Published online Jan 16, 2015. doi: 10.4253/wjge.v7.i1.37
Peer-review started: September 28, 2014
First decision: November 19, 2014
Revised: November 21, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: January 16, 2015
Processing time: 111 Days and 14 Hours
Endoscopic retrograde cholangiopancreatography had been a treatment modality of choice for both benign and malignant biliary tract obstruction for more than half century, with a very high clinical success rate and low complications. But in certain circumstances, such as advanced and locally advanced pancreatobiliary malignancies (pancreatic cancer, cholangiocarcinoma, ampullary tumor) and tight benign strictures, endoscopic retrograde cholangiopancreatography (ERCP) fails. Up to this point, the only alternative interventions for these conditions were percutaneous transhepatic biliary drainage or surgery. Endoscopic ultrasound guided interventions was introduced for a couple decades with the better visualization and achievement of the pancreatobiliary tract. And it’s still in the process of ongoing development. The inventions of new techniques and accessories lead to more feasibility of high-ended procedures. Endoscopic ultrasound guided biliary drainage was a novel treatment modality for the patient who failed ERCP with the less invasive technique comparing to surgical bypass. The technical and clinical success was high with acceptable complications. Regarded the ability to drain the biliary tract internally without an exploratory laparotomy, this treatment modality became a very interesting procedures for many endosonographers, worldwide, in a short period. We have reviewed the literature and suggest that endoscopic ultrasound-guided biliary drainage is also an option, and one with a high probability of success, for biliary drainage in the patients who failed conventional endoscopic drainage.
Core tip: Failure of endoscopic retrograde cholangiopancreatography occurs in 5%-10% of the cases from many etiologies. However, there are few alternative options for biliary drainage up to the present time. Percutaneous biliary drainage and surgical bypass have their own drawbacks. Endoscopic ultrasound guided biliary drainage (EUS-BD) is a new platform with a very high technical and clinical success rate with an acceptable complications. This review focused on the techniques, instruments including tips and tricks of this treatment modality. EUS-BD would become another alternative options for biliary drainage for both benign and malignant conditions in the future.
- Citation: Prachayakul V, Aswakul P. Endoscopic ultrasound-guided biliary drainage as an alternative to percutaneous drainage and surgical bypass. World J Gastrointest Endosc 2015; 7(1): 37-44
- URL: https://www.wjgnet.com/1948-5190/full/v7/i1/37.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i1.37
Endoscopic retrograde cholangiopancreatography (ERCP) was first introduced by Demling and Classen[1] in 1970 and is now the treatment of choice for pancreatobiliary diseases. It was originally used as a diagnostic tool, but since the development of magnetic resonance imaging (MRI) and computed tomography (CT), which provide superior soft tissue details of the pancreatobiliary tract, ERCP has been used exclusively for therapeutic purposes. Pancreaticobiliary obstructions are the most common cause of pancreatobiliary disease. Because of the development of ever better endoscopy instruments and technologies, the overall success rate of ERCP is now 90% to 95% with a complication rate of 5% to 7%[2-16]. Selective bile duct cannulation, if performed by experienced endoscopists, is an effective treatment for over 90% of cases of pancreatobiliary disease without anatomical obstructions. It is not effective in only 3% to 5% of cases, usually due to gastroduodenal obstruction, failed cannulation, distorted ampullae, altered anatomy, a periampullary diverticulum, or previous enteral stents. In cases of failed ERCP, patients are usually referred for either percutaneous transhepatic biliary drainage (PTBD) or surgical bypass. Both these procedures have high rates of undesirable complications. Endoscopic ultrasound-guided biliary drainage (EUS-BD) is a new technique that was developed within the last decade. It is an attractive alternative to PTBD or surgery when ERCP fails, but there is no strong evidence-based data on which procedure is best in this setting. We have reviewed the literature and summarize the advantages and disadvantages of PTBD, surgical bypass, and EUS-BD, including which technique is best for different clinical situations and how to maximize procedural success and reduce complications for each method.
