Published online Mar 16, 2014. doi: 10.4253/wjge.v6.i3.74
Revised: October 10, 2013
Accepted: November 12, 2013
Published online: March 16, 2014
Processing time: 8 Days and 20.4 Hours
AIM: To identify potential factors that can predict adverse short-term outcomes in patients with acute cholangitis undergoing endoscopic retrograde cholangiopancreatography (ERCP).
METHODS: Retrospective analysis of consecutive patients admitted to our center for acute cholangitis and underwent ERCP from 2001 to 2012. Involvement of two or more organ systems was termed as organ failure (OF). Cardiovascular failure was defined based on a systolic blood pressure of < 90 mmHg despite fluid replacement and/or requiring vasopressor treatment; respiratory failure if the Pa02/Fi02 ratio was < 300 mmHg and/or required mechanical ventilation; coagulopathy if the platelet count was < 80; and renal insufficiency if serum creatinine was > 1.9 mg/dL. Variables associated with short term adverse clinical outcomes defined as persistent OF and/or 30-d mortality was determined.
RESULTS: A total of 172 patients (median age 62 years, 56.4% female) were included. The median door to ERCP time was 17 h. Bile duct stones were the most common etiology (n = 67, 39.2%). In multivariate analysis, factors that were independently associated with persistent OF and/or 30-d mortality included American Society of Anesthesiology (ASA) physical classification score > 3 (OR = 7.70; 95%CI: 2.73-24.40), presence of systemic inflammatory response syndrome (OR = 3.67; 95%CI: 1.34-10.3) and door to ERCP time greater than 72 h (OR = 3.36; 95%CI: 1.12-10.20). Door to ERCP time greater than 72 h was also associated with 70% increase in the mean length of stay (P < 0.001). Every one point increase in the ASA physical classification and every 1 mg/dL increase in the pre-ERCP bilirubin level was associated with a 34% and 2% increase in the mean length of hospital stay, respectively. Transfer status did not impact clinical outcomes.
CONCLUSION: Higher ASA physical classification and delays in ERCP are associated with adverse clinical outcomes and prolonged length of hospital stay in patients with acute cholangitis undergoing ERCP.
Core tip: We investigated the effect of timing of endoscopic retrograde cholangiopancreatography (ERCP) on clinical outcomes defined as persistent organ failure and/or 30-d mortality, and length of hospital stay in patients with acute cholangitis. We observed that an American Society of Anesthesiology physical classification score > 3, presence of systemic inflammatory response syndrome and door to ERCP time greater than 72 h are associated with adverse clinical outcomes and prolonged length of hospital stay in patients with acute cholangitis.
- Citation: Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Sanaka MR, Vargo JJ, Parsi MA. Factors predicting adverse short-term outcomes in patients with acute cholangitis undergoing ERCP: A single center experience. World J Gastrointest Endosc 2014; 6(3): 74-81
- URL: https://www.wjgnet.com/1948-5190/full/v6/i3/74.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i3.74
Bacterial cholangitis occurs in the setting of partial or complete biliary obstruction and is often secondary to bile duct stones. The bacteria gain access to the biliary tree by retrograde ascent from the duodenum (ascending cholangitis). Management of acute bacterial cholangitis requires antibiotics and/or relief of biliary obstruction[1-3]. This is usually accomplished by endoscopic retrograde cholangiopancreatography (ERCP)[1]. Bacterial cholangitis carries high mortality, approaching 10%, in the setting of sepsis with organ failure (OF)[1].
A number of studies have shown that endoscopic treatment of acute cholangitis is safe and effective[1-6]. However, our practices are guided by limited data and expert opinion. In author's experience emergent ERCP after appropriate resuscitation, in the form of antibiotics, intravenous fluids and stabilization of hemodynamic parameters is recommended. The Tokyo guidelines, based on expert opinion, recommend ERCP within 24 h of presentation in patients with acute cholangitis[7]. This recommendation was reinforced in a community-based study in which patients who underwent ERCP within 24 h of presentation had a shorter hospital stay[8]. In a prospective study of 95 patients with acute cholangitis, delay in biliary drainage was associated with increased risk of cholangitis complications and mortality[9]. A recently published study also suggests that failed or delayed (greater than 72 h) ERCP for acute cholangitis is associated with prolonged hospital stay, increased cost of hospitalization, and a worse composite clinical outcome (death, persistent organ failure, and/or intensive care unit stay)[10]. In addition to the timing of ERCP, presence of resistant organisms and elevated blood urea nitrogen has been associated with OF in patients undergoing ERCP[11]. Another study from our institution suggested that delays in ERCP may be associated with increased risk of 30-d readmissions[12]. Given the lack of strong evidence based data on the timing of ERCP on short term cholangitis outcomes and other predictors of OF, we sought to study the effect of timing of ERCP on clinical outcomes defined as persistent OF and/or 30-d mortality, and length of hospital stay (LOS) in patients with acute cholangitis.
