not done, the sensitivity is lower when compared with the conventional EUS[41].
From a technical standpoint, EUS and IDUS are able to detect, with high precision, tumoral infiltration of the common bile duct and main pancreatic duct (Figure 2B). Despite ERCP can detect CBD invasion, we believe that it should only be performed after EUS, if EP is indicated. EUS and IDUS can provide high precision diagnostic information for staging ampullary tumors, and are useful in identifying lesions selected for EP. However, these tools have limitations, because the occurrence of super and understating and the difficulty in assessing focal infiltration are relevant. The improvement of endoscopic procedures is necessary for an accurate assessment of ampullary tumors[43].
From a practical standpoint, ERCP should be performed before EP, if EUS is not available or inconclusive as to ductal involvement. Although intraductal invasion is usually an indication for surgery, it has been demonstrated that, when tumoral infiltration reaches ± 1 cm into CBD and MPD, tumor is amenable to endoscopic resection[26,27,44].
Positron computerized tomography (PET/CT) and magnetic resonance imaging (MRI) are highly sensitive for detection of distant metastases. MRI and CT was superior to EUS for assessment of nodal involvement[45].
EP is performed after EUS staging confirming a less than 5.0 cm tumor confined to mucosa and/or submucosal (uT1), with intraductal tumoral infiltration less than 1 cm. It can be performed using the EUS device itself or a duodenoscope. With the duodenoscope rectified, a preferable monofilament polypectomy snare is used for grasping the tumor, always in the craniocaudal direction, i.e., the snare tip is positioned on cranial tumor apex.
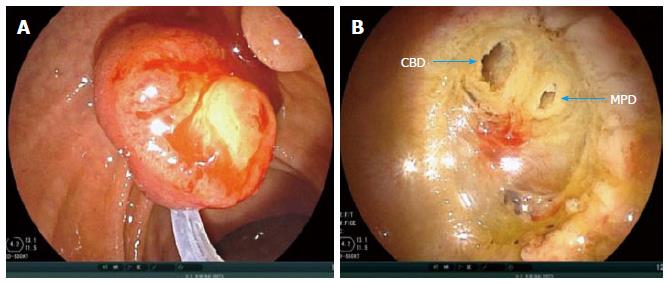
The snare is widely opened, duodenoscope is pushed in a craniocaudal direction, and tumor is grasped for en bloc resection (Figure 3). The papillary tumor is grasped at its base, always respecting a limit, up to 0.5 cm below the lesion border identified by FICE. Thereafter a constant tension is applied to the ring handle while using an electrocautery until tumor en bloc resection is completed. There are no specific equipment or a standard technique for EP.
There is also no guidance on the potency and mode of electronic current (cutting or coagulation). The authors prefer to use only cutting current (40 to 50 J) and the endocutter. Some authors recommend performing submucosal injection, ablative therapy after EP, and placement of a prophylactic pancreatic stent. The use of antibiotic prophylaxis before EP is not established[46]. The authors do not advocate its use.
Some experts use injection of contrast with methylene blue into MPD to identify the pancreatic orifice after tumor resection. This is not our practice. After complete removal of the lesion, which sometimes takes a few minutes, depending on its size and extension, a whitish rough area can be seen, which in some cases reveals the muscular layer of duodenal wall, as well two holes (biliary and pancreatic ducts).
Efforts should be exhaustive and mandatory to recover all resected tissue in all patients, for histopathological evaluation. Then CBD and MPD catheterization is performed, with contrast injection, to ensure easy recanalization after ampullary resection.
When en bloc resection is not feasible, a piecemeal resection is recommended. However, it should be noted that the en bloc resection is essential for the treatment of preneoplastic and/or malignant lesions, because this allows accurate histopathologic evaluation after tumoral resection[26].