Percutaneous transhepatic biliary drainage (PTBD) is a treatment option for patients for whom ERCP was not successful. The first report on PTBD was in 1961 by Catalano et al[17], and it was the treatment of choice for biliary drainage for more than two decades. The technical success rate for PTBD ranges from 75% to 100% and the clinical success rate ranges from 65% to 92%. The complication rate ranges from 9% to 31%[18-21]. Ho et al[22] published a review article on why PTBD should be considered first-line treatment for biliary drainage. Data showed that PTBD was superior than endoscopic biliary drainage in malignant hilar biliary obstruction with a technical success rate of 89% vs 41%, respectively (P < 0.001) and complication rates of 52% and 18%, respectively (P = 0.04). The data on the best type of drainage for distal CBD obstruction was inconclusive. PTBD is successful even in patients who have poor performance status. It also takes less procedural time and has few complications. The drawbacks are that it cannot be used in the presence of moderate to marked ascites and the fact that bile drainage is external, which impairs the patient’s quality of life and involves difficulty in taking care of the catheter.
Surgical bypass is another treatment option after failed ERCP or unresectable hilar cholangiocarcinoma. Glazer et al[23] published a meta-analysis of randomized controlled trials of immediate stent placement vs surgical bypass in the palliative management of malignant biliary obstruction and found that there was significantly less recurrent biliary obstruction after surgical bypass than after stent placement (RR 0.14, 95%CI: 0.03-0.63, P < 0.01). The technical success rates (RR 0.99, 95%CI: 0.93-1.05; P = 0.67) and complication rates (RR 1.54, 95%CI: 0.87-2.71; P = 0.14) were not significantly different. Despite the more invasive approach, surgery produced better drainage; the drainage was internal, which had less effect on the patient’s quality of life; and the interval to recurrent biliary occlusion was longer. Unfortunately, this technique is only suitable for patients who are good surgical candidates, which limits its use in cases of advanced malignant biliary obstruction.
EUS-BD has been increasingly used as a minimally invasive alternative to surgery or radiologic intervention for biliary drainage after failed ERCP. EUS-BD can be performed via the papillary or gastrointestinal lumen. In the transpapillary route, rendezvous retrograde or antegrade stenting is used. For gastrointestinal luminal access, choledochoduodenostomy or hepaticogastrostomy is used, depending on the desired site of access. Artifon et al[24] conducted a randomized trial of EUS-guided choledochoduodenostomy or percutaneous drainage for unresectable distal biliary obstruction after failed ERCP. Technical success and clinical success were 100% in both groups. The complication rate for PTBD was 15.3% and the complication rate for EUD-BD was 25% (P = 0.2), and the cost of the procedures was similar (7570 USD and 5573 USD respectively, P = 0.39). Khashab et al[25] also conducted a trial of PTBD (n = 51) and EUS-BD (n = 22) after failed ERCP. Their technical success rate was higher in the PTBD group than the EUS-BD group (100% vs 86.4%, P = 0.007), and their clinical success rates were 92.2% vs 86.4%, P = 0.40. PTBD was associated with higher adverse events (index procedure: 39.2% vs 15.7%), but stent patency and survival rate were equivalent in both groups. PTBD cost more than twice as much to perform as EUS-BD (P = 0.004), mainly because the re-intervention rate was higher (80.4% vs 15.7%, P < 0.001). Multicenter studies and other cases reports and case series[26-41] have confirmed the safety and efficacy of EUS-BD alone. In the authors’ opinions, there was no one best approach among these three platforms for patients who failed ERCP. We recommend surgical bypass for patients with both duodenal and biliary obstructions who are good surgical candidates, but EUS-BD might be better than PTBD in patients with a large volume of ascites or patients who refuse external drainage. First-line treatment options depend on each institution’s facilities, the clinician’s expertise, and the patient’s preferences after receiving enough information to accurately evaluate each procedure’s strengths, weaknesses, and impact on quality of life.
The use of endoscopic ultrasound-guided cholangiography was initially described by Wiersema et al[42] in 1996. The first EUS-guided biliary drainage was reported by Giovannini et al[43] in 2001. In 2004, Mallory et al[44] reported the first case of EUS-guided ERCP using the rendezvous technique.