The Cleveland Clinic electronic medical records database was queried for patients admitted to our hospital and diagnosed with acute cholangitis who underwent ERCP from January 2001 to August 2012. After detailed review of medical records, patients without evidence of acute cholangitis were excluded. Demographic, clinical, and procedural data and adverse events were collected. The study was approved by the Cleveland Clinic Institutional Review Board.
The major inclusion criterion was presence of acute cholangitis as the indication for ERCP. Diagnosis of acute cholangitis was based on recorded signs and symptoms or documented purulent drainage from the bile duct during ERCP and/or positive blood cultures in patients with risk factors for acute cholangitis. Patients who were transferred from other hospitals to our endoscopy center just for the purpose of ERCP with return to the referring hospital immediately after the procedure were excluded. ERCP procedures were performed by one of eight experienced interventional endoscopists.
The medical records were reviewed for demographic, clinical, laboratory, and procedural information. The demographic and clinical variables, included age, gender, smoking status, alcohol use, body mass index, etiology of cholangitis, Charlson comorbidity index (CCI), presence of systemic inflammatory response syndrome (SIRS), ASA physical classification, presence of renal insufficiency (defined as defined as a serum creatinine > 1.9 mg/dL), use of anti-platelet agents (aspirin and/or clopidogrel), use of statins and LOS. Information was also obtained on whether the patient was transferred from outside hospitals or directly admitted to our hospital. The ASA physical classification at the time of the procedure was determined by the anesthesiologist and excluded the reason for ERCP (cholangitis).
Complete laboratory information including blood count, serum bilirubin and liver enzymes, coagulation panel and serum creatinine prior to ERCP, and results of blood cultures including possible isolated organism(s) were obtained.
Procedural information included success or failure of ERCP, door to ERCP time, presence of purulent discharge from the biliary orifice on endoscopy, and biliary sphincterotomy with or without stent placement during ERCP. Information on need for precut sphincterotomy, papillary balloon dilation, presence of biliary stricture, biliary stricture dilation, pancreatic duct injection and post-ERCP adverse events were also collected.
All patients were started on intravenous antibiotics (with gram negative coverage) uniformly within 6 h of admission. In patients in whom cholangitis was not recognized at admission, broad spectrum antibiotics were started for fever of unknown origin within the time frame of 6 h.
Failed ERCP was defined as unsuccessful cannulation of the bile duct. Door to ERCP time was defined as the time from admission to performance of ERCP. Pancreatic duct injection was defined as opacification of any portion of the pancreatic duct with contrast during the procedure. Post-ERCP complications were defined per ASGE workshop[13].
Involvement of two or more organ systems was termed as OF. The criteria were adapted from a previous study on definition of OF[10,14]. We included the involvement of the following organ systems for defining OF. Cardiovascular failure was defined based on a systolic blood pressure of < 90 mmHg despite fluid replacement and/or requiring vasopressor treatment; respiratory failure if the Pa02/Fi02 ratio was < 300 mmHg and/or required mechanical ventilation; coagulopathy if the platelet count was < 80; and renal insufficiency if serum creatinine was > 1.9 mg/dL. Persistent OF was recorded when OF was present for greater than 48 h. The 30-d mortality data was calculated using the Social Security Death Index to confirm the date of death, in addition to in hospital records for those who died while in the hospital.
The primary study aim was to identify potential variables associated with adverse clinical outcomes defined as persistent OF and/or 30-d mortality. The secondary aim was to investigate factors associated with prolonged LOS.