The submucosal injection of diluted epinephrine is suggested as a means to lift the tumor from the wall, which at least theoretically may reduce the risk of bleeding. However, it is uncertain and questionable whether injection of adrenaline reduces the risk of bleeding and/or perforation[20,27,47]. The authors dismiss the submucosal injection of pharmacological agents, due to distortion of tumoral anatomy and its periphery, hindering an adequate grasping by the polypectomy snare. Moreover, a perforation following tumor resection may occur, due to a short distance between duodenal wall and pancreas, as seen by EUS.
If residual tumor tissue remains after resection, it should be destroyed! The use of coagulation with argon gas is the most widely used modality; it is safe because it is a non-contact technique, acting in tumor surface[12,46-48].
The use of stent in MPD, in order to reduce the risk of acute pancreatitis (AP) associated to EP, seems to be a consensus because it minimizes the risk of MPD stenosis, allowing the use of safer coagulation therapies. Anyway we must emphasize that this theory is unproven. Others advocate pancreatic stent placement only if MPD drainage is not sufficient after EP[49-52]. The only prospective, randomized, controlled study, to evaluate the role of prophylactic stent in MPD, to reduce AP after EP, showed a statistically significant decrease in the rate of AP after stent procedure[53].
Otherwise, the adequate MPD diameter and length for stenting are uncertain. In other work, for example, the authors suggests that routine use of prophylactic pancreatic stent in all patients is unnecessary and efforts should be directed to know which groups of patients actually benefit from its insertion[54]. Most pancreatic stents migrate spontaneously from MPD within 2 wk after insertion. Abdominal X-ray after 2 wk can confirm this finding. A stent, which remains “in situ” for more than 2 wk, should be removed endoscopically.
The placement of a prophylactic plastic biliary stent, to reduce the risk of cholangitis, has not been widely performed and cannot be uniformly recommended at the present moment, unless there is concern about inadequate biliary drainage after EP.
The EP is a “high risk” procedure, due to complications inherent to the method. They can be classified as early: AP, bleeding, perforation and cholangitis or late: papillary stenosis. The overall complication rate reported by major centers of tertiary care varies between 8% and 35%, and the most common complications are AP (5%-15%) and bleeding (2%-16%)[10,25,48,55]. Most episodes of bleeding can be controlled immediately by conservative treatment and endoscopic hemostasis and most episodes of AP are mild and resolve with conservative treatment only. The rate of pancreatic and/or biliary ductal stenosis varies between 0%-8%, and can be treated by sphincterotomy, stent placement, and balloon dilation.
The use of pancreatic stent can prevent an episode of AP and papillary stenosis[49-54]. Another interesting fact reported by a recent randomized study showed that prophylactic rectal indomethacin significantly reduced the incidence and severity of AP post-ERCP, providing an additional benefit in pancreatic temporary stenting[56]. The mortality after-EP is rare, but it has been reported to be 0.4% (range 0% to 7%)[57].
The results of the endoscopic treatment of ampullary tumors reported in the literature are shown in Table 1. The EP results are based on retrospective case series studies with heterogeneous groups. As there is no consensus on the definition of “success” after EP, it is difficult to compare the results of the reported studies. Conventionally, “success” can be defined as a complete tumor resection (as the proven absence of visible residual adenoma by endoscopy and histological analysis during a 3-6 mo follow up). In the literature the rate of the success varies between 46% to 92% in the different series. The complication rate after EP varies between 8% to 42% and the major problems are acute pancreatitis, perforation and bleeding. The most important complication after EP is the acute pancreatitis that could be diminished with the insertion of the plastic pancreatic stent. This is a controversial point, because in our experience if you have a dilated main pancreatic duct the use of the PPS is unnecessary[58].