Endoscopic ultrasound-guided biliary drainage can be classified into two major groups: the transpapillary approach (rendezvous retrograde and antegrade stent insertion) and the transmural approach (choledochoduodenostomy and hepaticogastrostomy)[45-48].
EUS-guided biliary drainage should be reserved for patients for whom ERCP was not successful. Some experts recommend the transpapillary (rendezvous) approach before the transmural approach[49-51]. Rendezvous technique is impossible if the ampulla is not accessible; but, even in patients with accessible ampullae, the rendezvous procedure can be difficult because it is necessary to change from the echoscope to the duodenoscope and the railroad technique during guide wire grasping is not always easy. In the authors’ opinion, the advantage of the procedure is that it’s not necessary to create a bilo-entereic tract, which can sometimes produce leakage and bleeding. In patients with surgically altered anatomy in which the anastomotic opening could not initially be seen and the access to the opening was not too difficult. When the position of the echoscope is good enough and dilatation and the guidewire can be passed down to the duodenum easily, rendezvous is a good option. If access is through the intrahepatic ducts (left lobe segments II or III) or extra-hepatic duct [common bile duct, (CBD)] the route depends on the location of the obstruction and the expertise of the endoscopist. If the site of obstruction is located above the proximal to mid-CBD, the intra-hepatic route is best. For distal obstruction with large CBD caliber, the extrahepatic route is the ideal choice.
Each route has advantages and disadvantages. It is easier to make the puncture using the extra-hepatic route, but the echoscope is in an upward curving position that makes it more difficult to control and easier to slip out. The puncture and guidewire placement are more difficult in the transmural route, but handling the scope is easier.
The transmural route of EUS-guided biliary drainage can be achieved through an EUS-guided choledochoduodenostomy or an EUS-guided hepaticogastrostomy. The site of puncture depends on the location of the obstruction. If the obstruction site is distally located, choledochoduodenostomy is procedure of choice, while hilar obstructions are best served by a hepaticogastrostomy. It is easier to perform the puncture and handle the scope in segment II of the left lobe of the liver[52,53] and the endoscopist who performed the procedure has to confirm that the puncture site is not in the esophagus in order to avoid higher risk of mediastinitis. Even though some experts use the right lobe[54], it is not yet standard of practice.
Where to puncture: We summarized the advantages and disadvantages of extrahepatic and intrahepatic duct puncture in Table 1.
| Route of access | |
| Extrahepatic route | Intrahepatic route |
| Easy approach (especially for large-caliber CBD) The puncture site is close to the scope More difficult scope positioning to achieve desired direction from the punctured duct (rendezvous) Easy guidewire negotiation and neo-tract creation (EUS-BD) Difficult scope handling | The duct to be punctured is far from the scope Easier scope positioning to achieve desired direction from the punctured duct Easy scope handling Difficult guidewire negotiation and neo-tract creation Higher risk of bleeding Higher risk of bile leakage |
There are two major ways to create a bilo-enteric tract: cauterization with a needle knife or small caliber cystotome especially 6 Fr in diameter[55-66] and non-cauterization with a tapered-tip catheter[67] or Soehendra stent retriever[68]. Neo-tract creation is followed by neo-tract dilation. The advantages and disadvantages of these two approaches are summarized in Table 2.
| Neo-tract creation methods | |
| Cauterization | Non-cauterization |
| Easy neo-tract creation with no need for forceful manipulation More tissue injury from thermal burn The procedure takes less time More complications, especially bile leakage or perforation | More difficult and forceful manipulation, especially when the intervening tissue is thick or the direction is inappropriate Less injury, smaller diameter of the neo-tract Lower risk of bile leakage or bleeding |
Neo-tract dilation can also be performed two ways: balloon dilation or graded dilation. Both methods are evaluated in Table 3.
| Dilatation methods | |
| Balloon dilation | Graded dilation |
| Radial force leads to bigger neo-tract diameter (easier but greater risk for bile leakage, bleeding and perforation) | Axial force creates a smaller neo-tract. More difficult, but less leakage and less bleeding) |
| Easier stent insertion | Stent insertion can be more difficult |
| Only a single dilation session is needed and there are fewer guidewire exchanges | More sessions of dilation are needed and there are more frequent guidewire exchanges |
There is no best approach. The technique of choice depends on the individual endoscopist’s expertise. If balloon dilation must be used, the authors recommend the small size (4 mm diameter) balloon dilator.