Descriptive statistics were computed for all factors. These include medians, 25% and 75%, range or mean and standard deviation for quantitative variables and frequencies and percentages for categorical factors. Continuous data are summarized as mean ± SD. Categorical data are summarized as frequency and group percentage. Normally distributed continuous variables were analyzed by using a t test, and continuous variables that were not normally distributed were analyzed by using the nonparametric (Wilcoxon) rank sum test. Logistic regression models were built for the primary outcome defined as persistent OF and/or mortality selecting from the various covariates associated with the end point at the 0.15 significance level and then choosing the best subset of covariates according to an overall model score test. The covariates in the model included age (forced covariate), gender, door to ERCP time (≤ 24, 24-48, 48-72 and > 72 h), CCI, ASA physical classification, anti-platelets, SIRS, biliary stent placement, total bilirubin levels, and presence of post-ERCP AEs. Logistic regression models were also built for prolonged LOS by backward selection by stepwise removal of variables at P > 0.05. The modeling of this outcome was most appropriate when performed on the Log2 (LOS), since this transformed variable had far less skewness and much more symmetry in its distribution. R 2.10.1 software (The R Foundation for Statistical Computing, Vienna, Austria) was used to perform all analyses.
Query of the electronic medical records yielded 202 patients who were diagnosed with acute cholangitis who underwent ERCP. Thirty patients were excluded because of absence of confirmation of acute cholangitis after detailed review of the medical records. The remaining 172 patients were included in the analysis of outcomes. Table 1 highlights the characteristics of the entire cohort. Sixty-four (37.2%) patients were transferred from outside hospitals for further management of acute cholangitis; while the rest were directly admitted to our hospital. One hundred and eleven (64.5%) were admitted to the gastroenterology primary service, while the rest were admitted to other services (hepatobiliary, general surgery and other specialty services).
| Variable | Value |
| Age (yr) | 61.0 ± 16.9 |
| Male | 97 (56.4) |
| Race (white) | 135 (80.8) |
| BMI (kg/m2) | 26.9 ± 5.9 |
| Smokers | 45 (37.5) |
| Alcohol consumption | 19 (12.8) |
| With primary sclerosing cholangitis | 48 (27.9) |
| With fever | 105 (61.8) |
| With SIRS | 33 (19.5) |
| Transfer patients | 64 (37.4) |
| Patients admitted to gastroenterology service | 111 (64.5) |
| Patients with post-liver transplantation | 4 (2.3) |
| Charlson comorbidity index | |
| 0 | 33 (19.4) |
| 1 | 37 (21.8) |
| 2 | 23 (13.5) |
| 3 | 43 (25.3) |
| > 4 | 34 (20.0) |
| American Society of Anesthesiology Physical Classification | |
| 1 | 2 (1.2) |
| 2 | 2 (1.2) |
| 3 | 101 (59.1) |
| 4 | 62 (36.3) |
| 5 | 4 (2.3) |
| Altered mental status | 8 (4.7) |
| Medications at admission | |
| Antibiotics | 126 (76.4) |
| Anti-platelets (aspirin and/or clopidogrel) | 37 (22.3) |
| Statins | 31 (18.7) |
| Etiology of cholangitis: | |
| Choledocholithiasis | 67 (38.9) |
| Primary sclerosing cholangitis | 45 (26.2) |
| Malignant stricture | 28 (16.3) |
| Benign stricture | 20 (11.6) |
| Others | 12 (6.9) |
| Laboratory values: | |
| Pre-procedural bilirubin (mg/dL) | 6.0 ± 5.0 |
| Pre-procedural platelet count (cells/cu.mm) | 189.4 ± 117.3 |
| Pre-procedural international normalized ratio | 1.2 ± 0.5 |
| Serum creatinine during hospitalization (mg/dL) | 1.3 ± 1.5 |
| With positive blood culture | 44 ± 31.7 |
| Median time between admission and ERCP (h, range) | 17 (1-240) |
| Who had ERCP within 24 h | 104 (60.5) |
| Who had ERCP within 24-48 h | 25 (14.5) |
| Who had ERCP within 48-72 h | 14 (8.1) |
| Who has ERCP > 72 h | 29 (16.9) |
| Purulent bile during ERCP | 68 (40.5) |
| Dominant stricture during ERCP | 32 (18.8) |
| Ampullary diverticulum | 9 (5.3) |
| Balloon dilatation | 69 (49.8) |
| Biliary sphincterotomy performed | 50 (35.2) |
| Biliary stent placed | 131 (77.1) |
| Pancreatic stent placed | 14 (8.2) |
| Stone extraction | 67 (39.2) |
| Failed ERCP | 2 (1.2) |
| Complications post ERCP | |
| Bleeding | 2 (1.2) |
| Pancreatitis | 3 (1.8) |
| Perforation | 2 (1.2) |
Univariate comparisons of continuous and categorical variables for patients with adverse clinical outcome (persistent OF or death) are shown in Table 2. Older age, CCI, higher ASA physical classification, presence of SIRS and fever were associated with worse clinical outcome. Anti-platelet agents and statin use at admission were not associated with adverse clinical outcome. Transfer status at admission did not impact the risk of adverse clinical outcome (P = 0.41). The presence of underlying PSC as the cause of cholangitis was associated with a decreased risk of adverse clinical outcomes, while post-liver transplant status was not associated with adverse clinical outcome.