| Ref. | Patients | Success/(%) | Complications/(%) | Mortality/(%) | Recidive/(%) | Surgery/(%) |
| Binmoeller et al[13] | 25 | 23/92 | 5/20 | 0/0 | 6/24 | 3/12 |
| Vogt et al[64] | 18 | 12/67 | 4/22 | 0/0 | 6/33 | NA |
| Zádorová et al[18] | 16 | 13/81 | 4/25 | 0/0 | 3/19 | 1/6.2 |
| Desilets et al[47] | 13 | 12/92 | 1/7.7 | 0/0 | 0/0 | 1/7.7 |
| Norton et al[48] | 26 | 12/46 | 5/19 | 0/0 | 2/7.7 | 1/3.8 |
| Bohnacker et al[20] | 87 | 74/85 | 29/33 | 0/0 | 15/17 | 17/19 |
| Catalano et al[14] | 103 | 83/80 | 10/9.7 | 0/0 | 10/9.7 | 16/15.5 |
| Cheng et al[15] | 55 | 39/71 | 12/22 | 0/0 | 9/16.3 | 4/7.2 |
| Han et al[21] | 33 | 20/60.6 | 11/33.3 | 0/0 | 2/6 | 2/6 |
| Ismail et al[65] | 61 | 56/92 | 15/24.5 | 0/0 | 12/19.6 | 9/14.7 |
| Napoleon et al[66] | 93 | 84/90 | 39/42 | 1/1 | 5/5.3 | NA |
| Ridtitid et al[67] | 182 | 134/73.6 | 34/18.6 | 0/0 | 16/8.7 | NA |
| Ardengh et al[58] | 41 | 38/92 | 11/26.8 | 0/0 | 3/7.3 | 4/9.7 |
Recurrence of benign lesions occur in up to 33% of patients depending on the tumor size, final histology, presence of intraductal tumor, coexistence of FAP and endoscopist experience[21,57,59-64]. If you use the endoscopic ultrasound before the EP you could find with precision the presence of intraductal tumor. In this case there are contraindication to submitted the patient to EP. Recurrent lesions are usually benign and most can be removed endoscopically.
EP is a safe and effective therapy and should be established as the first-line therapy for ampullary adenomas. The accurate staging of ampullary tumors is important for selecting patients to EP or surgical treatment. Compared to surgery, EP is associated with lower morbidity and mortality, and seems to be a preferable modality of treatment for small benign ampullary tumors with no intraductal extension. The EP procedure, when performed by an experienced endoscopist, leads to successful eradication in up to 85% of patients with ampullary adenomas.
P- Reviewer: Chen JQ, Fabre JM S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol. 2014;20:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 3. | Fujita R, Satake Y, Sugata F, Ono K, Soma S. Endoscopic papillectomy--excision of biliary calculi. Nihon Rinsho. 1978;Suppl:2190-2191. [PubMed] |
| 4. | Suzuki K, Kantou U, Murakami Y. Two cases with ampullary cancer who underwent endoscopic excision. Prog Dig Endosc. 1983;23:236-239. |
| 5. | Ohike N, Kim GE, Tajiri T, Krasinskas A, Basturk O, Coban I, Bandyopadhyay S, Morohoshi T, Goodman M, Kooby DA. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, Hochwald SN. Contemporary results with ampullectomy for 29 “benign” neoplasms of the ampulla. J Am Coll Surg. 2008;206:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301-309. [PubMed] |
| 10. | Patel R, Varadarajulu S, Wilcox CM. Endoscopic ampullectomy: techniques and outcomes. J Clin Gastroenterol. 2012;46:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Yamamoto Y, Nemoto T, Okubo Y, Nihonyanagi Y, Ishiwatari T, Takuma K, Tochigi N, Okano N, Wakayama M, Igarashi Y. Comparison between the location and the histomorphological/immunohistochemical characteristics of noninvasive neoplasms of the ampulla of Vater. Hum Pathol. 2014;45:1910-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Baillie J. Endoscopic ampullectomy. Am J Gastroenterol. 2005;100:2379-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 201] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Silvis SE. Endoscopic snare papillectomy. Gastrointest Endosc. 1993;39:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Wong RF, DiSario JA. Approaches to endoscopic ampullectomy. Curr Opin Gastroenterol. 2004;20:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Bassan M, Bourke M. Endoscopic ampullectomy: a practical guide. J Interv Gastroenterol. 2012;2:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006;38:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006;63:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Hernandez LV, Catalano MF. Endoscopic papillectomy. Curr Opin Gastroenterol. 