Published online Feb 16, 2014. doi: 10.4253/wjge.v6.i2.55
Revised: December 18, 2013
Accepted: January 7, 2014
Published online: February 16, 2014
Processing time: 205 Days and 11.1 Hours
AIM: To evaluate the feasibility of using an automated spring-loaded needle device for endoscopic ultrasound (EUS)-guided abdominal paracentesis (EUS-P) to see if this would make it easier to puncture the mobile and lax gastric wall for EUS-P.
METHODS: The EUS database and electronic medical records at Fukushima Medical University Hospital were searched from January 2001 to April 2011. Patients with a history of cancer and who underwent EUS-P using an automated spring-loaded needle device with a 22-gauge puncture needle were included. The needle was passed through the instrument channel and advanced through the gastrointestinal wall under EUS guidance into the echo-free space in the abdominal cavity and ascitic fluid was collected. The confirmed diagnosis of malignant ascites included positive cytology and results from careful clinical observation for at least 6 mo in patients with negative cytology. The technical success rate, cytology results and complications were evaluated.
RESULTS: We found 11 patients who underwent EUS-P with an automated spring-loaded needle device. In 4 cases, ascites was revealed only with EUS but not in other imaging modalities. EUS-P was done in 7 other cases because there was minimal ascitic fluid and no safe window for percutaneous abdominal aspiration. Ascitic fluid was obtained in all cases by EUS-P. The average amount aspirated was 14.1 mL (range 0.5-38 mL) and that was sent for cytological exam. The etiology of ascitic fluid was benign in 5 patients and malignant in 6. In all cases, ascitic fluid was obtained with the first needle pass. No procedure-related adverse effects occurred.
CONCLUSION: EUS-P with an automated spring-loaded needle device is a feasible and safe method for ascites evaluation.
Core tip: Even in patients with a minute amount of ascitic fluid, an automated spring-loaded needle device enabled us to perform endoscopic ultrasound (EUS)-guided abdominal paracentesis (EUS-P) readily, which has the potential to play an important role for staging of cancer since the establishment of malignant ascites denotes a more advanced stage of cancer.
- Citation: Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Shibukawa G, Sato A, Sato M, Ikeda T, Watanabe K, Nakamura J, Annangi S, Tasaki K, Obara K, Ohira H. An automated spring-loaded needle for endoscopic ultrasound-guided abdominal paracentesis in cancer patients. World J Gastrointest Endosc 2014; 6(2): 55-59
- URL: https://www.wjgnet.com/1948-5190/full/v6/i2/55.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i2.55
The existence of malignant ascites in cancer patients indicates a dismal prognosis[1-9]. Therefore, the etiology of ascitic fluid in cancer patients needs careful evaluation. Endoscopic ultrasound (EUS) can detect a minute or minimal amount of ascitic fluid that may be undetectable in other imaging modalities, including abdominal ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI)[10-15]. Moreover, EUS-guided abdominal paracentesis (EUS-P) has the potential to play an important role for staging of cancer since the establishment of malignant ascites denotes a more advanced stage of cancer[16-20]. Although EUS-P is a useful technique at times, we encountered technical difficulties during EUS-P, probably due to less counteracting force from extramural objects and a lax gastrointestinal wall.
An automated spring-loaded needle device which was designed to function analogously to spring-loaded biopsy needles used for percutaneous tissue sampling with high puncture speed was developed by Binmoeller et al[21,22] for cases in which penetration during EUS-FNA is difficult[23,24].
In this study, we aimed to evaluate the feasibility of using an automated spring-loaded needle device for EUS-P to see if this would make it easier to puncture the mobile and lax gastric wall for EUS-P.
The EUS database and electronic records at Fukushima Medical University were searched from January 2001 to April 2011 for patients with a history of cancer and for whom EUS-P was performed using an automated spring-loaded needle device. Before EUS-P, written informed consent was obtained from all patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review committee.