| Variable | OR (95%CI) | P value |
| Age | 1.15 (1.02-1.30) | 0.02 |
| Male | 0.90 (0.43-1.89) | 0.78 |
| Body mass index | 1.23 (0.88-1.71) | 0.23 |
| Smoking | 0.62 (0.22-1.72) | 0.36 |
| Primary sclerosing cholangitis | 0.27 (0.09-0.82) | 0.02 |
| Systemic inflammatory response syndrome | 5.46 (2.34-12.73) | < 0.001 |
| Charlson comorbidity index | 1.02 (0.85-1.23) | 0.04 |
| American Society of Anesthesiology physical classification | 6.94 (3.14-15.33) | < 0.001 |
| Altered mental status | 1.31 (0.25-6.80) | 0.61 |
| Statins at admission | 2.32 (0.97-5.57) | 0.06 |
| Anti-platelets meds at admission | 2.18 (0.93-5.07) | 0.07 |
| Door to ERCP timing (> 72 h) | 2.03 (0.83-4.95) | 0.12 |
| Pre-ERCP Bilirubin | 1.05 (0.98-1.12) | 0.20 |
| Positive blood cultures | 1.79 (0.79-4.10) | 0.17 |
| Presence of fever | 2.87 (1.17-7.04) | 0.02 |
| Post ERCP bleeding | 4.00 (0.24-65.59) | 0.37 |
| Presence of purulent bile at ERCP | 1.41 (0.45 -5.40) | 0.73 |
| Transfer patients | 0.72 (0.32-1.59) | 0.41 |
Factors that were independently associated with persistent OF and/or 30-d mortality included ASA physical classification > 3 (OR = 7.70; 95%CI: 2.73-24.40), presence of systemic inflammatory response syndrome (OR = 3.67; 95%CI: 1.34-10.3) and door to ERCP time greater than 72 h (OR = 3.36; 95%CI: 1.12-10.20) (Table 3). Of the 4 deaths, 2 occurred within the first 24 h and the other 2 deaths occurred with door to ERCP time of greater than 72 h. The two patients, who died within 24 h, succumbed in the intensive care unit with multi-organ failure and underwent ERCP on vasopressor support.
| Variable | OR (95%CI) | P value |
| American Society of Anesthesiology physical classification > 3 | 7.70 (2.73-24.4) | < 0.001 |
| Systemic inflammatory response syndrome | 3.67 (1.34-10.3) | 0.01 |
| Age (per 5 years) | 1.05 (0.89-1.26) | 0.54 |
| Door to ERCP time > 72 h | 3.36 (1.12-10.2) | 0.03 |
| Primary sclerosing cholangitis | 0.41 (0.09 -1.49) | 0.20 |
| Bile duct stent placement | 0.70 (0.23-2.19) | 0.52 |
| Charlson comorbidity index | 0.89 (0.69-1.13) | 0.37 |
ERCP was delayed for 72 h in 29/172 (16.9%) patients. In 20/29 patients, comorbidity-associated factors such as severe coagulopathy, hepatic encephalopathy or decompensated congestive heart failure precluded immediate ERCP. These patients required resuscitation prior to performance of any invasive procedure. In seven patients, cholangitis appeared to have been present for several days before it was recognized. Two other patients had metastatic cancer to the bile duct (primary breast and renal cell carcinoma). These patients had presented with fever of unknown origin and cholangitis was recognized only after 48 h. All these patients were on broad spectrum antibiotics started within 6 h of admission. There was no impact of service or day of admission on clinical outcomes of patients.