2008;24:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Ito K, Fujita N, Noda Y. Endoscopic diagnosis and treatment of ampullary neoplasm (with video). Dig Endosc. 2011;23:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Rattner DW, Fernandez-del Castillo C, Brugge WR, Warshaw AL. Defining the criteria for local resection of ampullary neoplasms. Arch Surg. 1996;131:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, Koshita S, Kanno Y, Ogawa T, Kato Y. Impact of technical modification of endoscopic papillectomy for ampullary neoplasm on the occurrence of complications. Dig Endosc. 2012;24:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Aiura K, Imaeda H, Kitajima M, Kumai K. Balloon-catheter-assisted endoscopic snare papillectomy for benign tumors of the major duodenal papilla. Gastrointest Endosc. 2003;57:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Bohnacker S, Seitz U, Nguyen D, Thonke F, Seewald S, deWeerth A, Ponnudurai R, Omar S, Soehendra N. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Kim JH, Kim JH, Han JH, Yoo BM, Kim MW, Kim WH. Is endoscopic papillectomy safe for ampullary adenomas with high-grade dysplasia? Ann Surg Oncol. 2009;16:2547-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Kim HK, Lo SK. Endoscopic approach to the patient with benign or malignant ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:347-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Petrone G, Ricci R, Familiari P, Inzani F, Matsuoka M, Mutignani M, Delle Fave G, Costamagna G, Rindi G. Endoscopic snare papillectomy: a possible radical treatment for a subgroup of T1 ampullary adenocarcinomas. Endoscopy. 2013;45:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Salmi S, Ezzedine S, Vitton V, Ménard C, Gonzales JM, Desjeux A, Grimaud JC, Barthet M. Can papillary carcinomas be treated by endoscopic ampullectomy? Surg Endosc. 2012;26:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Woo SM, Ryu JK, Lee SH, Lee WJ, Hwang JH, Yoo JW, Park JK, Kang GH, Kim YT, Yoon YB. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol. 2009;24:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Yoon LY, Moon JH, Choi HJ, Min SK, Cha SW, Cheon YK, Cho YD, Lee MS, Kim JS. Wire-guided endoscopic snare retrieval of proximally migrated pancreatic stents after endoscopic papillectomy for ampullary adenoma. Gut Liver. 2011;5:532-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Ogawa T, Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Koshita S, Kanno Y, Masu K, Ishii S. Endoscopic papillectomy as a method of total biopsy for possible early ampullary cancer. Dig Endosc. 2012;24:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Kwon J, Lee SE, Kang MJ, Jang JY, Kim SW. A case of gangliocytic paraganglioma in the ampulla of Vater. World J Surg Oncol. 2010;8:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Niido T, Itoi T, Harada Y, Haruyama K, Ebihara Y, Tsuchida A, Kasuya K. Carcinoid of major duodenal papilla. Gastrointest Endosc. 2005;61:106-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Itoi T, Tsuji S, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Ikeuchi N, Igarashi M, Gotoda T. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy (with videos). Gastrointest Endosc. 2009;69:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Elek G, Gyôri S, Tóth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 39. | Bourgeois N, Dunham F, Verhest A, Cremer M. Endoscopic biopsies of the papilla of Vater at the time of endoscopic sphincterotomy: difficulties in interpretation. Gastrointest Endosc. 1984;30:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Menzel J, Hoepffner N, Sulkowski U, Reimer P, Heinecke A, Poremba C, Domschke W. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT--a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Okano N, Igarashi Y, Hara S, Takuma K, Kamata I, Kishimoto Y, Mimura T, Ito K, Sumino Y. Endosonographic preoperative evaluation for tumors of the ampulla of vater using endoscopic ultrasonography and intraductal ultrasonography. Clin Endosc. 