Published online Aug 16, 2014. doi: 10.4253/wjge.v6.i8.359
Revised: May 26, 2014
Accepted: June 27, 2014
Published online: August 16, 2014
Processing time: 112 Days and 9 Hours
AIM: To evaluate whether virtual chromoendoscopy can improve the delineation of small bowel lesions previously detected by conventional white light small bowel capsule endoscopy (SBCE).
METHODS: Retrospective single center study. One hundred lesions selected from forty-nine consecutive conventional white light SBCE (SBCE-WL) examinations were included. Lesions were reviewed at three Flexible Spectral Imaging Color Enhancement (FICE) settings and Blue Filter (BF) by two gastroenterologists with experience in SBCE, blinded to each other’s findings, who ranked the quality of delineation as better, equivalent or worse than conventional SBCE-WL. Inter-observer percentage of agreement was determined and analyzed with Fleiss Kappa (κ) coefficient. Lesions selected for the study included angioectasias (n = 39), ulcers/erosions (n = 49) and villous edema/atrophy (n = 12).
RESULTS: Overall, the delineation of lesions was improved in 77% of cases with FICE 1, 74% with FICE 2, 41% with FICE 3 and 39% with the BF, with a percentage of agreement between investigators of 89% (κ = 0.833), 85% (κ = 0.764), 66% (κ = 0.486) and 79% (κ = 0.593), respectively. FICE 1 improved the delineation of 97.4% of angioectasias, 63.3% of ulcers/erosions and 66.7% of villous edema/atrophy with a percentage of agreement of 97.4% (κ = 0.910), 81.6% (κ = 0.714) and 91.7% (κ = 0.815), respectively. FICE 2 improved the delineation of 97.4% of angioectasias, 57.1% of ulcers/erosions and 66.7% of villous edema/atrophy, with a percentage of agreement of 89.7% (κ = 0.802), 79,6% (κ = 0.703) and 91.7% (κ = 0.815), respectively. FICE 3 improved the delineation of 46.2% of angioectasias, 24.5% of ulcers/erosions and none of the cases of villous edema/atrophy, with a percentage of agreement of 53.8% [κ = not available (NA)], 75.5% (κ = NA) and 66.7% (κ = 0.304), respectively. The BF improved the delineation of 15.4% of angioectasias, 61.2% of ulcers/erosions and 25% of villous edema/atrophy, with a percentage of agreement of 76.9% (κ = 0.558), 81.6% (κ = 0.570) and 25.0% (κ = NA), respectively.
CONCLUSION: Virtual chromoendoscopy can improve the delineation of angioectasias, ulcers/erosions and villous edema/atrophy detected by SBCE, with almost perfect interobserver agreement for FICE 1.
Core tip: One of the recent technical advances of small bowel capsule endoscopy (SBCE) technology is the possibility to enhance endoscopic imaging with computed virtual chromoendoscopy, using the Flexible Spectral Imaging Color Enhancement (FICE) or the Blue Filter modes. In our study, virtual chromoendoscopy, particularly FICE 1, improved the delineation of three main types of small bowel mucosal lesions: vascular (angioectasias), mucosal breaks (ulcers and erosions) and villous pattern (edema and atrophy), with substantial inter-observer agreement. Thus, we support the use of virtual chromoendoscopy as a complement to conventional white light SBCE for the evaluation of difficult to interpret endoscopic images.
- Citation: Cotter J, Magalhães J, Castro FD, Barbosa M, Carvalho PB, Leite S, Moreira MJ, Rosa B. Virtual chromoendoscopy in small bowel capsule endoscopy: New light or a cast of shadow? World J Gastrointest Endosc 2014; 6(8): 359-365
- URL: https://www.wjgnet.com/1948-5190/full/v6/i8/359.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i8.359
Small bowel capsule endoscopy (SBCE) is a well established diagnostic procedure for the evaluation of small bowel diseases, with a high diagnostic yield when compared to other small bowel imaging modalities[1-5]. Recently, SBCE diagnostic abilities have been further expanded with the incorporation of virtual chromoendoscopy into the versions 6, 7 and 8 of RAPID® Reader (Given® Imaging Ltd, Yoqneam, Israel)[6-8], using the Flexible Spectral Imaging Color Enhancement (FICE, Fujinon Corporation®, Saitama, Japan) and the Blue Filter (BF). FICE uses a spectral estimation technology, narrowing the bandwidth of white light that permits an automatic reconstruction of pre-acquired conventional endoscopic images into virtual images with different wavelengths of red, green and blue, in order to enhance vascular contrast and the resolution of surface patterns[9,10]. The BF is another setting of virtual chromoendoscopy consisting of colour enhancement within a short wavelength range (490-430 nm). Virtual chromoendoscopy works with the convenience of a quick push-button switch between white light and chromoendoscopy with no need for dye spraying[11]. Virtual chromoendoscopy has been extensively investigated in the upper and lower GI tract[9,12-14], and recently in double-balloon enteroscopy[15]. Despite the conflicting data, most studies support its use to improve the evaluation of size, borders and mucosal pattern of different types of lesions[9,11,16-18]. However, it is currently controversial whether virtual chromoendoscopy may increase the diagnostic yield and diagnostic accuracy of SBCE, and what are the optimal wavelength filters to be used[7,11,19].
The aim of this study was to evaluate whether the currently available virtual chromoendoscopy settings may improve the delineation of the most frequent small bowel mucosal lesions detected by conventional white light SBCE (SBCE-WL).
We conducted a retrospective single center study, which included forty nine consecutive SBCE examinations for the investigation of patients with iron deficiency anemia, overt or occult obscure digestive bleeding and suspected or known Crohn’s disease.
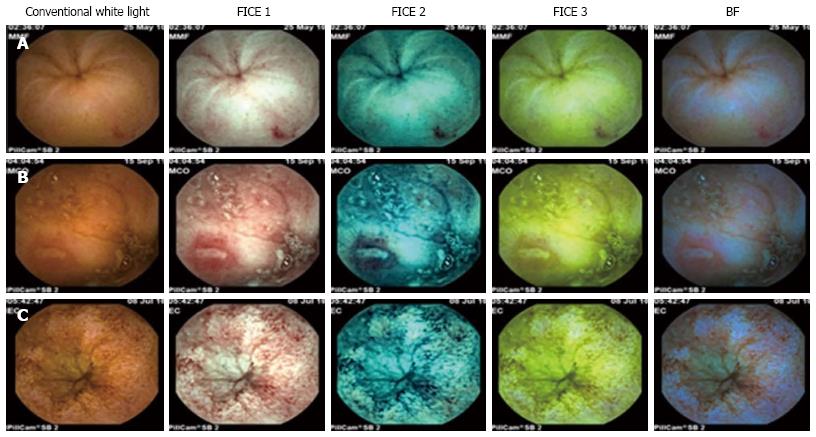
All patients followed a 24 h clear liquid diet and 12 h fasting prior to SBCE (PillCam® SB, Given® Imaging Ltd Yoqneam, Israel). No oral purge was administered. All videos were reviewed with conventional white light by a gastroenterologist with extensive experience on SBCE (> 500 procedures), who selected 100 consecutive lesions to enter the study, including vascular lesions (angioectasias, n = 39), mucosal breaks (ulcers/erosions, n = 49) and villous morphology changes (villous edema/atrophy, n = 12) (Figure 1). All lesions were described using the terminology proposed by the Given Capsule Endoscopy working group[20]. According to the methodology of the study, two gastroenterologists with experience in SBCE (more than 200 examinations) reviewed the selected lesions using all three FICE settings and the BF, and were blinded to each other’s evaluation. The settings used in the study were: FICE 1 (wavelength red 595 nm, green 540 nm, blue 535 nm), FICE 2 (wavelength red 420 nm, green 520 nm, blue 530 nm), FICE 3 (wavelength red 595 nm, green 570 nm, blue 415 nm) and BF (wavelength 490-430 nm). The sequence used by the reviewers was uniform, starting with FICE 1, then FICE 2, FICE 3 and finally the BF.
SBCE-WL and virtual chromoendoscopy images were compared regarding the contrast of mucosal surface and clear demarcation of the borders of the lesions. Each investigator rated the delineation of lesions with each setting of FICE and BF mode as follows: +2 (remarkably better delineation with enhanced delineation of lesion surface and/or borders), +1 (slight improvement), 0 (equivalent to conventional SBCE-WL), -1 (worse delineation or inability to characterize a specific lesion). Finally, the scores attributed by the investigators were added for each lesion, such that a final score ≥ 2 was classified as better delineation, a score between 0 and 1 was considered equivalent to conventional SBCE-WL, and a score ≤ -1 indicated worse delineation with virtual chromoendoscopy.
Inter-observer percentage of agreement was determined and analyzed using Fleiss Kappa coefficient, such that κ(k) < 0 indicated poor agreement, 0.00-0.20 slight agreement, 0.21-0.40 fair agreement, 0.41-0.60 moderate agreement, 0.61-0.80 substantial agreement, and 0.81-1.00 almost perfect agreement[21].
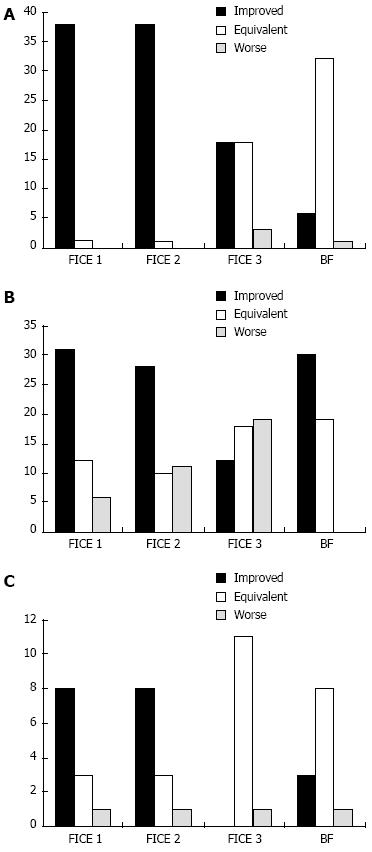
Overall, the delineation of small bowel mucosal lesions was improved in 77% of cases with FICE 1, 74% with FICE 2, 41% with FICE 3 and 39% with the BF, with a percentage of agreement between the two investigators of 89% [κ = 0.833 (P < 0.001), 95%CI: 0.741-0.925], 85% [κ = 0.764 (P < 0.001), 95%CI: 0.654-0.874], 66% [κ = 0.486 (P < 0.001), 95%CI: 0.345-0.627] and 79% [κ = 0.593 (P < 0.001), 95%CI: 0.438-0.748], respectively (Table 1). FICE 1 improved the delineation of 97.4% of vascular lesions (angioectasias), 63.3% of mucosal breaks (ulcers/erosions) and 66.7% of villous morphology changes (edema/atrophy), with a percentage of agreement of 97.4% [κ = 0.910 (P < 0.001), 95%CI: 0.736-1.084], 81.6% [κ = 0.714 (P < 0.001), 95%CI: 0.543-0.885 and 91.7% [κ = 0.815 (P < 0.001), 95%CI: 0.470-1.160], respectively. FICE 2 improved the delineation of 97.4% of angioectasias, 57.1% of ulcers/erosions and 66.7% of villous edema/atrophy, with a percentage of agreement of 89.7% [κ = 0.802 (P < 0.001), 95%CI: 0.620-0.984], 79.6% [κ = 0.703 (P < 0.001), 95%CI: 0.540-0.866] and 91.7% [κ = 0.815 (P < 0.001), 95%CI: 0.470-1.160], respectively. FICE 3 improved the delineation of 46.2% of angioectasias, 24.5% of ulcers/erosions and none of the cases of villous edema/atrophy, with a percentage of agreement of 53.8% (κ = NA), 75.5% (κ = NA) and 66.7% [κ = 0.304 (P = 0.098), 95%CI: -0.091-0.700], respectively. The BF improved the delineation of 15.4% of angioectasias, 61.2% of ulcers/erosions and 25% of villous edema/atrophy, with a percentage of agreement of 76.9% [κ = 0.558 (P < 0.001), 95%CI: 0.264-0.852], 81.6% [κ = 0.570 (P < 0.001), 95%CI: 0.333-0.807] and 25.0% (κ = NA), respectively. The detailed outcomes in terms of quality of delineation per type of lesion with each setting of virtual chromoendoscopy are summarized in graphical representation for angioectasias (Figure 2A), ulcers/erosions (Figure 2B) and villous edema/atrophy (Figure 2C).
| Angioectasias (n = 39) | Ulcers/erosions (n = 49) | Villous edema/atrophy (n = 12) | Overall (n = 100) | |
| FICE 1 | ||||
| Improved delineation | 38/39 (97.4%) | 31/49 (63.3%) | 8/12 (66.7%) | 77/100 (77.0%) |
| Percentage of agreement, κ | 97.4%, κ = 0.910 | 81.6%, κ = 0.714 | 91.7%, κ = 0.815 | 89.0%, κ = 0.833 |
| FICE 2 | ||||
| Improved delineation | 38/39 (97.4%) | 28/49 (57.1%) | 8/12 (66.7%) | 74/100 (74.0%) |
| Percentage of agreement, κ | 89.7%, κ = 0.802 | 79.6%, κ = 0.703 | 91.7%, κ = 0.815 | 85.0%, κ = 0.764 |
| FICE 3 | ||||
| Improved delineation | 18/39 (46.2%) | 12/49 (24.5%) | 0/12 (0.0%) | 41/100 (41.0%) |
| Percentage of agreement, κ | 53.8%, κ = NA | 75.5%, κ = NA | 66.7%, κ = 0.304 | 66.0%, κ = 0.486 |
| BF | ||||
| Improved delineation | 6/39 (15.4%) | 30/49 (61.2%) | 3/12 (25.0%) | 39/100 (39.0%) |
| Percentage of agreement, κ | 76.9%, κ = 0.558 | 81.6%, κ = 0.570 | 25.0%, κ = NA | 79.0%, κ = 0.593 |
Currently available data on the use of virtual chromoendoscopy on SBCE are scarce, with conflicting results reported in the literature regarding its accuracy and clinical value[7,11,22-24]. Moreover, there is ongoing discussion on what should be the optimal settings to improve the detection and/or delineation of different types of lesions[7,19]. Some important questions have been addressed[11], such as whether virtual chromoendoscopy may improve the detection rate of clinically relevant lesions, and whether it may contribute to a better characterization of lesions detected with conventional SBCE-WL. We should underline that a significant number of non-pathological or clinically irrelevant lesions may be detected when FICE is used, such as small red spots or prominent folds that may be erroneously interpreted as angioectasias when FICE is used[22]. Our study did not address this issue, since we did not perform a comparative evaluation of the full video using white light vs virtual chromoendoscopy; indeed, all images of the lesions selected to enter the study had been previously identified with SBCE-WL, as we aimed to evaluate whether virtual chromoendoscopy could improve the delineation of the most common lesions in the small bowel detected by the capsule.
We observed that, overall, FICE 1 and FICE 2 improved the delineation of small bowel lesions in up to 77% and 74% of the cases, respectively, with almost perfect interobserver agreement for FICE 1 [κ = 0.833 (P < 0.001), 95%CI: 0.741-0.925] and substantial interobserver agreement for FICE 2 [κ = 0.764 (P < 0.001), 95%CI: 0.654-0.874]. Conversely, the interobserver agreement was moderate with FICE 3 [κ = 0.486 (P < 0.001), 95%CI: 0.345-0.627)] and BF [κ = 0.593 (P < 0.001), 95%CI: 0.438-0.748], and these settings only improved the delineation of lesions in 41% and 39%, respectively. FICE 1 and FICE 2 were particularly useful improving the delineation of angioectasias (97.4% with both settings) and, to a lesser degree, ulcers/erosions (63.3% and 57.1%, respectively) and villous edema/atrophy (66.7% with both settings). Overall, FICE 1 and FICE 2 were superior to FICE 3 and BF for all types of lesions, which is in line with other published data[6,7,25] (Table 2). Interestingly, in the case of ulcers/erosions, the BF yielded good results, comparable to FICE 1 and FICE 2, improving the delineation of 61.2% of lesions, although with a lower interobserver agreement [κ = 0.570 (P < 0.001), 95%CI: 0.333-0.807].
| Ref. | Center | Study type | No. of patients | Outcome | Results |
| Imagawa et al[7] | Single center | Retrospective | 122 patients | Delineation | 145 lesions |
| FICE 1: improved delineation in 87.0% (20/23) of angioectasias, 53.3% (26/47) of ulcers/erosions and 25.3% (19/75) of tumors | |||||
| FICE 2: improved delineation in 87.0% (20/23) of angioectasias, 25.5% (12/47) of ulcers/erosions and 20.0% (15/75) of tumors | |||||
| FICE 3: no improvement | |||||
| Imagawa et al[6] | Single center | Prospective | 50 patients | Detection rate | FICE 1: increased detection rate of angioectasias (48 vs 17, P = 0.0003) |
| FICE 2: increased detection rate of angioectasias (45 vs 17, P < 0.0001) | |||||
| FICE 3: increased detection rate of angioectasias (24 vs 17, P = ns) | |||||
| Detection of ulcers, erosions and tumors did not differ significantly between conventional SBCE-WL and SBCE-FICE | |||||
| Gupta et al[22] | Single center | Retrospective | 60 patients | Detection rate | 157 lesions detected with SBCE-FICE vs 114 with SBCE-WL (P = 0.15) |
| 5/55 angioectasias were better characterized with SBCE-FICE | |||||
| More P0 diagnosed with SBCE-FICE (39 vs 8, P < 0.001) | |||||
| Intra-class κ correlations with SBCE-FICE: 0.88 (P2 lesions); 0.61 (P1 lesions) | |||||
| Intra-class κ correlations with SBCE-WL: 0.92 (P2 lesions); 0.79 (P1 lesions) | |||||
| For P2 lesions, the sensitivity was 94% vs 97% and specificity was 95% vs 96% for SBCE-FICE and SBCE-WL, respectively | |||||
| Krystallis et al[19] | Single center | Retrospective | 200 patients | Delineation | 167 lesions including angioectasias (n = 18), erosions/ulcers (n = 60), villi oedema (n = 17), cobblestone (n = 11), blood lumen (n = 15), lesions of unknown clinical significance (n = 46) |
| FICE 1: improved delineation in 34%; κ = 0.646 | |||||
| FICE 2: improved delineation in 8.6%; κ = 0.617 | |||||
| FICE 3: improved delineation in 7.7%; κ = 0.669 | |||||
| Blue mode: improved delineation in 83%; κ = 0.786 | |||||
| Duque et al[8] | Single center | Prospective | 20 patients | Detection rate | 150 lesions |
| SBCE-FICE: increased detection rate (95 vs 75), κ = 0.650 | |||||
| SBCE-FICE did not miss any lesion identified by CE-WL and allowed the identification of a higher number of angioectasias (35 vs 32, P = 0.25) and erosions (41 vs 24, P < 0.001) | |||||
| Nakamura et al[25] | Single center | Prospective | 50 patients | Detection rate (QuickView) | SBCE-WL: sensitivity 80%, specificity 100% |
| SBCE-FICE: sensitivity 91% specificity 86% | |||||
| SBCE-FICE resulted in more false positive findings and lower specificity | |||||
| Sakai et al[26] | Single center | Prospective | 12 patients | Detection rate | 142 lesions including angioectasias (n = 60) and ulcers/erosions (n = 82) |
| Angioectasias were detected with CE-WL (26/60), SBCE-FICE 1 (40/60), SBCE-FICE 2 (38/60), SBCE-FICE 3 (31/60) | |||||
| Ulcers/erosions were detected with SBCE-WL (38/82), SBCE-FICE 1 (62/82), SBCE-FICE 2 (60/82), SBCE-FICE 3 (20/82) | |||||
| SBCE-FICE 1and 2 significantly increased the detection rate of angioectasias (P = 0.0017 and P = 0.014, respectively) and ulcers/erosions (P = 0.0012 and P = 0.0094, respectively) | |||||
| In poor bowel visibility conditions, SBCE-FICE yielded a high rate of false-positive findings | |||||
| Cotter et al | Single center | Retrospective | 49 patients | Delineation | 100 lesions including angioectasias (n = 39), ulcers/erosions (n = 49), villous edema/atrophy (n = 12) |
| FICE 1: image improvement in 77% (κ = 0.833) | |||||
| FICE 2: image improvement in 74% (κ = 0.764) | |||||
| FICE 3: image improvement in 66% (κ = 0.486) | |||||
| BF: image improvement in 79% (κ = 0.593) | |||||
| FICE 1 improved the delineation of 97.4% of angioectasias, 63.3% of ulcers/erosions and 66.7% of villous edema/atrophy | |||||
| FICE 2 improved the delineation of 97.4% of angioectasias, 57.1% of ulcers/erosions and 66.7% of villous edema/atrophy | |||||
| FICE 3 improved the delineation of 46.2% of angioectasias, 24.5% of ulcers/erosions and none of the cases of villous edema/atrophy | |||||
| BF improved the delineation of 15.4% of angioectasias, 61.2% of ulcers/erosions and 25.0% of villous edema/atrophy |
The outcomes per type of lesion may be summarized as follows: the delineation of angioectasias was improved with either FICE 1 or FICE 2 in almost all cases (97.4%); the delineation of ulcers/erosions was improved in 57%-63% of the cases with either FICE 1 (63.3%), FICE 2 (57.1%) or BF (61.2%); the delineation of villous edema/atrophy was improved with either FICE 1 or FICE 2 in approximately two thirds (66.7%) of the cases. As in other published studies[7,19,23], we found FICE 3 to be ineffective for the vast majority of small bowel mucosal lesions. The results of our study suggest that FICE 1 (wavelengths red 595 nm, green 540 nm, blue 535 nm) seems to achieve the optimal appearance of vascular and mucosal contrast for small bowel lesions, with the highest interobserver agreement among all settings of FICE, and thus it should generally be the setting of choice when using virtual chromoendoscopy. Imagawa et al[7] had reported that both FICE 1 and FICE 2 could improve the delineation of ulcers and erosions, however the detection rate of such lesions was similar between white light and virtual chromoendoscopy[6]. Similarly to our study, Krystallis et al[19] reported a better delineation of ulcers using the BF. Duque et al[8] reported an improvement in the diagnosis of erosions using FICE 2, due to the enhancement of its inflammatory halo. Regarding villous edema/atrophy, in our study it was better visualized with FICE 1 and FICE 2, while other authors[19] have found edema to be better visualized with the BF mode.
In summary, our results suggest that virtual chromoendoscopy, and particularly FICE 1, may be used in those cases where the characterization or interpretation of small bowel lesions is not straightforward with conventional SBCE-WL. On the other hand, in our study virtual chromoendoscopy did not lead to reclassification of any of the lesions detected with conventional SBCE-WL, and we did not evaluate whether it could contribute to increase the diagnostic yield of SBCE by identifying new lesions previously undetected with SBCE-WL, as we evaluated pre-selected lesions, which had already been previously diagnosed. Moreover, in the absence of a gold standard, it is not possible to accurately assess the false positives rate of these new techniques. Thus, at this point, although virtual chromoendoscopy has been shown to improve the delineation of small bowel lesions previously diagnosed by conventional SBCE-WL, the impact of this technology on the detection rate, accuracy of diagnosis and improved clinical outcome warrants further investigation. Our data support the current use of virtual chromoendoscopy as a complement to conventional white light SBCE for the evaluation of difficult to interpret endoscopic images.
One of the recent technical advances of small bowel capsule endoscopy (SBCE) is the possibility to enhance endoscopic imaging with computed virtual chromoendoscopy, using the Flexible Spectral Imaging Color Enhancement (FICE) or the Blue Filter (BF) modes. However, it is currently controversial whether virtual chromoendoscopy may increase the diagnostic yield and diagnostic accuracy of SBCE, and what are the optimal wavelength filters to be used.
The authors aimed to evaluate whether different settings of FICE or the Blue Filter could improve the delineation of the most frequent small bowel mucosal lesions detected by conventional white light small bowel capsule endoscopy (SBCE-WL), namely the three main types of small bowel mucosal lesions: vascular (angioectasias), mucosal breaks (ulcers and erosions) and villous pattern (edema and atrophy).
Virtual chromoendoscopy improved the delineation of three main types of small bowel mucosal lesions: vascular (angioectasias), mucosal breaks (ulcers and erosions) and villous pattern (edema and atrophy). FICE 1 (wavelengths red 595 nm, green 540 nm, blue 535 nm) seems to achieve the optimal appearance of vascular and mucosal contrast for small bowel lesions, with the highest interobserver agreement among all settings of FICE, and thus it should generally be the setting of choice when using virtual chromoendoscopy.
The results suggest that virtual chromoendoscopy, and particularly FICE 1, may be used in those cases where the characterization or interpretation of small bowel lesions is not straightforward with conventional SBCE-WL. Authors’ support the use of virtual chromoendoscopy as a complement to conventional white light SBCE for the evaluation of difficult to interpret endoscopic images.
FICE (Fujinon Corporation®, Saitama, Japan) is a computed virtual chromoendoscopy modality that uses a spectral estimation technology, narrowing the bandwidth of white light that permits an automatic reconstruction of pre-acquired conventional endoscopic images into virtual images with different wavelengths of red, green and blue, in order to enhance vascular contrast and the resolution of surface patterns. BF is a different setting of virtual chromoendoscopy consisting of colour enhancement within a short wavelength range (490-430 nm).
In a retrospective study, the authors have evaluated virtual chromoendoscopy SBCE in the delineation of small bowel lesions previously detected by white light SBCE. The virtual chromoendoscopy included 3 types of FICE and a blue filter. This is an interesting report.
P- Reviewer: Koulaouzidis A, Moussata D, Muguruma N, Tsuji Y S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-1248; quiz 1249. |
| 2. | Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13:4372-4378. |
| 3. | Apostolopoulos P, Liatsos C, Gralnek IM, Giannakoulopoulou E, Alexandrakis G, Kalantzis C, Gabriel P, Kalantzis N. The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy. 2006;38:1127-1132. |
| 4. | Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc. 2002;56:349-353. |
| 5. | Fukumoto A, Tanaka S, Shishido T, Takemura Y, Oka S, Chayama K. Comparison of detectability of small-bowel lesions between capsule endoscopy and double-balloon endoscopy for patients with suspected small-bowel disease. Gastrointest Endosc. 2009;69:857-865. |
| 6. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: a pilot study. Scand J Gastroenterol. 2011;46:1133-1137. |
| 7. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved visibility of lesions of the small intestine via capsule endoscopy with computed virtual chromoendoscopy. Gastrointest Endosc. 2011;73:299-306. |
| 8. | Duque G, Almeida N, Figueiredo P, Monsanto P, Lopes S, Freire P, Ferreira M, Carvalho R, Gouveia H, Sofia C. Virtual chromoendoscopy can be a useful software tool in capsule endoscopy. Rev Esp Enferm Dig. 2012;104:231-236. |
| 9. | Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562-569. |
| 10. | Pohl J, Aschmoneit I, Schuhmann S, Ell C. Computed image modification for enhancement of small-bowel surface structures at video capsule endoscopy. Endoscopy. 2010;42:490-492. |
| 11. | Spada C, Hassan C, Costamagna G. Virtual chromoendoscopy: will it play a role in capsule endoscopy? Dig Liver Dis. 2011;43:927-928. |
| 12. | Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73-78. |
| 13. | Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, Nokubi M, Horie H, Yamamoto H. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc. 2009;69:734-741. |
| 14. | East JE, Tan EK, Bergman JJ, Saunders BP, Tekkis PP. Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther. 2008;28:854-867. |
| 15. | Neumann H, Fry LC, Bellutti M, Malfertheiner P, Mönkemüller K. Double-balloon enteroscopy-assisted virtual chromoendoscopy for small-bowel disorders: a case series. Endoscopy. 2009;41:468-471. |
| 16. | McGill S, Soetikno R, Kaltenbach T. Image-enhanced endoscopy in practice. Can J Gastroenterol. 2009;23:741-746. |
| 17. | Coriat R, Chryssostalis A, Zeitoun JD, Deyra J, Gaudric M, Prat F, Chaussade S. Computed virtual chromoendoscopy system (FICE): a new tool for upper endoscopy? Gastroenterol Clin Biol. 2008;32:363-369. |
| 18. | Pohl J, Ell C. Impact of virtual chromoendoscopy at colonoscopy: the final requiem for conventional histopathology? Gastrointest Endosc. 2009;69:723-725. |
| 19. | Krystallis C, Koulaouzidis A, Douglas S, Plevris JN. Chromoendoscopy in small bowel capsule endoscopy: Blue mode or Fuji Intelligent Colour Enhancement? Dig Liver Dis. 2011;43:953-957. |
| 20. | Korman LY, Delvaux M, Gay G, Hagenmuller F, Keuchel M, Friedman S, Weinstein M, Shetzline M, Cave D, de Franchis R. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951-959. |
| 21. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. |
| 22. | Gupta T, Ibrahim M, Deviere J, Van Gossum A. Evaluation of Fujinon intelligent chromo endoscopy-assisted capsule endoscopy in patients with obscure gastroenterology bleeding. World J Gastroenterol. 2011;17:4590-4595. |
| 23. | Nogales Rincón O, Merino Rodríguez B, González Asanza C, Fernández-Pacheco PM. [Utility of capsule endoscopy with flexible spectral imaging color enhancement in the diagnosis of small bowel lesions]. Gastroenterol Hepatol. 2013;36:63-68. |
| 24. | Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol. 2013;19:3726-3746. |
| 25. | Nakamura M, Ohmiya N, Miyahara R, Ando T, Watanabe O, Kawashima H, Itoh A, Hirooka Y, Goto H. Usefulness of flexible spectral imaging color enhancement (FICE) for the detection of angiodysplasia in the preview of capsule endoscopy. Hepatogastroenterology. 2012;59:1474-1477. |
| 26. | Sakai E, Endo H, Kato S, Matsuura T, Tomeno W, Taniguchi L, Uchiyama T, Hata Y, Yamada E, Ohkubo H. Capsule endoscopy with flexible spectral imaging color enhancement reduces the bile pigment effect and improves the detectability of small bowel lesions. BMC Gastroenterol. 2012;12:83. |