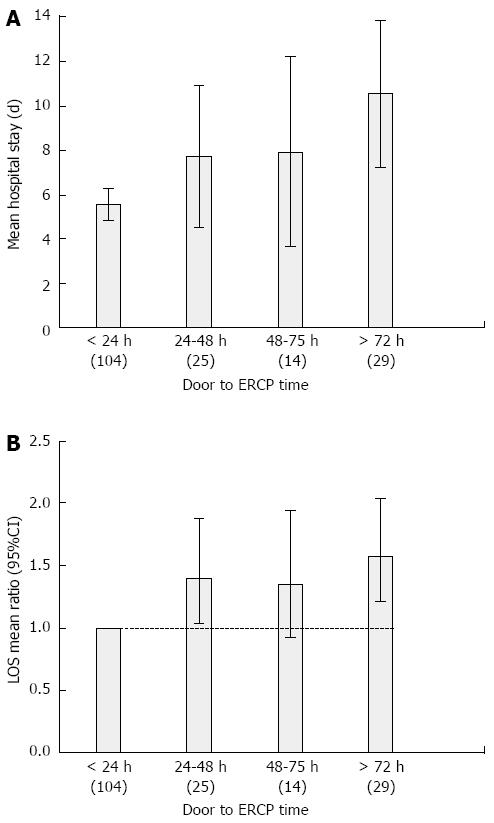
In unadjusted analysis, increasing door to ERCP time was directly associated with an increase in LOS (Table 4, Figure 1A). Patients who underwent ERCP after 72 h were more than three and half times more likely to have a LOS beyond 10 d as compared to those who underwent an ERCP within 24 h of admission. Higher ASA physical classification and presence of persistent cardiovascular, renal and respiratory failure were associated with prolonged LOS beyond 10 d. The transfer status of the patient was not associated with prolonged LOS (Table 4). On multi-variate analysis, door to ERCP time greater than 72 h was associated with 70% increase in the mean LOS. Every 1 point increase in the ASA physical classification score, and every 1 mg/dL increase in pre-ERCP serum bilirubin levels was associated with a 34% and 2% increase in the mean LOS respectively (Figure 1B). Patients who developed post-ERCP adverse events had a 91% increase in the mean LOS (Table 5).
| Variable | OR (95%CI) | P value |
| Age | 1.05 (0.92-1.19) | 0.47 |
| Male | 0.98 (0.42-2.31) | 0.97 |
| Body mass index | 1.07 (0.71-1.60) | 0.74 |
| Smoking | 1.13 (0.37-3.41) | 0.83 |
| Primary sclerosing cholangitis | 0.60 (0.21-1.71) | 0.34 |
| Systemic inflammatory response syndrome | 2.02 (0.75-5.40) | 0.16 |
| Charlson comorbidity index | 1.04 (0.85-1.28) | 0.69 |
| American Society of Anesthesiology physical classification > 3 | 3.12 (1.47-6.63) | 0.003 |
| Altered mental status | 2.03 (0.39-10.67) | 0.33 |
| Statins at admission | 0.90 (0.28-2.88) | 1 |
| Anti-platelets meds at admission | 1.03 (0.35-3.01) | 1 |
| Door to ERCP time | ||
| ≤ 24 h (reference) | - | - |
| 24-48 h | 2.66 (1.12-6.34) | 0.03 |
| 48-72 h | 2.82 (1.17-6.82) | 0.02 |
| > 72 h | 3.57 (1.39-9.17) | 0.03 |
| Pre-ERCP bilirubin | 1.07 (0.99-1.15) | 0.08 |
| Positive blood cultures | 1.70 (0.69-4.21) | 0.25 |
| Presence of fever | 1.04 (0.43-2.53) | 0.94 |
| Post-ERCP pancreatitis | 3.00 (0.26-34.39) | 0.38 |
| Serum creatinine at admission | 1.48 (1.13-1.94) | 0.004 |
| Presence of persistent cardiovascular failure | 3.18 (1.15-8.79) | 0.04 |
| Presence of persistent respiratory failure | 13.81 (2.38-80.11) | 0.004 |
| Presence of persistent renal failure | 4.81 (1.74-13.29) | 0.004 |
| Transfer patients | 0.93 (0.39-2.25) | 0.87 |
| Variable | OR (95%CI) | P value |
| American Society of Anesthesiology physical classification (every 1 class) | 1.34 (1.19-1.52) | < 0.001 |
| Door to ERCP time | ||
| ≤ 24 h (reference) | - | - |
| 24-48 h | 1.22 (0.97-1.54) | 0.09 |
| 48-72 h | 1.24 (0.92-1.66) | 0.16 |
| > 72 h | 1.70 (1.36-2.12) | < 0.001 |
| Pre-ERCP bilirubin (every 1 mg/dL) | 1.02 (1.01-1.04) | 0.02 |
| Post-ERCP bleeding or pancreatitis | 1.91 (1.12-3.24) | 0.02 |
| Charlson comorbidity index | 1.01 (0.96-1.06) | 0.74 |
| Positive blood culture | 1.18 (0.96-1.46) | 0.12 |
Although the safety and efficacy of endoscopic treatment in patients with acute cholangitis has been demonstrated in a number of studies[1-7], the literature on timing of endoscopy and its impact on cholangitis outcomes remains limited. In our study, delayed ERCP was associated with adverse clinical outcomes including persistent OF and/or 30-d mortality. Despite adjustments for disease severity, there was a significant association between door to ERCP time and LOS. It is also important to realize that the presence of co-morbidities contributed to this delay in ERCP.
In a previously published study of 90 patients, older age, increased serum bilirubin levels and ERCP delay of 72 h were associated with adverse clinical outcomes of intensive care unit (ICU) stay, persistent OF, and death[10] In our study, after adjustments for ASA physical classification and CCI, neither age nor serum bilirubin levels were predictors of adverse outcomes. This discrepancy is likely due to different study designs and statistical analyses. The previous study did not make adjustments for variables such as ASA physical classification, and transfer status of the admitted patients which could potentially impact clinical outcomes[10]. In addition, the sample size was limited to study the defined variables, Furthermore, the decision to include ICU admissions in the composite outcome may be biased as ICU admissions may have different thresholds in different hospitals. To avoid this selection bias, we did not include ICU stay in our definition of clinical outcomes. It should also be pointed out that the associated conditions that led to delays in ERCP, such as hemodynamic instability or unrecognized cholangitis, may be contributing to worse outcomes and prolonged LOS in these patients.
The number of SIRS criteria has in previous studies been associated with the risk of death from sepsis of various etiologies[15,16]. Our study results are in line with those of prior studies in which SIRS criteria have been used to predict outcomes in acute cholangitis[17,18]. In a large study from Hong Kong, a heart rate above 100/min was associated with requirement for emergent ERCP[16]. Similar findings were reported in a French study[18].
Previous studies have suggested that older age is a risk factor associated with significant mortality in acute cholangitis[19-21]. In our study, older age was associated with adverse outcomes only on univariate analysis. However, on multivariate analysis when adjustments were made for co-morbidities including the ASA physical classification, age lost its significance. We believe that this discrepancy is mainly because in prior studies comorbidities were not taken into consideration.
In our study, higher ASA physical classification was associated with adverse short term outcomes and increase in LOS in patients with acute cholangitis. Previous studies have shown correlation of ASA physical classification with hospital LOS, postoperative infections, and overall morbidity and mortality rates following surgical procedures[22,23]. Higher ASA physical classifications were also shown to correlate with a higher incidence of cardiopulmonary events in a large study of the Clinical Outcomes Research Initiative database[24]. Thus the ASA physical classification may be an important prognostic indictor in patients with acute cholangitis. As expected, most of the patients in our cohort were ASA physical classification 3 or 4 reflecting the nature of most tertiary center practices.
It was not surprising that patients with renal insufficiency and those who developed persistent OF and post-ERCP AEs had a longer duration of hospital stay. It is possible that the same factors that increase the risk of AEs may also influence prolonged hospital stay. It may also reflect a delayed general recovery after developing an AE in patients with underlying comorbidities who undergo emergent ERCP for acute cholangitis. In a large study published in the abstract form, elevated blood urea nitrogen was identified as an important predictor of mortality[11]. In our patient cohort, higher pre-ERCP bilirubin was also associated with prolonged hospital stay. In a recent study, elevated bilirubin was identified as a predictor of worse outcomes and OF[10]. Bilirubin at high concentrations has been shown to induce inflammation, apoptosis, and oxidative stress[25,26]. In a large study, higher bilirubin at admission was associated with subsequent development of sepsis-related acute respiratory distress syndrome and death[27].
Some studies in acute pancreatitis have shown worse outcomes in patients transferred from other hospitals compared to those admitted directly[28,29]. The proposed reason is that transfer patients are sicker resulting in worse outcomes. We did not observe any association between transfer status and clinical outcomes in acute cholangitis. In our cohort only 7/64 transferred patients had a door to ERCP time of greater than 72 h. Based on their CCI and ASA physical classification, transferred patients were not sicker than those directly admitted to our hospital and had a similar incidence of OF during their hospitalization and similar LOS.
The large sample size, statistical adjustments for multiple clinical covariates, and the uniformity of practice patterns for the treatment of cholangitis in a single tertiary care institution are the strengths of this study. Our study has several limitations. First, this is a retrospective study with all inherent limitations of a retrospective design. Second, we studied the association of door to ERCP time with clinical outcomes. Since some patients may have had symptoms for several days before clinical presentation, the time from symptom development to ERCP may be a better factor to study than door to ERCP time. We could not obtain information on symptom to ERCP time because of retrospective nature of the study. However, despite these limitations, our study presents compelling evidence that door to ERCP time and comorbidities are important predictive factors for adverse outcomes in patients with acute cholangitis.
To conclude, delay in performing ERCP and comorbidities are associated with adverse clinical outcomes and prolonged LOS in patients with acute cholangitis. Future strategies to address these issues and to improve outcomes in patients with acute cholangitis need evaluation.
Predictors of adverse short-term outcomes in patients with acute cholangitis are unclear. There is lack of strong evidence based data on the timing of endoscopic retrograde cholangiopancreatography (ERCP) on short term cholangitis outcomes and other predictors of organ failure.
A recently published study suggests that failed or delayed (greater than 72 h) ERCP for acute cholangitis is associated with prolonged hospital stay, increased cost of hospitalization, and a worse composite clinical outcome (death, persistent organ failure, and/or intensive care unit stay). There is lack of strong evidence based data on the timing of ERCP on short term cholangitis outcomes. In this study, the authors have demonstrated that higher American Society of Anesthesiology (ASA) physical classification and delays in ERCP are associated with adverse clinical outcomes and prolonged length of hospital stay.
Although a previous study demonstrated that ERCP delay of 72 h was associated with adverse clinical outcomes of intensive care unit (ICU) stay, persistent organ failure (OF), and death, adjustments for variables such as ASA physical classification, and transfer status of the admitted patients which could potentially impact clinical outcomes was not done. This study has clearly demonstrated that higher ASA physical classification and delays in ERCP are associated with adverse clinical outcomes and prolonged length of hospital stay.
By understanding how delay in ERCP impacts outcomes, this study may represent a future strategy for improving timing of therapeutic intervention in the treatment of patients with acute cholangitis.
Involvement of two or more organ systems was termed as OF. Door to ERCP time was defined as the time from admission to performance of ERCP.
This paper reports a carefully performed retrospective single center study analyzing factors predicting adverse short-term outcomes in patients with acute cholangitis undergoing ERCP. According to the dataset which is based upon 172 patients, higher ASA physical classification and delay in ERCP are both associated with adverse clinical outcome and prolonged length of hospital stay in patients with acute cholangitis. Overall this is a well written and clinically important study.
P- Reviewer: Langner C S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Leese T, Neoptolemos JP, Baker AR, Carr-Locke DL. Management of acute cholangitis and the impact of endoscopic sphincterotomy. Br J Surg. 1986;73:988-992. |
| 2. | Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, Wong J. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586. |
| 3. | Leung JW, Chung SC, Sung JJ, Banez VP, Li AK. Urgent endoscopic drainage for acute suppurative cholangitis. Lancet. 1989;1:1307-1309. |
| 4. | Gogel HK, Runyon BA, Volpicelli NA, Palmer RC. Acute suppurative obstructive cholangitis due to stones: treatment by urgent endoscopic sphincterotomy. Gastrointest Endosc. 1987;33:210-213. |
| 5. | Sharma BC, Agarwal DK, Baijal SS, Saraswat VA, Choudhuri G, Naik SR. Endoscopic management of acute calculous cholangitis. J Gastroenterol Hepatol. 1997;12:874-876. |
| 6. | Lam SK. A study of endoscopic sphincterotomy in recurrent pyogenic cholangitis. Br J Surg. 1984;71:262-266. |
| 7. | Miura F, Takada T, Kawarada Y, Nimura Y, Wada K, Hirota M, Nagino M, Tsuyuguchi T, Mayumi T, Yoshida M. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:27-34. |
| 8. | Chak A, Cooper GS, Lloyd LE, Hammar PJ, Issa K, Rosenthal GE. Effectiveness of ERCP in cholangitis: a community-based study. Gastrointest Endosc. 2000;52:484-489. |
| 9. | Boender J, Nix GA, de Ridder MA, Dees J, Schütte HE, van Buuren HR, van Blankenstein M. Endoscopic sphincterotomy and biliary drainage in patients with cholangitis due to common bile duct stones. Am J Gastroenterol. 1995;90:233-238. |
| 10. | Khashab MA, Tariq A, Tariq U, Kim K, Ponor L, Lennon AM, Canto MI, Gurakar A, Yu Q, Dunbar K. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10:1157-1161. |
| 11. | Lee BS, Hwang JH, Lee SH, Jang SE, Jang ES, Jo HJ, Shin CM, Park YS, Kim JW, Jung SH. Risk factors of organ failure in patients with bacteremic cholangitis. Dig Dis Sci. 2013;58:1091-1099. |
| 12. | Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Butt M, Sanaka MR, Vargo JJ, Parsi MA. Delay in performing ERCP and adverse events increase the 30-day readmission risk in patients with acute cholangitis. Gastrointest Endosc. 2013;78:81-90. |
| 13. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. |
| 14. | Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652. |
| 15. | Alberti C, Brun-Buisson C, Goodman SV, Guidici D, Granton J, Moreno R, Smithies M, Thomas O, Artigas A, Le Gall JR. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168:77-84. |
| 16. | Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117-123. |
| 17. | Hui CK, Lai KC, Yuen MF, Ng M, Lai CL, Lam SK. Acute cholangitis--predictive factors for emergency ERCP. Aliment Pharmacol Ther. 2001;15:1633-1637. |
| 18. | Gigot JF, Leese T, Dereme T, Coutinho J, Castaing D, Bismuth H. Acute cholangitis. Multivariate analysis of risk factors. Ann Surg. 1989;209:435-438. |
| 19. | Sugiyama M, Atomi Y. Treatment of acute cholangitis due to choledocholithiasis in elderly and younger patients. Arch Surg. 1997;132:1129-1133. |
| 20. | Pitt HA, Cameron JL, Postier RG, Gadacz TR. Factors affecting mortality in biliary tract surgery. Am J Surg. 1981;141:66-72. |
| 21. | Agarwal N, Sharma BC, Sarin SK. Endoscopic management of acute cholangitis in elderly patients. World J Gastroenterol. 2006;12:6551-6555. |
| 22. | Sauvanet A, Mariette C, Thomas P, Lozac’h P, Segol P, Tiret E, Delpero JR, Collet D, Leborgne J, Pradère B. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201:253-262. |
| 23. | Prause G, Offner A, Ratzenhofer-Komenda B, Vicenzi M, Smolle J, Smolle-Jüttner F. Comparison of two preoperative indices to predict perioperative mortality in non-cardiac thoracic surgery. Eur J Cardiothorac Surg. 1997;11:670-675. |
| 24. | Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27-34. |
| 25. | Rodrigues CM, Solá S, Brito MA, Brites D, Moura JJ. Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J Hepatol. 2002;36:335-341. |
| 26. | Alexandra Brito M, Silva RF, Brites D. Bilirubin toxicity to human erythrocytes: a review. Clin Chim Acta. 2006;374:46-56. |
| 27. | Zhai R, Sheu CC, Su L, Gong MN, Tejera P, Chen F, Wang Z, Convery MP, Thompson BT, Christiani DC. Serum bilirubin levels on ICU admission are associated with ARDS development and mortality in sepsis. Thorax. 2009;64:784-790. |