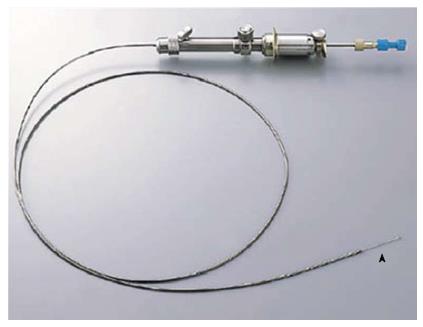
EUS-P was performed using a curved linear-array echoendoscope (GF-UCT240-AL5 or GF-UC240P-AL5; Olympus Medical Systems Corp., Tokyo, Japan) in conjunction with SSD-5500 (Aloka Co. Ltd., Tokyo, Japan), or using a FG-36UX (Pentax Corp., Tokyo, Japan) in conjunction with EUB-6000 (Hitachi Ltd., Tokyo, Japan). The needle device in all patients was an automated spring-loaded powershot needle (NA-11J-KB; Olympus Medical Systems Corp., Tokyo, Japan) with a 22-gauge puncture needle (Figure 1). In patients in whom EUS-FNA was performed for other lesions, EUS-P was initially performed to prevent potential seeding or dissemination and needles were changed to prevent contamination after this procedure.
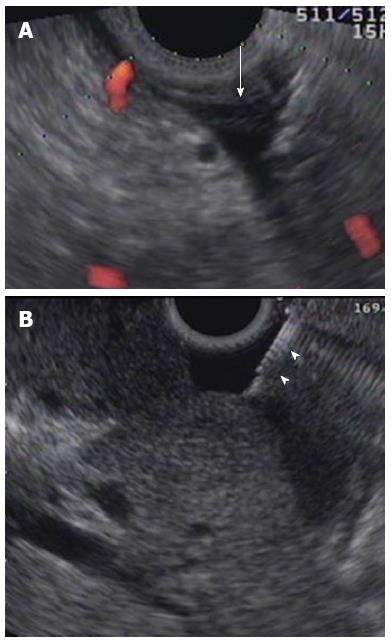
Ascitic fluid was defined as the presence of extra-luminal free fluid (anechoic space on EUS), as viewed from the stomach or duodenum. The needle was passed through the instrument channel and advanced through the gastrointestinal (GI) wall under EUS guidance into the echo-free space in the abdominal cavity (Figure 2). In patients with GI lesions, puncture points were determined carefully to avoid tissue contamination from primary lesions. After being guided into the target lesion, the stylet was removed. The needle was retracted to maintain its position within the fluid and avoid sucking up against adjacent bowel and/or omentum while aspirating. Subsequently, the suction syringe was released and the needle was withdrawn into the catheter. It was then removed completely. Aspirated ascitic fluid was sprayed on the glass slide or in the tube and submitted for pathological examination.
The cytological criteria used for reporting EUS-P results were based on the guidelines of the Papanicolaou Society of Cytopathology for fine needle aspiration and reporting. We regarded Class I-II as benign, Class III as atypical/indeterminate, and Class IV/V as malignant[25].
The confirmed diagnosis of malignant ascites included positive cytology and results from careful clinical observation for at least 6 mo in patients with negative cytology.
Eleven patients (7 males and 4 females) were enrolled. The average age of patients was 66.4 years (range 50-78 years). Primary malignancy was pancreatic adenocarcinoma in 6 patients, cholangiocarcinoma in 2 patients, and breast cancer, gastric cancer and malignant lymphoma in 1 patient, respectively. Six patients with pancreatic adenocarcinoma underwent EUS-FNA for primary lesions at the same time of EUS-P. In 4 others with synchronous malignancy, primary lesions were diagnosed with other modalities (e.g., endoscopic retrograde cholangiopancreatography, endoscopy). In one patient with a history of breast cancer 5 years prior to EUS-P, concomitant malignancy was not detected. The clinical characteristics are summarized in Table 1.