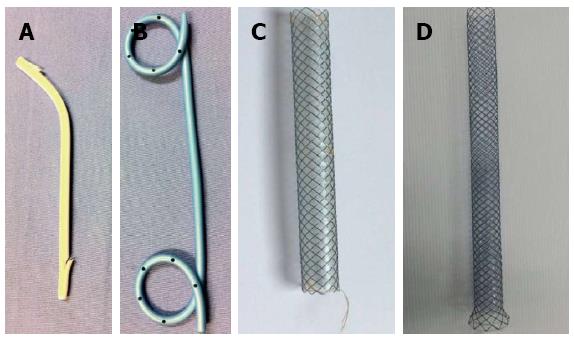
In the early years of EUS-guided biliary drainage, the most commonly used stent was plastic; but many experts used fully covered, self-expandable metal stents (FCSEMS) instead of plastic stents and reported good outcomes[69-71]. Many types of metallic stents were developed for this purpose. Even though metal stents create a wider lumen with better drainage ability, they are more expensive and there is a risk of migration. Recently, Galasso et al[72] developed a stent suitable for EUS-guided hepaticogastrostomy called the Gio-Bor stent. It is a half-covered SEMS stent (Figure 1). The authors recommend an FCSEMS or partial CSEMS stent 40 to 60 mm in length for EUS-CD and 80 to 100 mm in length for EUS-HG. The small introducer (7 Fr) FCSEMS and partial CSEMS are shorter procedures and need fewer guidewire exchanges. However, there was a multicenter Japanese study[38] demonstrate that higher bile leakage was associated with plastic stent placement, therefore there was a trend towards to preference of using covered SEMS to prevent this complication.
How to locate the puncture site: The site of puncture should be evaluated both endosonographically and fluoroscopically. Endosonographic tracing of the left intrahepatic bile duct was important in guiding the tip of needle and helping the endoscopist select the segment most suitable for puncture and easy guidewire negotiation. The fluoroscopic view can also help the endoscopist assess the best angle for bile duct puncture and easy neo-tract creation. Interestingly, if the scope’s tip is perpendicular to the gastroduodenal wall, it will make the dilation process more difficult, so we recommend a slightly tangential angle. If the tip of the scope is too angulated, it will make the puncture more difficult. The distance between the punctured duct and the probe should be no more than 1-2 cm. Before starting the puncture, check Doppler color flow to avoid the intervening vessel.
Using a 0.025 stiff guidewire (VisiGlide) or a 0.035 hydrophilic tip guidewire will make guidewire negotiation easier. The direction of the needle tip will directly affect guidewire manipulation. If the direction of the needle is opposite to the desired guidewire direction, manipulation will be really difficult. Moving the guidewire back and forth just a little bit (jiggling maneuver) will help change the guidewire direction. Using guidewires designed for manual twisting maneuvers or that have accessories, such as Terumo or ViziGlide guidewires, will make guidewire manipulation easier.
Most endoscopists who perform EUS-guided biliary drainage have experience with guidewire shearing or knotting during the procedure. Saxena et al[73] and Khashub et al[74] recommend flushing the channel with water and using a special type of needle, such as an access needle, which is designed to resolve these problems. However, in the authors’ experience, this specially designed needle was not sharp enough in some situations and did not prevent guidewire shearing. We found that the way to prevent shearing and knotting was to push, not pull, the guidewire back, even if the desired duct was not yet punctured, and to exchange the needle for the small-sized dilator or tapered-tip catheter after the guidewire was looped and continue the guidewire negotiation later on. We have had no problem with shearing or knotting if we followed these guidelines.
