Published online Nov 16, 2014. doi: 10.4253/wjge.v6.i11.555
Revised: June 30, 2014
Accepted: October 1, 2014
Published online: November 16, 2014
Processing time: 200 Days and 14.9 Hours
AIM: To compare endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) for early gastric cancer (EGC).
METHODS: Computerized bibliographic search was performed on PubMed/Medline, Embase, Google Scholar and Cochrane library databases. Quality of each included study was assessed according to current Cochrane guidelines. Primary endpoints were en bloc resection rate and histologically complete resection rate. Secondary endpoints were length of procedure, post-treatment bleeding, post-procedural perforation and recurrence rate. Comparisons between the two treatment groups across all the included studies were performed by using Mantel-Haenszel test for fixed-effects models (in case of low heterogeneity) or DerSimonian and Laird test for random-effects models (in case of high heterogeneity).
RESULTS: Ten retrospective studies (8 full text and 2 abstracts) were included in the meta-analysis. Overall data on 4328 lesions, 1916 in the ESD and 2412 in the EMR group were pooled and analyzed. The mean operation time was longer for ESD than for EMR (standardized mean difference 1.73, 95%CI: 0.52-2.95, P = 0.005) and the “en bloc” and histological complete resection rates were significantly higher in the ESD group [OR = 9.69 (95%CI: 7.74-12.13), P < 0.001 and OR = 5.66, (95%CI: 2.92-10.96), P < 0.001, respectively]. As a consequence of its greater radicality, ESD provided lower recurrence rate [OR = 0.09, (95%CI: 0.05-0.17), P < 0.001]. Among complications, perforation rate was significantly higher after ESD [OR = 4.67, (95%CI, 2.77-7.87), P < 0.001] whereas the bleeding incidences did not differ between the two techniques [OR = 1.49 (0.6-3.71), P = 0.39].
CONCLUSION: In the endoscopic therapy of EGC, ESD showed a superior efficacy but higher complication rate with respect to EMR.
Core tip: Endoscopic submucosal dissection (ESD) represents a promising approach to the therapy of Early Gastric Cancer. Preliminar studies showed better outcomes in terms of complete en bloc and histologic resection rate with respect to classical Endoscopic Mucosal Resection (EMR). Some concerns raise due to higher complication rate (particularly perforation) and longer operation times related to the complexity of the procedure. The current meta-analysis outline the superiority of ESD in obtaining higher radical resection rate and lower recurrence rates compared to EMR but confirms the aforementioned concerns on higher incidences of perforation and bleeding (in this case non significantly) after ESD.
-
Citation: Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection
vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc 2014; 6(11): 555-563 - URL: https://www.wjgnet.com/1948-5190/full/v6/i11/555.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i11.555
Early gastric cancer (EGC) is a malignant tumor confined to the mucosa or the submucosa regardless of lymph node metastases[1].
As the diagnostic rate of EGC increases, endoscopy has become the treatment of choice for the radical cure of EGC. Since the 1980s endoscopic mucosal resection (EMR) has been proposed as a replacement for invasive surgery because of favorable long-term outcomes and improved quality of life for patients[2,3]. EMR is routinely performed due to its safety profile, low cost, patient tolerance and rapid post-procedural recovery.
Endoscopic submucosal dissection (ESD) was developed in the late 1990s to enable the en bloc removal of lesions larger than 2 cm[4]. In fact, only a complete pathological specimen may allow clinicians to achieve a reliable diagnosis and hence to plan the correct therapeutic strategy.
ESD uses the technique of improved needle-knife under endoscopy to exfoliate the diseased submucosa with coagulation corrent.
Previous systematic reviews and meta-analyses have reported higher resection rates but also a relatively higher rate of complications such as bleeding and perforation after ESD because of its large wound incidence and difficulties[5,6]. Since these reviews date early 2010s, systematic analyses including pooled data of recent studies on this topic are lacking.
Aim of the current meta-analysis is to update the state of the art of endoscopic therapies of EGC in light of the last studies published in this field.
Computerized bibliographic search was performed on PubMed/Medline, Embase, Google Scholar, Cochrane library databases using the following key words: “EMR”, “ESD”, “endoscopic mucosal resection”, “endoscopic submucosal dissection” and “early gastric cancer”. Complementary manual search was performed by checking the references of all the main review articles on this topic, in order to identify possible additional studies. Moreover, the abstracts of main oncological and endoscopic congresses were retrieved.
Eligible studies were randomized controlled trials, prospective or retrospective cohort and case-control studies and international congress abstracts comparing EMR and ESD for the treatment of EGC in human patients published until April 2014. The search was restricted to English-language articles. Studies were excluded if they had not compared data between the two treatments. Case reports or studies with insufficient data were also excluded. Included studies were selected independently by two investigators (AF and MA). Disagreements were solved by discussion and following a third opinion (MdM).
The quality of the included studies was assessed according to the guidelines of the Cochrane Reviewer’s Handbook recommended by the Cochrane Collaboration[7].
Primary endpoints were: en bloc resection rate (i.e., no piecemeal removal of the lesion)[8] and histologically complete resection rate (no neoplastic cells in lesion edges).
Secondary endpoints were: length of procedure (from marking to removal of the tumor); post-treatment bleeding; post-procedural perforation; and recurrence rate (diagnosed by histology within the treated area during follow-up).
Pooled data of continuous variables were expressed as standardized mean difference while data of categorical ones as odds ratio (OR) and 95%CI.
Comparisons between the two treatment groups across all the included studies were performed by using Mantel-Haenszel test for fixed-effects models[9] (in case of low heterogeneity) or DerSimonian and Laird test for random-effects models[10] (in case of high heterogeneity). The level of heterogeneity between the included studies was assessed following the guidelines of the Cochrane Collaboration[7]. Once heterogeneity was noted, between-study sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates according to the study characteristics.
Since the use of scales for evaluating quality or risk of bias is explicitly discouraged in Cochrane reviews[7], in assessing study quality a domain-based evaluation was performed, in which critical assessments were made separately for different domains[7].
Publication biases were assessed using funnel plots visually and performing Begg and Mazumdar’s test based on the rank correlation between the observed effect sizes and observed standard errors[11]. Whenever publication biases were found, “trim and fill” method was performed aiming at obviating to these biases.
Significance threshold was assessed at a P-value < 0.05.
All calculations were performed using Review Manager (version 5.0 for Windows; the Cochrane Collaboration, Oxford, United Kingdom) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
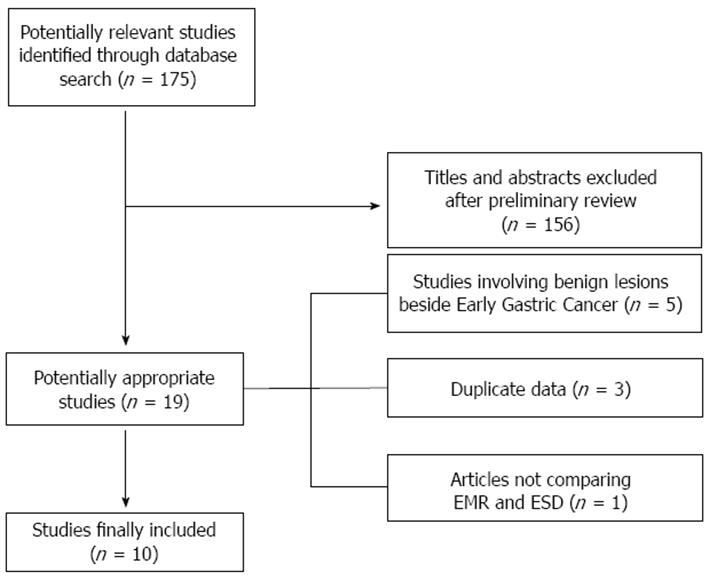
Figure 1 shows the flow chart of the search strategy conducted in this meta-analysis.
Initially, we identified 175 potentially relevant studies. After a preliminary review, 156 papers were excluded, because they were animal studies, case reports, comment letters or descriptive reviews.
Among 19 potentially appropriate articles, we excluded 5 studies[12-16] because did not examine EGC, 3 studies[17-19] because based on the same data and another one due to the lack of comparative results between the two treatments[20]. Finally, 10 studies[8,21-29] were included in the meta-analysis.
Main characteristics of included studies are shown in Table 1. The current meta-analysis analyzed reports of a total of 4328 lesions, 1916 in the ESD and 2412 in the EMR group. The included papers were all retrospective case-control studies, whereof 8 were full text articles[8,21-23,26-29] and 2 were abstracts[24,25].