| Patient | Age (yr)/sex | Primary malignancy | Aspirated ascitic fluid (mL) | Ascitic fluid in US/CT/MRI | Malignant ascitic fluid |
| 1 | 64/M | ML | 38 | + | - |
| 2 | 50/M | PDAC | 20 | + | + |
| 3 | 78/F | CC | 6 | + | - |
| 4 | 70/M | PDAC | 0.5 | - | + |
| 5 | 76/ M | GC, LC | 15 | + | - |
| 6 | 55/M | PDAC | 2 | - | + |
| 7 | 62/F | CC | 25 | + | + |
| 8 | 77/M | PDAC | 10 | + | - |
| 9 | 66/F | BC | 30 | + | + |
| 10 | 70/F | PDAC | 3 | - | + |
| 11 | 63/M | PDAC | 5 | - | - |
Ascitic fluid was obtained in all patients. The average amount was 14.1 mL (range 0.5-38 mL). The etiology of ascitic fluid was benign in 5 patients and malignant in 6. Among these 11 patients, ascitic fluid was revealed with US or CT in 63.6% (7 out of 11 patients) but there was no appropriate percutaneous route for image-guided abdominal paracentesis. By contrast, in 4 other patients (36.4%), ascites was detected with only EUS. EUS-FNA was performed for pancreatic mass lesions in all these patients and ascites was an incidental finding. The average amount of ascites obtained with EUS-P was only 2.6 mL (range 0.5-5 mL) in these patients. No complications occurred in any of these procedures.
Our results show that EUS-P with an automated spring-loaded needle device can be a useful technique to obtain a minute amount of ascitic fluid in cancer patients. Furthermore, EUS showed its ability to detect a scant amount of ascitic fluid which US and CT could not detect in 4 patients with pancreatic ductal adenocarcinoma. In these patients, the amount of aspirated fluid was only 2.6 mL on average. Two of them were diagnosed as malignant and this result changed their management.
Regarding the technical aspects, it is sometimes difficult to penetrate the mobile and lax gastrointestinal wall with a standard EUS-FNA needle. It may be a greater problem in patients who require EUS-P because there is less counteracting force from extramural objects. To solve this problem, we aimed to evaluate the feasibility of using an automated spring-loaded needle device with high puncture speed for EUS-P and to show its high technical success rate to obtain a minimum amount of ascitic fluid[21-24]. Limitations of our study were that it was retrospective and had a small number of cases. Additionally, with the lack of a control group with a standard EUS-FNA needle, we were unable to conclude which was the optimal type of needle for EUS-P.
Otherwise, based on our experience, we conclude that both EUS and EUS-P are useful in the management of cancer patients with gastrointestinal or other malignancies to detect and aspirate minute/minimal amounts of ascites that may not be visible by other imaging modalities or when percutaneous aspiration may not be feasible due to minimal fluid and lack of a suitable path. The automated spring-loaded needle device may provide a technical advantage, especially in cases that are difficult to penetrate the lax and mobile gastrointestinal wall.
We would like to acknowledge the following individuals: Toshiyuki Hoshi from the Department of Pathology at Fukushima Medical University Hospital for dedicated support for pathological evaluation; and Somashekar Gopala Krishna from the Department of Gastroenterology, Hepatology and Nutrition at UT MD Anderson Cancer Center for critical revision.
The etiology of ascitic fluid in cancer patients needs careful evaluation. Since the authors sometimes have difficulties in the detection and sampling of small amounts of ascitic fluid, the ability of endoscopic ultrasound (EUS) and EUS-guided abdominal paracentesis (EUS-P) has the potential to play an important role for staging of cancer. Although EUS-P is a useful technique at times, the authors encountered technical difficulties during EUS-P, probably due to less counteracting force from extramural objects and a lax gastrointestinal wall.
The importance of EUS-P for cancer staging has not been well recognized yet. Regarding the technical aspects, it is sometimes difficult to penetrate the mobile and lax gastrointestinal wall with a standard EUS-FNA needle. It may be a greater problem in patients who require EUS-P because there is less counteracting force from extramural objects.
The authors aimed to evaluate the feasibility of using an automated spring-loaded needle device with high puncture speed for EUS-P and to show its high technical success rate to obtain minimum amount of ascitic fluid.
This method can be useful in every patient with ascites who requires cancer staging to determine their treatment strategy.
EUS: a medical procedure in which endoscopy is combined with ultrasound to obtain images of the internal organs in the chest and abdomen. Paracentesis: a form of body fluid sampling procedure in which the peritoneal cavity is punctured by a needle to sample peritoneal fluid.
The results show that EUS-P with an automated spring-loaded needle device can be a useful technique to obtain a minute amount of ascitic fluid in cancer patients. The article is interesting, unique and worthy of publication.
P- Reviewers: Leitman IM, Spasojevic SD S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260-265. |
| 2. | Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364-367. |
| 3. | Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26-9; discussion 29-30. |
| 4. | Lee YT, Ng EK, Hung LC, Chung SC, Ching JY, Chan WY, Chu WC, Sung JJ. Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastases in gastric cancer patients. Gut. 2005;54:1541-1545. |
| 5. | Smith EM, Jayson GC. The current and future management of malignant ascites. Clin Oncol (R Coll Radiol). 2003;15:59-72. |
| 6. | Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945-949. |
| 7. | Fang N, Zhang HQ, He B, Xie M, Lu S, Wan YY, Wang NR. Clinicopathological characteristics and prognosis of gastric cancer with malignant ascites. Tumour Biol. 2013;Epub ahead of print. |
| 8. | Mohan HM, O’Connor DB, O’Riordan JM, Winter DC. Prognostic significance of detection of microscopic peritoneal disease in colorectal cancer: a systematic review. Surg Oncol. 2013;22:e1-e6. |
| 9. | Zervos EE, Osborne D, Boe BA, Luzardo G, Goldin SB, Rosemurgy AS. Prognostic significance of new onset ascites in patients with pancreatic cancer. World J Surg Oncol. 2006;4:16. |
| 10. | Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71-78. |
| 11. | Power DG, Schattner MA, Gerdes H, Brenner B, Markowitz AJ, Capanu M, Coit DG, Brennan M, Kelsen DP, Shah MA. Endoscopic ultrasound can improve the selection for laparoscopy in patients with localized gastric cancer. J Am Coll Surg. 2009;208:173-178. |
| 12. | Chu KM, Kwok KF, Law S, Wong KH. A prospective evaluation of catheter probe EUS for the detection of ascites in patients with gastric carcinoma. Gastrointest Endosc. 2004;59:471-474. |
| 13. | Sultan J, Robinson S, Hayes N, Griffin SM, Richardson DL, Preston SR. Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for oesophagogastric cancer. Br J Surg. 2008;95:1127-1130. |
| 14. | Twine CP, Barry JD, Blackshaw GR, Crosby TD, Roberts SA, Lewis WG. Prognostic significance of endoscopic ultrasound-defined pleural, pericardial or peritoneal fluid in oesophageal cancer. Surg Endosc. 2009;23:2229-2236. |
| 15. | Tsendsuren T, Jun SM, Mian XH. Usefulness of endoscopic ultrasonography in preoperative TNM staging of gastric cancer. World J Gastroenterol. 2006;12:43-47. |
| 16. | DeWitt J, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609-615. |
| 17. | Kaushik N, Khalid A, Brody D, McGrath K. EUS-guided paracentesis for the diagnosis of malignant ascites. Gastrointest Endosc. 2006;64:908-913. |
| 18. | Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336-339. |
| 19. | Peter S, Eltoum I, Eloubeidi MA. EUS-guided FNA of peritoneal carcinomatosis in patients with unknown primary malignancy. Gastrointest Endosc. 2009;70:1266-1270. |
| 20. | Chang KJ, Albers CG, Nguyen P. Endoscopic ultrasound-guided fine needle aspiration of pleural and ascitic fluid. Am J Gastroenterol. 1995;90:148-150. |
| 21. | Binmoeller KF, Jabusch HC, Seifert H, Soehendra N. Endosonography-guided fine-needle biopsy of indurated pancreatic lesions using an automated biopsy device. Endoscopy. 1997;29:384-388. |
| 22. | Binmoeller KF, Rathod VD. Difficult pancreatic mass FNA: tips for success. Gastrointest Endosc. 2002;56:S86-S91. |
| 23. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. |
| 24. | Irisawa A, Hikichi T, Bhutani MS, Ohira H. Basic technique of FNA. Gastrointest Endosc. 2009;69:S125-S129. |
| 25. | Nguyen GK, Suen KC, Villanueva RR. Needle aspiration cytology of pancreatic cystic lesions. Diagn Cytopathol. 1997;17:177-182. |