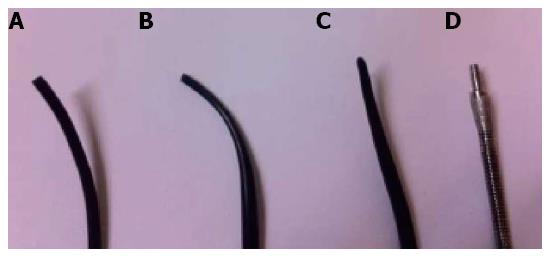
The distance between the puncture site and the desired duct is a very important factor in neo-tract creation. If the distance is longer, it is more difficult to penetrate through the tissue and pierce the bile duct. Another factor is the stiffness of the tissue between the puncture site and the bile duct. If the patient has liver fibrosis, the tissue is stiffer and this can make creation of a neo-tract more difficult. If difficulty is encountered, we recommended that the endoscopist should, firstly, re-check the position of the scope tip to make sure it is not perpendicular to the gastric wall. If graded dilation is being performed, change the dilating catheter to a smaller size or a catheter with a tapered tip, use a tapered-tip cannulation catheter, or re-shape the tip of the catheter by cutting it to a needle shape. Dilating with a Soehendra stent retriever, which has a drilling effect, might also be useful (Figure 2). If all of the above methods fail, cauterization may be necessary. Different types of catheter tips are shown in Figure 3.
Complications can occur if the needle knife is used with the Odd ratio of 12.4[75]. To minimize possible tissue damage during neo-tract creation, only open the knife half of its full length and cauterize until it enters the duct. In process of dilation, the dilator should be inserted after the knife is used. For cystotome usage, it very important to push the cystotome catheter against the mural and bile duct wall firmly, before starting the cauterization (this technique would help to enter the bile duct easily).
Generally, the least chance of bile leakage and bleeding if the diameter of neotract is as small as possible. Therefore, the authors recommend not to dilate the neo-tract larger than the size of stent introducer (always not more than 8.5 Fr). For graded dilation technique, 8.5 Fr size is suitable for Soehendra dilator and only 7 Fr size is suitable for Soehendra stent retriever whereas smaller balloon especially not more than 4 mm in diameter is suitable for balloon dilation.
The development of single step device which might be more suitable to each specific procedure would be helpful the help endoscopist to overcome the cubersome techniques such as multiple guidewire exchanges and would make the procedure time shorter; Smaller introducer (7 Fr) of smaller sized covered SEMSs (6 or 8 mm in diameter) would be benefit for less complications and shorter procedure time; Randomized control trial that EUS-BD as the treatment of choice in some particular conditions such as surgical altered anatomy would be interesting; The possibility of using EUS-BD as the preferable options than transpapillary drainage should be widely discussed and prospective study should be conducted.
EUS-guided biliary drainage is safe and effective when performed by an experienced endoscopist, and is an alternative to PTBD and surgical bypass after failed ERCP. Unfortunately, it use is still limited to tertiary care hospitals with advanced-complex endoscopy units. Clinicians will need to choose a treatment method based on each patient’s status, preferences, and the facilities of the hospitals in their area.
P- Reviewer: Murata A S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Demling L, Classen M. [Duodenojejunoscopy]. Dtsch Med Wochenschr. 1970;95:1427-1428 passim. |
| 2. | Dumonceau JM, Vonlaufen A. Pancreatic endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy. 2007;39:124-130. |
| 3. | Fabbri C, Luigiano C, Lisotti A, Cennamo V, Virgilio C, Caletti G, Fusaroli P. Endoscopic ultrasound-guided treatments: are we getting evidence based--a systematic review. World J Gastroenterol. 2014;20:8424-8448. |
| 4. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. |
| 5. | Qian Y, Huang J, Zhang Y, Fan ZN. Cannulation of the intradiverticular papilla using a duodenoscope: is it a safe method? World J Gastroenterol. 2014;20:10217-10218. |
| 6. | Song BJ, Kang DH. Prevention of postendoscopic retrograde cholangiopancreatography pancreatitis: the endoscopic technique. Clin Endosc. 2014;47:217-221. |
| 7. | Zang J, Zhang C, Gao J. Guidewire-assisted transpancreatic sphincterotomy for difficult biliary cannulation: a prospective randomized controlled trial. Surg Laparosc Endosc Percutan Tech. 2014;24:429-433. |
| 8. | Okabe Y, Ishida Y, Kuraoka K, Ushijima T, Tsuruta O. Endoscopic bile duct and/or pancreatic duct cannulation technique for patients with surgically altered gastrointestinal anatomy. Dig Endosc. 2014;26 Suppl 2:122-126. |
| 9. | Skinner M, Popa D, Neumann H, Wilcox CM, Mönkemüller K. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560-572. |
| 10. | Choudhary A, Winn J, Siddique S, Arif M, Arif Z, Hammoud GM, Puli SR, Ibdah JA, Bechtold ML. Effect of precut sphincterotomy on post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. World J Gastroenterol. 2014;20:4093-4101. |
| 11. | Artifon EL, Moura RN, Otoch JP. Difficult cannulation: what should I do before EUS guided access? Rev Gastroenterol Peru. 2014;34:53-57. |
| 12. | Myung DS, Park CH, Koh HR, Lim SU, Jun CH, Ki HS, Park SY, Rew JS. Cap-assisted ERCP in patients with difficult cannulation due to periampullary diverticulum. Endoscopy. 2014;46:352-355. |
| 13. | Kubiliun NM, Elmunzer BJ. Preventing pancreatitis after endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am. 2013;23:769-786. |
| 14. | Ito K, Horaguchi J, Fujita N, Noda Y, Kobayashi G, Koshita S, Kanno Y, Ogawa T, Masu K, Hashimoto S. Clinical usefulness of double-guidewire technique for difficult biliary cannulation in endoscopic retrograde cholangiopancreatography. Dig Endosc. 2014;26:442-449. |
| 15. | Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2013;45:605-618. |
| 16. | Kobayashi G, Fujita N, Imaizumi K, Irisawa A, Suzuki M, Murakami A, Oana S, Makino N, Komatsuda T, Yoneyama K. Wire-guided biliary cannulation technique does not reduce the risk of post-ERCP pancreatitis: multicenter randomized controlled trial. Dig Endosc. 2013;25:295-302. |
| 17. | Catalano D, Mariosa L, Miracco A, Mauro R. [Percutaneous cholangiography with biliary catheterization and drainage]. Rass Int Clin Ter. 1961;41:255-267. |
| 18. | Leng JJ, Zhang N, Dong JH. Percutaneous transhepatic and endoscopic biliary drainage for malignant biliary tract obstruction: a meta-analysis. World J Surg Oncol. 2014;12:272. |
| 19. | Garcarek J, Kurcz J, Guziński M, Janczak D, Sasiadek M. Ten years single center experience in percutaneous transhepatic decompression of biliary tree in patients with malignant obstructive jaundice. Adv Clin Exp Med. 2012;21:621-632. |
| 20. | Audisio RA, Morosi C, Bozzetti F, Cozzi G, Bellomi M, Pisani P, Pestalozza A, Gennari L, Severini A. The outcome of cholangitis after percutaneous biliary drainage in neoplastic jaundice. HPB Surg. 1993;6:287-293. |
| 21. | Stanley J, Gobien RP, Cunningham J, Andriole J. Biliary decompression: an institutional comparison of percutaneous and endoscopic methods. Radiology. 1986;158:195-197. |
| 22. | Ho CS, Warkentin AE. Evidence-based decompression in malignant biliary obstruction. Korean J Radiol. 2012;13 Suppl 1:S56-S61. |
| 23. | Glazer ES, Hornbrook MC, Krouse RS. A meta-analysis of randomized trials: immediate stent placement vs. surgical bypass in the palliative management of malignant biliary obstruction. J Pain Symptom Manage. 2014;47:307-314. |
| 24. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. |
| 25. | Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI. A Comparative Evaluation of EUS-Guided Biliary Drainage and Percutaneous Drainage in Patients with Distal Malignant Biliary Obstruction and Failed ERCP. Dig Dis Sci. 2014;Epub ahead of print. |
| 26. | Dhir V, Artifon EL, Gupta K, Vila JJ, Maselli R, Frazao M, Maydeo A. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430-435. |
| 27. | Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80-87. |
| 28. | Iqbal S, Friedel DM, Grendell JH, Stavropoulos SN. Outcomes of endoscopic-ultrasound-guided cholangiopancreatography: a literature review. Gastroenterol Res Pract. 2013;2013:869214. |
| 29. | Artifon EL, Ferreira FC, Sakai P. Endoscopic ultrasound-guided biliary drainage. Korean J Radiol. 2012;13 Suppl 1:S74-S82. |
| 30. | Luz LP, Al-Haddad MA, Sey MS, DeWitt JM. Applications of endoscopic ultrasound in pancreatic cancer. World J Gastroenterol. 2014;20:7808-7818. |
| 31. | Altonbary AY, Deiab AG, Bahgat MH. Endoscopic ultrasound-guided choledechoduodenostomy for palliative biliary drainage of obstructing pancreatic head mass. Endosc Ultrasound. 2014;3:137-140. |
| 32. | Will U, Fueldner F, Kern C, Meyer F. EUS-Guided Bile Duct Drainage (EUBD) in 95 Patients. Ultraschall Med. 2014;Epub ahead of print. |
| 33. | Hamada T, Isayama H, Nakai Y, Kogure H, Yamamoto N, Kawakubo K, Takahara N, Uchino R, Mizuno S, Sasaki T. Transmural biliary drainage can be an alternative to transpapillary drainage in patients with an indwelling duodenal stent. Dig Dis Sci. 2014;59:1931-1938. |
| 34. | Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7:94-102. |
| 35. | Kumta NA, Kedia P, Kahaleh M. Endoscopic ultrasound-guided biliary drainage: an update. Curr Treat Options Gastroenterol. 2014;12:154-168. |
| 36. | Takada J, Carmo AM, Artifon EL. EUS-guided biliary drainage for malignant biliary obstruction in patients with failed ERCP. J Interv Gastroenterol. 2013;3:76-81. |
| 37. | Alvarez-Sánchez MV, Jenssen C, Faiss S, Napoléon B. Interventional endoscopic ultrasonography: an overview of safety and complications. Surg Endosc. 2014;28:712-734. |
| 38. | Kawakubo K, Isayama H, Kato H, Itoi T, Kawakami H, Hanada K, Ishiwatari H, Yasuda I, Kawamoto H, Itokawa F. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328-334. |
| 39. | Khashab MA, Kumbhari V, Kalloo AN, Saxena P. EUS-guided biliary drainage by using a hepatogastrostomy approach. Gastrointest Endosc. 2013;78:675. |
| 40. | Tonozuka R, Itoi T, Sofuni A, Itokawa F, Moriyasu F. Endoscopic double stenting for the treatment of malignant biliary and duodenal obstruction due to pancreatic cancer. Dig Endosc. 2013;25 Suppl 2:100-108. |
| 41. | Sarkaria S, Lee HS, Gaidhane M, Kahaleh M. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver. 2013;7:129-136. |
| 42. | Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102-106. |
| 43. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. |
| 44. | Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100-107. |
| 45. | Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372-1379. |
| 46. | Sarkaria S, Sundararajan S, Kahaleh M. Endoscopic ultrasonographic access and drainage of the common bile duct. Gastrointest Endosc Clin N Am. 2013;23:435-452. |
| 47. | Artifon EL. Endoscopic ultrasound-guided biliary drainage. Endosc Ultrasound. 2013;2:61-63. |
| 48. | Park do H. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am. 2012;22:271-80, ix. |
| 49. | Shami VM, Kahaleh M. Endoscopic ultrasound-guided cholangiopancreatography and rendezvous techniques. Dig Liver Dis. 2010;42:419-424. |
| 50. | Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60-65. |
| 51. | Kawakubo K, Isayama H, Sasahira N, Nakai Y, Kogure H, Hamada T, Miyabayashi K, Mizuno S, Sasaki T, Ito Y. Clinical utility of an endoscopic ultrasound-guided rendezvous technique via various approach routes. Surg Endosc. 2013;27:3437-3443. |
| 52. | Khashab MA, Dewitt J. EUS-guided biliary drainage: is it ready for prime time? Yes! Gastrointest Endosc. 2013;78:102-105. |
| 53. | Perez-Miranda M, De la Serna-Higuera C. EUS access to the biliary tree. Curr Gastroenterol Rep. 2013;15:349. |
| 54. | Park SJ, Choi JH, Park do H, Choi JH, Lee SS, Seo DW, Lee SK, Kim MH. Expanding indication: EUS-guided hepaticoduodenostomy for isolated right intrahepatic duct obstruction (with video). Gastrointest Endosc. 2013;78:374-380. |
| 55. | Varadarajulu S, Hawes RH. EUS-guided biliary drainage: taxing and not ready. Gastrointest Endosc. 2013;78:742-743. |
| 56. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. |
| 57. | Belletrutti PJ, Gerdes H, Schattner MA. Successful endoscopic ultrasound-guided transduodenal biliary drainage through a pre-existing duodenal stent. JOP. 2010;11:234-236. |
| 58. | Artifon EL, Takada J, Okawa L, Moura EG, Sakai P. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP. 2010;11:597-600. |
| 59. | Prachayakul V, Aswakul P, Kachintorn U. EUS-guided choledochoduodenostomy for biliary drainage using tapered-tip plastic stent with multiple fangs. Endoscopy. 2011;43 Suppl 2 UCTN:E109-E110. |
| 60. | Panpimanmas S, Ratanachu-ek T. Endoscopic ultrasound-guided hepaticogastrostomy for hilar cholangiocarcinoma: the first trial in Thailand. J Med Assoc Thai. 2011;94 Suppl 2:S129-S134. |
| 61. | Artifon EL, Takada J, Okawa L, Ferreira F, Santos M, Moura EG, Otoch JP, Sakai P. Successful endoscopic ultrasound-guided overstenting biliary drainage through a pre-existing proximal migrated metal biliary stent. Rev Gastroenterol Mex. 2011;76:270-274. |
| 62. | Jürgensen C, Wentrup R, Zeitz M. Endoscopic ultrasound (EUS)-guided transduodenal drainage of an obstructed jejunal loop after hepaticojejunostomy as treatment for recurrent biliary sepsis. Endoscopy. 2013;45 Suppl 2 UCTN:E40-E41. |
| 63. | Sharaiha RZ, Kalloo AN, Khashab MA. EUS-guided hepatoesophagostomy for transesophageal biliary drainage (with video). Gastrointest Endosc. 2012;76:227-228. |
| 64. | Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010;71:413-419. |
| 65. | Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246-251. |
| 66. | Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005;100:1743-1747. |
| 67. | Prachayakul V, Aswakul P. A novel technique for endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2013;19:4758-4763. |
| 68. | Vila JJ, Goñi S, Arrazubi V, Bolado F, Ostiz M, Javier Jiménez F. Endoscopic ultrasonography-guided transgastric biliary drainage aided by Soehendra stent retriever. Am J Gastroenterol. 2010;105:959-960. |
| 69. | Artifon EL, Safatle-Ribeiro AV, Ferreira FC, Poli-de-Figueiredo L, Rasslan S, Carnevale F, Otoch JP, Sakai P, Kahaleh M. EUS-guided antegrade transhepatic placement of a self-expandable metal stent in hepatico-jejunal anastomosis. JOP. 2011;12:610-613. |
| 70. | Eum J, Park do H, Ryu CH, Kim HJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos). Gastrointest Endosc. 2010;72:1279-1284. |
| 71. | Lai LH, Chan FK, Sung JJ, Chan AW, Lee KF. EUS-guided transduodenal biliary drainage. Gastrointest Endosc. 2010;72:186-187; discussion 187. |
| 72. | Galasso D, Bories E, Caillol F, Forero Pineros EA, Pesenti C, Giovannini M. Feasibility of endoscopic ultrasound-guided hepaticogastrostomy in a patient with previous gastric banding. Endoscopy. 2013;45 Suppl 2 UCTN:E233-E234. |
| 73. | Saxena P, Aguila G, Kumbhari V, Khashab MA. Untying the knot: technique of unraveling a guidewire knot created during EUS-guided biliary drainage. Endoscopy. 2014;46 Suppl 1 UCTN:E140-E141. |
| 74. | Khashab MA, Dewitt J. Treatment and prevention of wire shearing during EUS-guided biliary drainage. Gastrointest Endosc. 2012;76:921-923. |
| 75. | Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-1284. |