| Ref. | Country | Patients | Lesions | Article | Baseline consistency | Follow-up | End-points |
| Min et al[21] 2009 | South Korea | ESD 243EMR 203 | ESD 243EMR 203 | Full text | No | 29 (4-44) m | Operation time, en bloc resection rate, histologic curative resection rate, bleeding, perforation,recurrence |
| Oka et al[22]2006 | Japan | ESD 185EMR 711 | ESD 195EMR 825 | Full text | No | ESD 19.4 ± 9.2 mEMR 83.2 ± 34.6 m | Operation time, en bloc resection rate, histologic curative resection rate, bleeding, perforation, recurrence |
| Oda et al[8]2006 | Japan | 655 | ESD 303EMR 411 | Full text | No | 39 (5-60) m | En bloc resection rate, histologic curative resection rate, perforation, recurrence (residual) |
| Catalano et al[23] 2009 | Italy | 45 | ESD 12EMR 36 | Full text | Not Recorded | 31 (12-71) m | Operation time, en bloc resection rate, histologic curative resection rate, bleeding, perforation, recurrence |
| Odashima et al[24] 2006 | Japan | ESD 57EMR 80 | ESD 57EMR 80 | Abstract | Not Recorded | Not Recorded | Histologic curative resection rate |
| Hoteya et al[25] 2007 | Japan | Not Recorded | ESD 304EMR 350 | Abstract | Not Recorded | Not Recorded | En bloc resection rate, histologic curative resection rate, delayed bleeding rate |
| Hoteya et al[26] 2010 | Japan | Not Recorded | ESD 40EMR 22 | Full text | No | Not Recorded | Operation time, en bloc resection rate, histologic curative resection rate, perforation, recurrence |
| Nakamoto et al[27] 2009 | Japan | ESD 106EMR 71 | ESD 122EMR 80 | Full text | Yes | 54 (12-89) m | Operation time, en bloc resection rate, histologic curative resection rate, perforation, bleeding |
| Watanabe et al[28] 2010 | Japan | ESD 219EMR 146 | ESD 219EMR 146 | Full text | Yes | ESD 14.3 mEMR 17.8 m | En bloc resection rate, histologic curative resection rate, perforation, recurrence |
| Tanabe et al[29] 2014 | Japan | ESD 421EMR 359 | ESD 421EMR 359 | Full text | No | ESD 65 mEMR 73 m | Recurrence, overall survival |
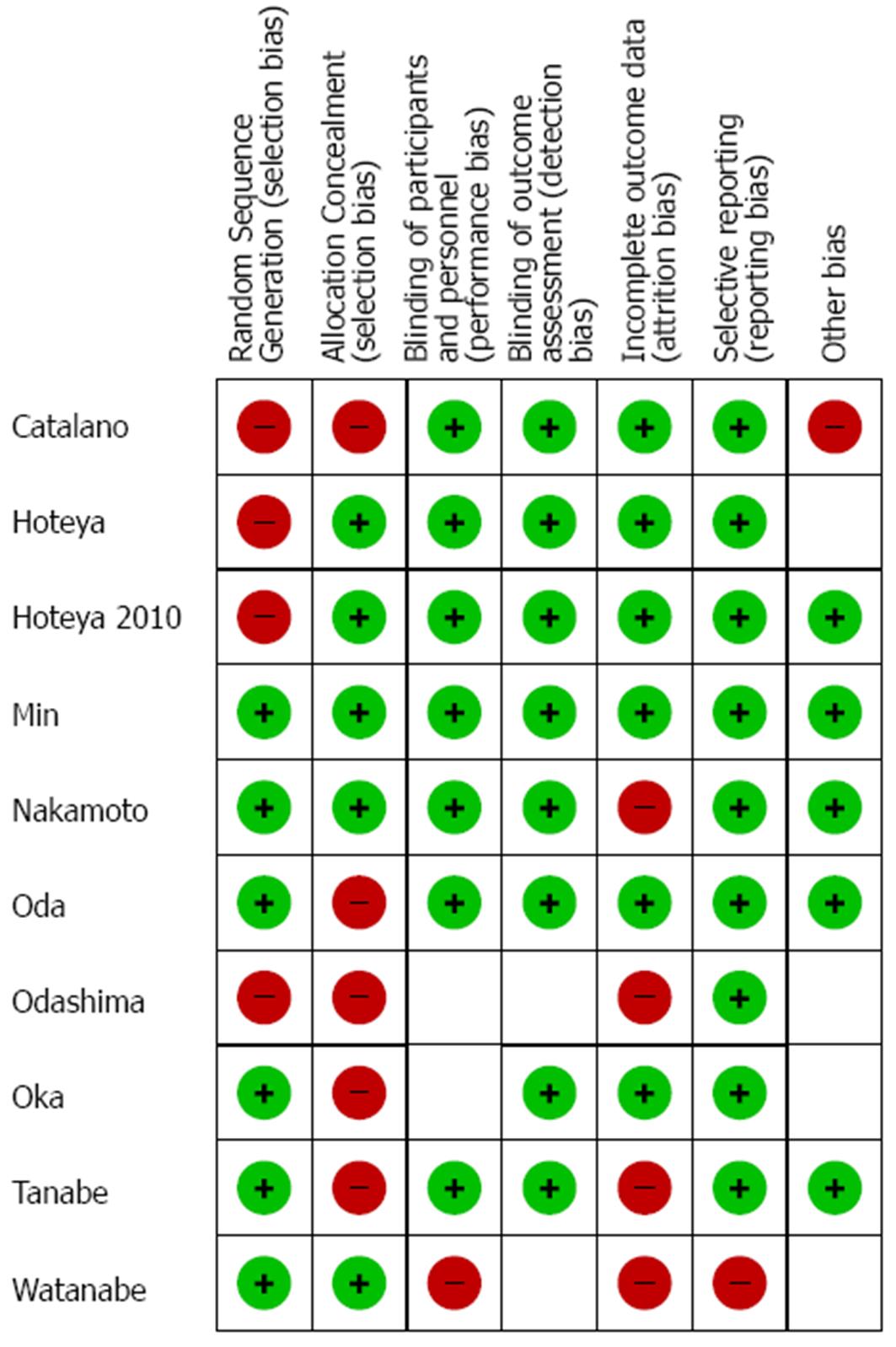
In two studies data on the number of patients were not available[25,26], while in three articles[24-26] median follow-up times were not recorded. The studies in which demographic and tumoral baseline parameters were reported did not show significant differences between treatment groups thus obviating to selection biases that could have affect final outcomes. Quality assessment of each study can be seen in Figure 2.
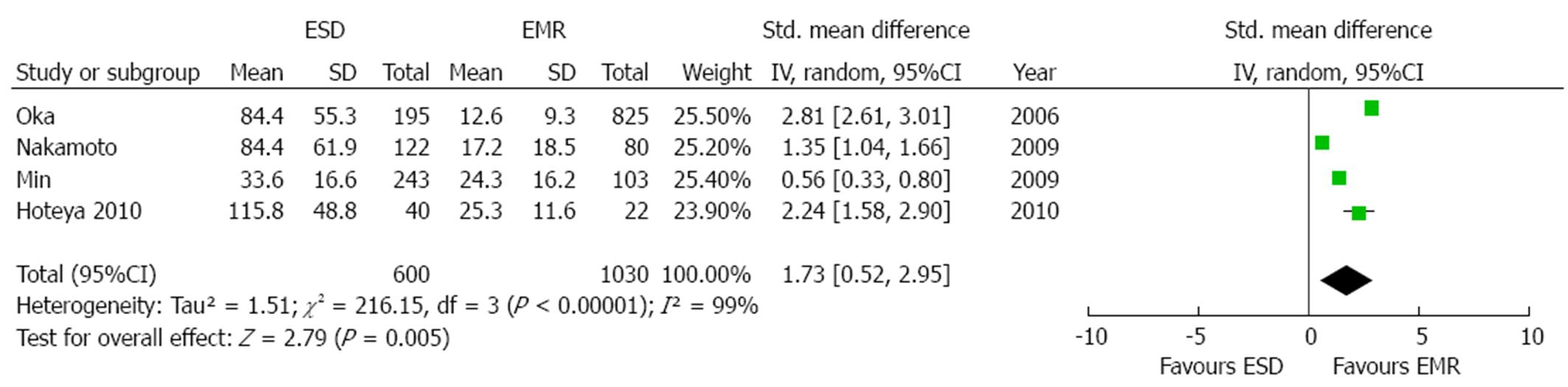
Mean length of the procedures was described in four studies[21,22,26,27]. As an high grade of heterogeneity was found (P < 0.001; I2 = 99%), a random effect model was applied. Mean operation time was significantly longer in the ESD group [overall standardized mean difference 1.73 (95%CI: 0.52-2.95), P = 0.005, Figure 3]. In order to explore the source of heterogeneity, we performed a sensitivity analysis both by eliminating the study with the smaller sample size[26] and by removing from the analysis the low-quality study[22]. However, in both cases the results were in favor of a significantly shorter duration of the procedure in the EMR patients [standardized mean difference: 1.58 (0.12-3.03), P = 0.03 and 1.33 (0.52-2.14), P = 0.001, respectively] without obviating to the aforementioned heterogeneity (P < 0.001, I2 = 99% and P≤ 0.001, I2 = 94%, respectively).
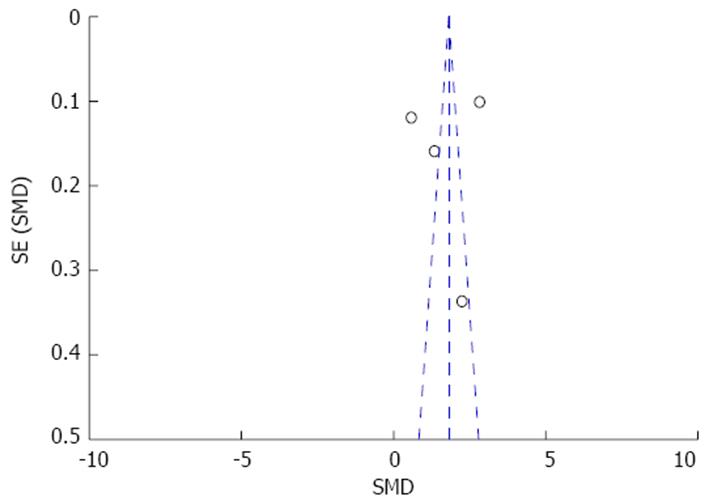
Eventual sources of publication bias were explored by means of visual examination of funnel plot (Figure 4) and of Begg and Mazumdar’s test (P = 0.03). After editing funnel plot by trim and fill method, the standardized mean difference in operation time was smaller with the missing studies filled in, but the results still remained significant (P = 0.04).
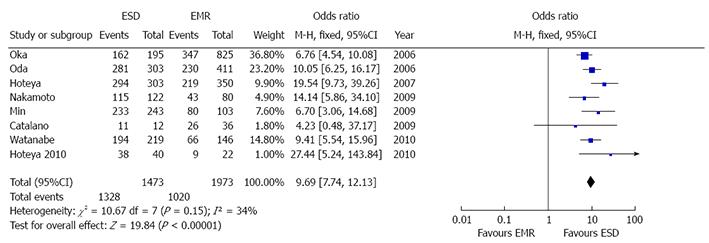
Eight studies reported the en bloc resection rate[8,21-23,25-28]. As no heterogeneity was found (P = 0.15, I2 = 34%), a fix model was performed (Figure 5). Overall en bloc resection rate resulted significantly higher in ESD patients [OR 9.69 (7.74-12.13), P < 0.001] with 1328 out of 1437 patients in ESD vs 1020/1973 in EMR group who underwent complete resection of the lesion. No publication bias was found (P = 0.34).
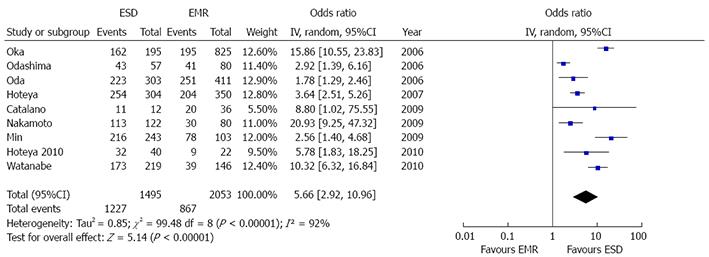
Nine studies reported histological resection rates[8,21-28]. A high grade of heterogeneity was found (P < 0.001, I2 = 92%), hence random model with inverse variance analysis was performed (Figure 6). ESD patients obtained a significantly higher histological resection rate [OR = 5.66 (2.92-10.96), P < 0.001]. In fact, 1227/1495 ESD patients and 867/2053 EMR patients reached this gold standard of endoscopic intervention.
Sensitivity examination of data was performed by means of subgroup analysis, considering separately studies including only small EGCs (i.e., < 10 mm)[21,22,27,28] and those based on greater lesions[8,23-26]. In both cases the better performances of ESD with respect to EMR resulted even amplified [OR = 9.28 (4.6-18.72), P < 0.001 and OR = 15.21 (11.2-19.98), P < 0.001, respectively]. A low grade of heterogeneity in the former studies and no heterogeneity at all in the latter was found (P < 0.1, I2 = 52% and P = 0.11, I2 = 45%, respectively).
Evidence of publication bias was found (P = 0.01), hence trim and fill method was applied without losing the statistical significance of the rates previously reported (P < 0.001).
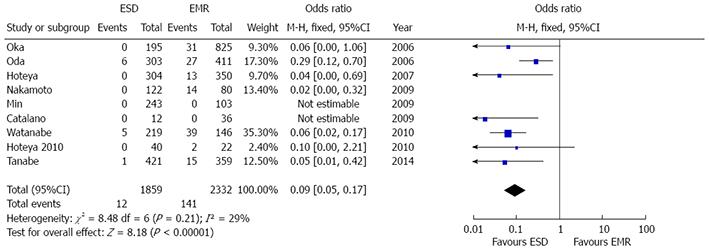
Data on local recurrence rate were reported in nine studies[8,21-23,25-29]. Recurrence rate resulted significantly lower after ESD [OR = 0.09 (0.05-0.17), P < 0.001] and low grade of heterogeneity was found (P = 0.21, I2 = 29%) (Figure 7). No evidence of publication bias was found (P = 0.11).
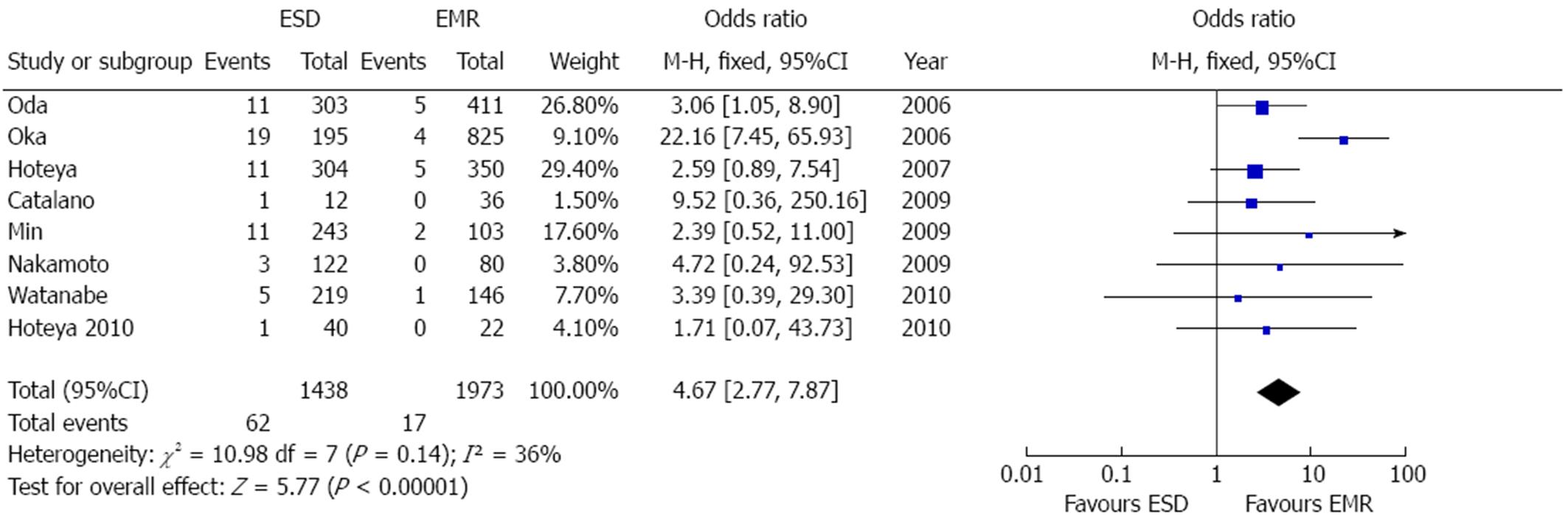
Eight articles examined treatment-related perforation rate[8,21-23,25-28]. Perforations were significantly more common after ESD [OR = 4.67 (2.77-7.87), P < 0.001] (Figure 8). Further sensitivity analyses were not needed due to the low grade of heterogeneity (P = 0.14, I2 = 36%). Overall, 62/1438 cases of perforation after ESD and 17/1973 after EMR were reported. There was no evidence of publication bias (P = 0.14).
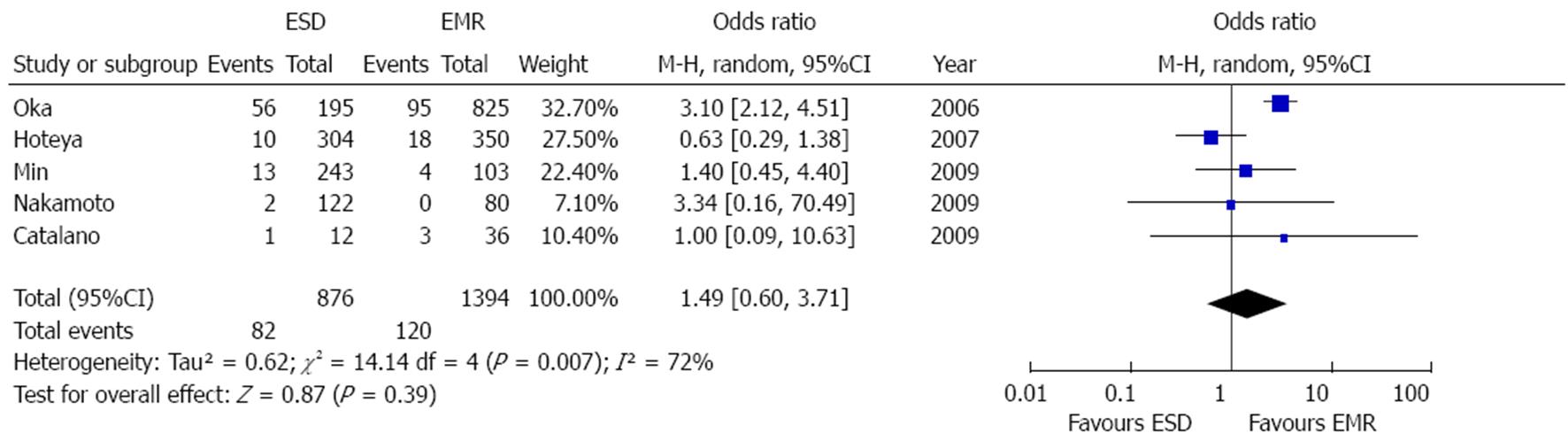
Five studies reported post-treatment bleeding rate[21-23,25,27]. As shown in Figure 9, bleeding rate was higher, although not significantly, after ESD [OR = 1.49 (0.6-3.71), P = 0.39]. Since high grade of heterogeneity was found (P = 0.007, I2 = 72%), a random effect model was built and sensitivity analyses were performed. In particular, restricting the analysis to the two studies reporting delayed bleeding rate after procedure[21,25], OR slightly decreased (1.04, 95%CI: 0.62-1.75) and no heterogeneity resulted. No evidence of publication bias was found (P = 0.09).
ESD represents a promising alternative to classical EMR for the treatment of EGCs. Two previous meta-analyses clearly demonstrated the superiority of ESD in terms of resection (both en bloc and histological) rate and recurrence rate with higher percentages of complications due to the complexity of the procedure[5,6]. Aim of this meta-analysis is to validate such results in light of the recently published studies on this topic.
A total of 10 studies analyzing the endoscopic treatment of 4328 lesions (1916 in the ESD and 2412 in the EMR group) were included in the current review. ESD was found to provide better outcomes in terms of lower recurrence rate and higher en bloc resection rate. As a consequence, ESD reached more frequently the complete histological removal of the treated lesions, which is the gold standard of each endoscopic interventional procedure. In this setting, the high OR [5.66 (2.92-10.96), P < 0.001] in favor of ESD stands for a clear superiority of such procedure for the treatment of non metastatic mucosal cancerous lesions of the stomach. The robustness of this finding was not affected from the high grade of heterogeneity because stratifying the analysis by the diameter of the lesions, no changes in the final outcomes were found. In fact, ESD resulted superior with respect to EMR in all the reviewed studies, regardless of the size of the treated lesions.
Unfortunately, broad and robust data on overall survival after endoscopic treatment of EGC are still too few for being included in a meta-analysis, hence this outcome could not be explored. However, the low aggressiveness and scarce attitude to extragastric spread of EGC should result in slight differences of survival outcomes between the two treatments. In fact, a recent study did not show significant differences in terms of overall survival between ESD and EMR despite lower recurrence rate after submucosal dissection[29].
Indubitably, ESD is a more complex procedure that should be performed in high-volume centers. Our meta-analysis confirms the results of previous systematic reviews finding higher procedure-related complication (both perforation and bleeding) rates and longer operation times in ESD patients. Among complications, particular concerns raise on regard to perforations. In fact our meta-analysis shows a significant association between ESD and perforation rate [OR = 4.67 (2.77-7.87), P < 0.001], thus meaning higher morbidity with respect to classical mucosal resection. Interestingly, bleeding rate showed only a non-significant trend in favor of EMR and restricting the analysis to delayed bleeding (the more insidious and difficult to treat), the two procedures resulted in similar rates (OR = 1.04, 95%CI: 0.62-1.75).
There are some limitations in our study. First, only retrospective non randomized studies are currently available in the literature, hence the absence of randomization may introduce patient selection biases. Second, the number of included studies is small. Third, estimation of overall survival was not feasible because of lack of long-term outcomes data in most of the studies included in the meta-analysis. Finally, only a low-quality, small Western study from Italy was included and most articles were from Eastern Asia, where high-experienced interventional endoscopists in high-volume centers deal with more EGC patients, hence the conclusions of the meta-analysis could be not applicable in Europe and United States.
Despite these limitations, our study has a number of strengths. It provides a comprehensive and simultaneous assessment of therapeutic efficacy, procedural complexity and safety profile of the two treatments. Second, any possible source of heterogeneity and publication bias that could have influenced the final results was explored by means of appropriate statistical tools and all the findings were confirmed performing sensitivity analysis.
In conclusion, ESD is a more effective therapy for EGC with higher en bloc and histologically complete resection rate and lower local recurrence in comparison to EMR, regardless of tumor size. On the other hand, its safety profile is affected from higher perforation rate which may limit its application in low-volume centers.
Broad randomized controlled trials from both Eastern and Western countries reporting also overall survival data are warranted in order to validate such findings.
Early gastric cancer (EGC) is a malignant tumor confined to the mucosa or the submucosa of the stomach regardless of lymph node metastases. Endoscopic submucosal dissection (ESD) was developed to overcome the problem caused by incomplete resection by conventional Endoscopic mucosal resection (EMR) for EGC. Aim of this meta-analysis is to compare the efficacy outcomes, expressed in terms of en bloc and histological complete resection rate and recurrence rate, and safety profile of ESD with respect to EMR for early gastric cancer.
Most of the published literature in the field outlines the superior efficacy as well as the higher complication rate of ESD with respect to EMR.
This study provides an updated comprehensive comparison of therapeutic efficacy, procedural complexity and safety profile of the two treatments. Second, any possible source of heterogeneity and publication bias that could have influenced the final results was explored by means of appropriate statistical tools and all the findings were confirmed performing sensitivity analysis.
The results could be useful for endoscopists involved in gastric cancer management but they should be confirmed by large randomized controlled trials with long-term follow-up and should be validated both in Eastern and Western countries.
EGC is a malignant tumor confined to the mucosa or the submucosa regardless of lymph node metastases. EMR has been proposed as a replacement for invasive surgery because of favorable long-term outcomes and improved quality of life for patients. EMR is widely accepted because of its minimal invasion, low cost, patient tolerance and better quality of life after the operation. ESD was developed in the late 1990s to enable the en bloc removal of lesions larger than 2 cm.
This study is a meta-analysis to compare the efficacy outcomes between ESD and EMR for early gastric cancer. The authors describe that ESD shows a superior complete resection rate but higher complication rate with respect to EMR. It has already well known that ESD provide the higher curability but higher complication rate compared to EMR. However, I believe this paper can give gastroenterologists useful information in treatments of early gastric cancer, as a review article of meta-analysis of previously published data.
P- Reviewer: Naito Y, Oda I, Tohda G S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
| 1. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. |
| 2. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. |
| 3. | Jung HY. Extended approach of EMR/ESD in stomach cancer. J Korean Gastric Cancer Assoc. 2008;8:5-8. |
| 4. | Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin N Am. 2014;24:213-233. |
| 5. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. |
| 6. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. |
| 7. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration 2011. Available from: http://handbook.cochrane.org/. |
| 8. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. |
| 9. | Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics. 1986;42:311-323. |
| 10. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. |
| 11. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. |
| 12. | Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821-826. |
| 13. | Wan JK, Joo YC, Ik SC, Ik SC, In SJ, Bong MK, Su JJH, Chang BR, Jin OK, Joon SL. The result of endoscopic mucosal resection and endoscopic submucosal dissection of gastric tumors over 15 years. Gastrointest Endosc. 2007;65:AB97. |
| 14. | Shimura T, Yamada T, Sasaki M, Kataoka H, Joh T. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection for intramucosal gastric neoplasms. Gastrointest Endosc. 2007;65:AB169. |
| 15. | Watanabe K, Ogata S, Watanabe K, Kawazoe S, Tsunada S, Iwakiri R, Fujimoto K. Clinical outcomes of endoscopic mucosal resection for gastric tumors: historical comparison between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2005;61:AB244. |
| 16. | Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol. 2009;24:1102-1106. |
| 17. | Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-782. |
| 18. | Hoteya S, Iizuka T, Yahagi N. Feasibility of endoscopic submucosal dissection for early gastric cancer arising from remnant stomach, compared with conventional endoscopic mucosal resection. Gastrointest Endosc. 2008;67:AB277. |
| 19. | Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28:639-647. |
| 20. | Jee YS, Hwang SH, Rao J, Park DJ, Kim HH, Lee HJ, Yang HK, Lee KU. Safety of extended endoscopic mucosal resection and endoscopic submucosal dissection following the Japanese Gastric Cancer Association treatment guidelines. Br J Surg. 2009;96:1157-1161. |
| 21. | Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009;41:201-209. |
| 22. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. |
| 23. | Catalano F, Trecca A, Rodella L, Lombardo F, Tomezzoli A, Battista S, Silano M, Gaj F, de Manzoni G. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc. 2009;23:1581-1586. |
| 24. | Odashima M, Otaka M, Jin M, Wada I, Horikawa Y, Matsuhashi T, Ohba R, Hatakeyama N, Oyake J, Watanabe S. Improved curative resection rates of early gastric cancers by endoscopic mucosal resection (EMR) using endoscopic submucosal dissection method (ESD). Gastrointest Endosc. 2006;63:AB187. |
| 25. | Hoteya S, Iizuka T, Hashimoto M, Mizuno H, Otsuka T, Noguchi T, Kikuchi D, Hirayama Y, Kawano K, Yahagi N. The safety and efficacy of the endoscopic submucosal dissection for early gastric cancers,comparedwith conventional endoscopic mucosal resection. Gastrointest Endosc. 2007;65:AB358. |
| 26. | Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Clinical advantages of endoscopic submucosal dissection for gastric cancers in remnant stomach surpass conventional endoscopic mucosal resection. Dig Endosc. 2010;22:17-20. |
| 27. | Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746-750. |
| 28. | Watanabe T, Kume K, Taip M, Shibata M, Kubo H, Ejiri Y, Otsuki M. Gastric mucosal cancer smaller than 7mm can be treated with conventional endoscopic mucosal resection as effectively as with endoscopic submucosal dissection. Hepatogastroenterology. 2010;57:668-673. |
| 29. | Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130-136. |