2014;47:174-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Moon JH. Endoscopic diagnosis of ampullary tumors using conventional endoscopic ultrasonography and intraductal ultrasonography in the era of endoscopic papillectomy: advantages and limitations. Clin Endosc. 2014;47:127-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Kim JH, Moon JH, Choi HJ, Lee HS, Kim HK, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. Endoscopic snare papillectomy by using a balloon catheter for an unexposed ampullary adenoma with intraductal extension (with videos). Gastrointest Endosc. 2009;69:1404-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, Stotland B, Rosato EF, Morris JB, Eckhauser F. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Menees SB, Schoenfeld P, Kim HM, Elta GH. A survey of ampullectomy practices. World J Gastroenterol. 2009;15:3486-3492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Baillie J. Endoscopic ampullectomy: does pancreatic stent placement make it safer? Gastrointest Endosc. 2005;62:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Lee SK, Kim MH, Seo DW, Lee SS, Park JS. Endoscopic sphincterotomy and pancreatic duct stent placement before endoscopic papillectomy: are they necessary and safe procedures? Gastrointest Endosc. 2002;55:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Napoléon B, Alvarez-Sanchez MV, Leclercq P, Mion F, Pialat J, Gincul R, Ribeiro D, Cambou M, Lefort C, Rodríguez-Girondo M, Scoazec JY. Systematic pancreatic stenting after endoscopic snare papillectomy may reduce the risk of postinterventional pancreatitis. Surg Endosc. 2013;27:3377-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, Irie J. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Chang WI, Min YW, Yun HS, Lee KH, Lee JK, Lee KT, Rhee PL. Prophylactic pancreatic stent placement for endoscopic duodenal ampullectomy: a single-center retrospective study. Gut Liver. 2014;8:306-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Jun DW, Choi HS. Is the endoscopic papillectomy safe procedure in periampullary tumors? Korean J Gastroenterol. 2005;46:247-250. [PubMed] |
| 56. | Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 57. | Heinzow HS, Lenz P, Lenze F, Domagk D, Domschke W, Meister T. Feasibility of snare papillectomy in ampulla of Vater tumors: meta-analysis and study results from a tertiary referral center. Hepatogastroenterology. 2012;59:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Ardengh JC, Baron TH, Kemp PR, Mota GA, Taglieri E, Micelli-Neto O, Orsini ET, Santos JS. Impact of technical modification of EUS-guided endoscopic papillectomy for ampullary neoplasm on the rate of post- resection acute pancreatitis. Gastrointest Endosc. 2013;77:AB 374. |
| 59. | Ahn DW, Ryu JK, Kim J, Yoon WJ, Lee SH, Kim YT, Yoon YB. Endoscopic papillectomy for benign ampullary neoplasms: how can treatment outcome be predicted? Gut Liver. 2013;7:239-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 62. | Jung MK, Cho CM, Park SY, Jeon SW, Tak WY, Kweon YO, Kim SK, Choi YH. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Kim SH, Moon JH, Choi HJ, Kim DC, Lee TH, Cheon YK, Cho YD, Park SH, Kim SJ. Usefulness of pancreatic duct wire-guided endoscopic papillectomy for ampullary adenoma for preventing post-procedure pancreatitis. Endoscopy. 2013;45:838-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Vogt M, Jakobs R, Benz C, Arnold JC, Adamek HE, Riemann JF. Endoscopic therapy of adenomas of the papilla of Vater. A retrospective analysis with long-term follow-up. Dig Liver Dis. 2000;32:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Ismail S, Marianne U, Heikki J, Jorma H, Leena K. Endoscopic papillectomy, single-centre experience. Surg Endosc. 2014;28:3234-3239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Napoleon B, Gincul R, Ponchon T, Berthiller J, Escourrou J, Canard JM, Boyer J, Barthet M, Ponsot P, Laugier R. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Ridtitid W, Tan D, Schmidt SE, Fogel EL, McHenry L, Watkins JL, Lehman GA, Sherman S, Coté GA. Endoscopic papillectomy: risk factors for incomplete resection and recurrence during long-term follow-up. Gastrointest Endosc. 2014;79:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |