Published online Aug 16, 2014. doi: 10.4253/wjge.v6.i8.373
Revised: June 5, 2014
Accepted: June 27, 2014
Published online: August 16, 2014
Processing time: 136 Days and 21.8 Hours
AIM: To show the safety and effectiveness of endoscopic ultrasound (EUS)-guided drainage of pelvic abscess that were inaccessible for percutaneous drainage.
METHODS: Eight consecutive patients with pelvic abscess that were not amenable to drainage under computed tomography (CT) guidance were referred for EUS-guided drainage. The underlying cause of the abscesses included diverticulitis in 4, postsurgical surgical complications in 2, iatrogenic after enema in 1, and Crohn’s disease in 1 patient. Abscesses were all drained under EUS guidance via a transrectal or transsigmoidal approach.
RESULTS: EUS-guided placement of one or two 7 Fr pigtail stents was technically successful and uneventful in all 8 patients (100%). The abscess was perisigmoidal in 2 and was multilocular in 4 patients. All procedures were performed under conscious sedation and without fluoroscopic monitoring. Fluid samples were successfully retrieved for microbiological studies in all cases and antibiotic policy was adjusted according to culture results in 5 patients. Follow-up CT showed complete recovery and disappearance of abscess. The stents were retrieved by sigmoidoscopy in only two patients and had spontaneously migrated to outside in six patients. All drainage procedures resulted in a favourable clinical outcome. All patients became afebrile within 24 h after drainage and the mean duration of the postprocedure hospital stay was 8 d (range 4-14). Within a median follow up period of 38 mo (range 12-52) no recurrence was reported.
CONCLUSION: We conclude that EUS-guided drainage of pelvic abscesses without fluoroscopic monitoring is a minimally invasive, safe and effective approach that should be considered in selected patients.
Core tip: For pelvic abscesses that are not amenable to percutaneous drainage, EUS-guided drainage affords a safe and efficient alternative method. The procedure was performed in eight patients under conscious sedation and without a radiological monitoring. One or two plastic stents (7 Fr) were placed after dilatation of the tract with a balloon in four patients. Revising this technique by using a cystotome in other four patients appeared feasible and without adverse events. Abscess resolution was documented by imaging examination in all patients. This outcome was not affected although spontaneous stent dislodgment or migration occurred in the majority of patients.
- Citation: Hadithi M, Bruno MJ. Endoscopic ultrasound-guided drainage of pelvic abscess: A case series of 8 patients. World J Gastrointest Endosc 2014; 6(8): 373-378
- URL: https://www.wjgnet.com/1948-5190/full/v6/i8/373.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i8.373
Infected pelvic fluid collections may occur as a complication of intestinal and gynaecological inflammatory diseases or abdominal surgery.
Unless drainage is promptly achieved, a pelvic abscess is unlikely to heal with conservative measures only, including antibiotics. Historically the treatment of pelvic abscess has been either laparotomy with lavage or blind surgical incision and drainage through the rectal or vaginal wall. Over time management has evolved from operative through percutaneous drainage into endoscopic ultrasound (EUS)-guided transrectal drainage. Case series demonstrate how EUS-guided drainage has passed through various stages of modifications[1-5]. The present case series documents the value of this approach in daily clinical practice and highlights recent developments in this field.
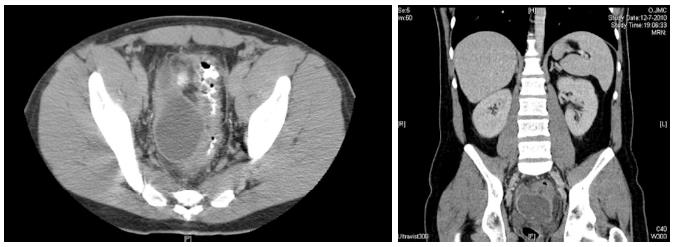
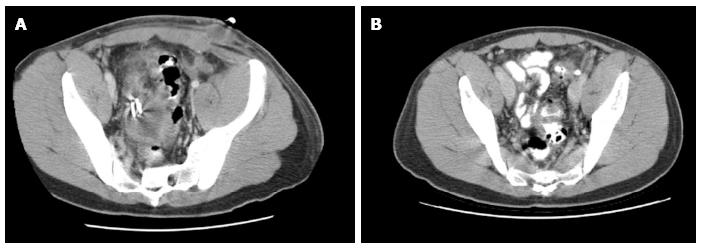
This is a retrospective analysis of a prospectively collected data of a single centre case series of patients who underwent EUS-guided drainage of pelvic abscess in the period between December 2010 and December 2012. A dedicated pelvic computed tomography (CT) scan was performed before the drainage procedure to determine the exact size and location of the abscess (Figure 1).
EUS-guided drainage was indicated when pelvic abscess was not amenable to drainage by CT guidance due to a lack of adequate and safe window for puncture. At the time of puncture all patients were receiving intravenous antibiotics (amoxicillin plus clavulanic acid or ciprofloxacin) and none had coagulation disorders. Informed consent was obtained from all patients before the procedure. Colon preparation was achieved by administration of polyethyleneglycol (Klean-Prep, Norgine BV, the Netherlands) and natrium phosphate enema (Colex Klysma, Tramedico BV, the Netherlands).
All procedures were performed under conscious sedation by administering a combination of intravenous midazolam and fentanyl. No fluoroscopic monitoring was used during the procedure.
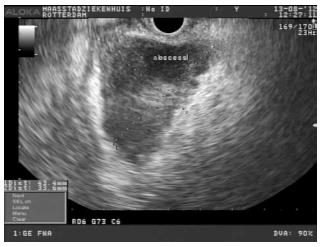
A therapeutic curvilinear array echoendoscope (EG-160; Olympus®, Tokyo, Japan) with a working channel of 3.2 mm was inserted up to 25 cm from the anal verge. Perirectal and perisigmoidal abscesses (< 15 cm or > 15 cm from anal verge respectively) and the area of contact between the rectal (colonic) wall and abscess wall were located by EUS (Figure 2). Colour doppler was used to exclude the presence of intervening blood vessels in the contact zone.
The abscess cavity was punctured using a 19-A gauge needle (EchoTip; Cook Medical®, Limerick, Ireland) and fluid was aspirated to confirm the location. A sample of aspirated material was sent for microbiological culture. A 0.035-inch guidewire was inserted through the needle and coiled in the cavity under EUS control.
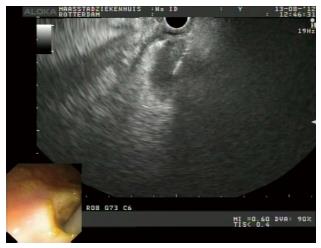
The tract between the rectum and the abscess cavity was created by two different methods. Initially (patients 1-4), a needle knife was inserted into the tract to facilitate the insertion of a biliary balloon (Cook Medical®, Limerick, Ireland). The balloon was then inflated to 8 mm to dilate the tract. In subsequent cases (patients 5-8), the collection was punctured with a 19-gauge FNA needle (Cook Medical®, Limerick, Ireland) through which a guidewire was advanced. After removing the FNA needle, a 10 Fr cystotome (Cook Medical®, Limerick, Ireland) was passed over the guidewire under EUS control into the cavity using electro cautery (Figure 3).
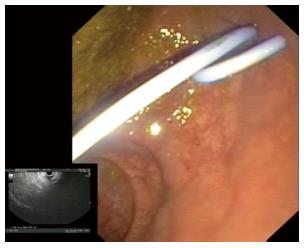
The drainage was accomplished by the placement of a 7 Fr double pigtail stent across the dilated tract into the abscess cavity (Figure 4). On indication a second stent was inserted after reintroducing the guidewire through a cannula that was passed adjacent to the primary stent under EUS control confirming its adequate positioning in the cavity by fluid/pus aspiration.
Patients continued their antibiotics or were switched according to culture results. Follow-up pelvic CT was performed to assess the response to treatment one week after the procedure. When abscess resolution was verified the stent was endoscopically removed by outpatient sigmoidoscopy. If resolution was not complete (Figure 5) the stent(s) were left in place and pelvic CT was repeated later to confirm abscess resolution prior to stent retrieval.
Technical success was defined as the ability to insert at least one 7 Fr pigtail stent to drain the abscess under EUS guidance. Recurrence was defined as the need for repeat EUS-guided drainage of a pelvic abscess after the stent retrieval. Clinical success was defined as complete resolution of the abscess without recurrence or a need for further surgery. The institutional review board of our hospital approved the study.
EUS-guided pelvic abscess drainage was performed in 8 patients (6 men; median age 55.5 years; range 21-74). The clinical features, technical details and outcomes of individual patients who underwent EUS-guided pelvic abscess drainage are shown in Table 1. The abscess was perisigmoidal in 2 and was multilocular in 4 patients. The median size of the abscess was 73 mm (range 45-90) and 43 mm (range 37-55) in the large and small axis respectively.
| Patient no. | Abscess location | Etiology | Abscess size (mm) | Abscess type | Cystotome | Stent (s) | Stent spontaneous discharge | Outcome | |
| Large axis | Small axis | ||||||||
| 1 | Perirectal | Diverticulitis | 90 | 55 | Multilocular | - | 2 | - | Complete resolution |
| 2 | Perirectal | Crohn’s disease | 45 | 37 | Unilocular | - | 1 | + | Complete resolution |
| 3 | Perisigmoidal | Diverticulitis | 75 | 43 | Multilocular | - | 2 | - | Complete resolution |
| 4 | Perirectal | Appendectomy | 65 | 46 | Unilocular | - | 1 | + | Complete resolution |
| 5 | Perirectal | Iatrogenic (enema injury) | 72 | 39 | Unilocular | + | 1 | + | Complete resolution |
| 6 | Perisigmoidal | Diverticulitis | 74 | 46 | Multilocular | + | 1 | + | Complete resolution |
| 7 | Perirectal | Diverticulitis | 53 | 43 | Multilocular | + | 1 | + | Complete resolution |
| 8 | Perirectal | Prostate surgery | 86 | 50 | Unilocular | + | 1 | + | Complete resolution |
Stent placement was technically successful in all patients without any adverse events. One patient underwent the procedure twice because during the first attempt the abscess appeared immature without successful fluid aspirate and it was decided not to place a stent. A repeated puncture one week later resulted in evident fluid aspirate and a stent was placed. One stent was placed in six patients and two stents were placed in two patients, one of whom received both transabdominal drain and transrectal stents.
All patients became afebrile within 24 h after drainage and the median hospital stay was 8 d (range 4-14). The fluid aspirate microbiological cultures showed a mono- or multibacterial growth of Gram-negative (Escherichia coli; Citrobacter braakii; Pseudomonas aeruginosa) and Gram-positive bacteria (Haemolytic streptococcus groep F.; Enterococcus faecium; Staphylococcus aureus). Antibiotic policy was adjusted according to culture results in 5 patients.
In only two patients the stents were removed during sigmoidoscopy while in the remainder the plastic stent dislocated and spontaneously fell out within one week of its placement. Abscess resolution and spontaneous discharge of the stent was confirmed by means of CT scan one week after the drainage procedure in 6 patients. Stents were endoscopically removed in two patients, respectively 4 and 6 wk after placement at the time when complete resolution of the abscess was confirmed on CT scan.
Within a median follow up period of 38 mo (range 12-52) no recurrence was reported in any patient. Two patients underwent surgery 2 and 3 mo after drainage procedure. One patient had an ileocecal resection for Crohn’s disease and another patient a sigmoid resection for recurrent diverticulitis. One patient died 10 mo after the procedure due to metastases from breast cancer.
This case series shows that minimally invasive EUS-guided drainage is effective in achieving resolution of pelvic abscesses. Multilocular abscesses also responded favourably to this method indicating internal communications between the different pockets. The procedures were safely performed without fluoroscopy and under conscious sedation. Fluid/pus aspirations for microbiological studies were obtained in all cases to guide antibiotic policy. After EUS guided drainage complete recovery occurred in all patients and in the majority of cases the stent(s) migrated spontaneously. None of the abscesses recurred. In two patients who were operated at a later stage, preoperative abscess drainage under EUS guidance facilitated surgical resection. Application of a cystotome over a guidewire using electrocautery to create a tract proved feasible and safe under endosonographic control.
Pelvic abscess can develop secondary to various intestinal diseases including complicated diverticulitis, appendicitis or Crohn’s disease. Gynaecological conditions such as pelvic inflammatory disease and abdominal surgery including low anterior resection for rectal cancer, prostate or obstetrical surgery are also known causes. The most common reported cause is acute diverticulitis causing colonic perforation[6].
When complicated by intra-abdominal rupture, pelvic abscesses can present as a life threatening abdominal emergency with high morbidity and mortality. In addition, conservative treatment alone is seldom effective in achieving complete resolution rendering drainage an unavoidable step in the management of pelvic abscess.
Historically the treatment of pelvic abscess has been either laparotomy with lavage or blind surgical incision and drainage through the rectal or vaginal wall[7]. Later minimally invasive imaging-guided drainage procedures, either computed tomography or ultrasonography, were introduced and established their effectiveness and safety profile[8-11]. However, a small proportion of patients remain inaccessible via the transabdominal approach due to lack of an appropriate window for drainage by intervening small bowel loops or blood vessels. In addition, abscess recurrence and/or fistula formation after percutaneous drainage may also complicate the patient’s disease course and compromise a surgical approach. Surgical intervention is not infrequent practiced when the abscess is complex and multiloculated, when abscess is inaccessible for minimally invasive drainage, or when a physician experienced in these minimally invasive procedures is not available[11].
The therapeutic application of endoscopic ultrasound (EUS) has gained a wide popularity because of its safety and effectiveness in draining peripancreatic fluid collections via the stomach or the duodenum. Accordingly, EUS-guided drainage of deep pelvic abscess could offer an alternative to surgery in selected patients. Since the introduction of this procedure, case series of patients with pelvic abscesses have reported a high success rate of EUS guided drainage without major complications.
The first report in 2003 described successful EUS-guided stent (8.5-10 Fr) placement in 9 of 12 patients and cyst fluid aspiration only in three. Surgical intervention was required in one patient after stent drainage and in two patients in whom the collection was only aspirated. All procedures were performed under general anaesthesia and fluoroscopic monitoring[2]. A subsequent report described the EUS-guided introduction of drainage catheter (10 Fr) attached to a flushing system for 4-8 d in four patients with pelvic abscess[3]. In this report, procedures were performed under conscious sedation and fluoroscopic control. Patients with multiloculated abscess were excluded. The same group reported their experience in another 4 patients who successfully underwent the combined placement of one or two double pigtail stents (7 Fr) and a single pigtail drainage catheter (10 Fr) with favourable outcome[4]. The same centre reported successful placement of either double pigtail stents (n = 15) or double pigtail stents in combination with a single pigtail flushing catheter (n = 10) in patients with deep pelvic abscess < 8 cm or > 8 cm respectively[5]. The flushing drain was removed after 36-72 h and the remaining stents 2 wk after their insertion. Six patients had perisigmoidal abscess. Three patients required a second intervention to replace an inadvertently dislodged drainage catheter and in one patient surgery could not be avoided.
A recent report showed the safety and success of EUS-guided drainage of pelvic abscess without fluoroscopic monitoring in 14 patients[1]. Four patients had pericolonic abscess. Three patients underwent only aspiration after EUS-guided puncture, two patients underwent dilatation with balloon and aspiration, and a single double pigtail stent (10 Fr) was placed in nine patients that were removed one week later. All except one recovered completely and one patient needed further surgery within one week after aspiration procedure.
In this series we show that EUS-guided placement of one (or more) 7 Fr stent for drainage of pelvic abscesses is safe and has an excellent clinical outcome. Importantly, we did not place any additional flushing catheter and all procedures were completed under conscious sedation without fluoroscopic monitoring. It must be emphasized that the placement of a second stent without fluoroscopic monitoring can be cumbersome or even be associated with adverse consequences and therefore extra caution has to be exercised. The cystotome already has shown its value in the EUS-guided drainage of peripancreatic fluid collections[12,13], and can also be applied to create a tract in case of pelvic abscesses. Despite spontaneous stent migration within one week in 6 out of 8 patients, complete recovery and no relapse occurred in these patients. The spontaneous migration of a stent can be related to the insertion of a relatively small calibre 7 Fr pigtail stent in a tract that has been dilated with a balloon or cystotome, the relatively thin muscular layer of rectal (colonic) wall or secondary to peristaltic movements and propulsion of faeces.
It has been argued that transrectal stents can clog easily, particularly because of faecal matter or pus[5]. For this reason, some physicians introduce a nasocystic flushing catheter to continuously irrigate the cavity for some days to enhance resolution of the abscess. According to the results of the present case series as well as others, this step does not seem to be essential to successfully manage pelvic abscesses[1,2]. Although in this series a single 7 Fr pigtail stent seemed to suffice in the majority of patients, placement of larger calibre or multiple stents could be helpful to assure adequate drainage in certain individuals.
In conclusion, EUS-guided placement of a single (or more) 7 Fr stent for the drainage of pelvic abscesses without fluoroscopic monitoring is safe and has an excellent clinical outcome.
Deep pelvic abscess can develop as a result of different inflammatory conditions or operations of the distal urogenital or gastrointestinal tract. A proper drainage that is essential for recovery can usually be achieved by percutaneous drainage under radiological monitoring when the abscess is accessible. Endoscopic ultrasound (EUS)-guided transrectal drainage offered alternative drainage route when the latter is not possible.
The literature addressing this issue is scarce. This study establishes the earlier reported safety and efficacy of this technique in a limited number of case series and widens the total number of patients described to be treated by this method.
The study reports the technical and procedural modifications indicating that a short term drainage with a plastic stent may be sufficiently effective leading to recovery. Furthermore, the study shows for the first time that a cystotome can be employed safely to dilate the drainage tract in this setting without adverse events.
EUS-guided drianage of deep pelvic abscess not amenable to percutaneous drainage using a cystotome can be safely applied in clinical practice to treat selected cases.
Pelvic abscess, endoscopic ultrasound guided drianage.
The manuscript describe EUS-guided pelvic abscess drainage in 8 patients. This is a promising technique that has been used recently.
P- Reviewer: Poli-Neto OB, Stanojevic GZ S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Puri R, Eloubeidi MA, Sud R, Kumar M, Jain P. Endoscopic ultrasound-guided drainage of pelvic abscess without fluoroscopy guidance. J Gastroenterol Hepatol. 2010;25:1416-1419. |
| 2. | Giovannini M, Bories E, Moutardier V, Pesenti C, Guillemin A, Lelong B, Delpéro JR. Drainage of deep pelvic abscesses using therapeutic echo endoscopy. Endoscopy. 2003;35:511-514. |
| 3. | Varadarajulu S, Drelichman ER. EUS-guided drainage of pelvic abscess (with video). Gastrointest Endosc. 2007;66:372-376. |
| 4. | Trevino JM, Drelichman ER, Varadarajulu S. Modified technique for EUS-guided drainage of pelvic abscess (with video). Gastrointest Endosc. 2008;68:1215-1219. |
| 5. | Varadarajulu S, Drelichman ER. Effectiveness of EUS in drainage of pelvic abscesses in 25 consecutive patients (with video). Gastrointest Endosc. 2009;70:1121-1127. |
| 6. | Kriwanek S, Armbruster C, Beckerhinn P, Dittrich K. Prognostic factors for survival in colonic perforation. Int J Colorectal Dis. 1994;9:158-162. |
| 7. | Benigno BB. Medical and surgical management of the pelvic abscess. Clin Obstet Gynecol. 1981;24:1187-1197. |
| 8. | Gerzof SG, Robbins AH, Johnson WC, Birkett DH, Nabseth DC. Percutaneous catheter drainage of abdominal abscesses: a five-year experience. N Engl J Med. 1981;305:653-657. |
| 9. | Hovsepian DM. Transrectal and transvaginal abscess drainage. J Vasc Interv Radiol. 1997;8:501-515. |
| 10. | Brusciano L, Maffettone V, Napolitano V, Izzo G, Rossetti G, Izzo D, Russo F, Russo G, del Genio G, del Genio A. Management of colorectal emergencies: percutaneous abscess drainage. Ann Ital Chir. 2004;75:593-597. |
| 11. | Gerzof SG, Johnson WC, Robbins AH, Nabseth DC. Expanded criteria for percutaneous abscess drainage. Arch Surg. 1985;120:227-232. |