Published online Jul 16, 2013. doi: 10.4253/wjge.v5.i7.346
Revised: April 27, 2013
Accepted: June 18, 2013
Published online: July 16, 2013
Processing time: 132 Days and 24 Hours
AIM: To evaluate the effects of choice of insertion route and ultrathin endoscope types.
METHODS: This prospective study (January-June 2012) included 882 consecutive patients who underwent annual health checkups. Transnasal esophagogastroduodenoscopy (EGD) was performed in 503 patients and transoral EGD in 235 patients using six types of ultrathin endoscopes. Patients were given a choice of insertion route, either transoral or transnasal, prior to EGD examination. For transoral insertion, the endoscope was equipped with a thin-type mouthpiece and tongue depressor. Conscious sedation was not used for any patient. EGD-associated discomfort was assessed using a visual analog scale (VAS; no discomfort 0- maximum discomfort 10).
RESULTS: Rates of preference for transnasal insertion were significantly higher in male (male/female 299/204 vs 118/117) and younger patients (56.8 ± 11.2 years vs 61.3 ± 13.0 years), although no significant difference was found in VAS scores between transoral and transnasal insertion (3.9 ± 2.3 vs 4.1 ± 2.5). Multivariate analysis revealed that gender, age, operator, and endoscope were independent significant predictors of VAS for transnasal insertion, although gender, age, and endoscope were those for transoral insertion. Further analysis revealed only the endoscopic flexibility index (EFI) as an independent significant predictor of VAS for transnasal insertion. Both EFI and tip diameter were independent significant predictors of VAS for transoral insertion.
CONCLUSION: Flexibility of ultrathin endoscopes can be a predictor of EGD-associated discomfort, especially in transnasal insertion.
Core tip: To evaluate the effects of choice of insertion route and ultrathin endoscope types for unsedated surveillance esophagogastroduodenoscopy (EGD), this prospective study was conducted including 882 consecutive patients who underwent annual health checkup using six types of ultrathin endoscopes in a single institute. EGD-associated discomfort was assessed using a visual analog scale (VAS) by patients themselves. Statistical analysis of VAS revealed the following two points; Transnasal insertion of ultrathin endoscopy for unsedated EGD can be preferable for younger males rather than elder females. Flexibility of ultrathin endoscopes can be a reliable predictor of reduction in transnasal EGD-associated discomfort rather than thinness of tip.
- Citation: Ono S, Niimi K, Fujishiro M, Nakao T, Suzuki K, Ohike Y, Kodashima S, Yamamichi N, Yamazaki T, Koike K. Ultrathin endoscope flexibility can predict discomfort associated with unsedated transnasal esophagogastroduodenoscopy. World J Gastrointest Endosc 2013; 5(7): 346-351
- URL: https://www.wjgnet.com/1948-5190/full/v5/i7/346.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i7.346
With improvements in resolution and image enhancement, gastrointestinal endoscopic technology has advanced considerably, detecting an increasing number of superficial neoplasms during surveillance esophagogastroduodenoscopy (EGD)[1-5]. New endoscopic treatments for superficial neoplasms, including endoscopic submucosal dissection, have been reported to be effective and less invasive compared with traditional open surgical exploration and treatment[6-10]. Against the backdrop of such concerns, importance of detecting them in early stage has been emphasized more than ever to achieve curative resection endoscopically.
Although identification of patients at high risk for superficial esophageal squamous cell carcinoma (SESCC) and early gastric cancer (EGC) has been reported as useful, diagnoses must still be confirmed by histopathological assessment of biopsy specimens obtained via endoscopy[11-13]. However, EGD-associated discomfort is a major problem for many patients, who are reluctant to undergo subsequent EGD procedures. Although sedation is possible for reduction of EGD-associated discomfort, cost and various adverse events associated with use of sedative agents must be considered among the risks and benefits of this option[14-17].
Use of an ultrathin endoscope may also reduce unsedated EGD-associated discomfort. Transnasal insertion of ultrathin endoscopes is reported to be a promising alternative in terms of patient satisfaction and cardiopulmonary function[18-21]. Although various types of ultrathin endoscopes are available at present, predictors of discomfort associated with EGD performed using ultrathin endoscopes have not been determined.
This prospective study was conducted to identify predictors of discomfort associated with unsedated EGD performed using ultrathin endoscopes.
This study was conducted at the Center for Epidemiology and Preventive Medicine of the University of Tokyo after receiving ethics committee approval. From January to June 2012, 882 consecutive patients who underwent annual health checkups were included in this study. Subjects were given a choice of insertion route, either transoral or transnasal, prior to EGD examination. The subjects were prepared for transnasal insertion using the modified spray method, which involves spraying 0.05% naphazoline nitrate into each nostril, followed by injection of a viscous gel of 2% lidocaine hydrochloride[22]. Conscious sedation was not used for any patient.
Six ultrathin endoscopes (A: GIF-XP260N, B: GIF-XP260NS, C: EG-530NW, D: EG-580NW, E: EG16-K10, and F: prototype EG17-K10) from three manufacturers (Olympus Corp., Tokyo, Japan; Fujifilm Holdings Corp., Tokyo, Japan; and Hoya Corp., Tokyo, Japan) were utilized in this study. Prototype EG17-K10 was equipped as part of a collaborative effort by the University of Tokyo Hospital and Hoya Corporation. Profiles of these endoscopies are shown in Table 1. All endoscopes were utilized for this study after being used for more than one hundred EGDs.
| A | B | C | D | E | F | |
| EFI (mm) | 224 | 192.4 | 175.2 | 174.8 | 146 | 166.6 |
| Tip diameter (mm) | 5 | 5.4 | 5.9 | 5.9 | 5.2 | 5.4 |
| Transnasal insertion | ||||||
| Insertion success rate | 58/59 | 110/112 | 119/123 | 112/118 | 47/47 | 57/57 |
| Nasal bleeding rate | 0/58 | 2/110 | 2/119 | 2/112 | 1/47 | 0/57 |
| VAS | 4.2 ± 2.7 | 4.0 ± 2.1 | 4.0 ± 2.4 | 4.0 ± 2.3 | 3.2 ± 2.2 | 3.8 ± 2.3 |
| Examination time (s) | 351.0 ± 58.8 | 345.8 ± 62.2 | 324.9 ± 61.1 | 340.0 ± 48.1 | 376.7 ± 61.7 | 349.1 ± 57.3 |
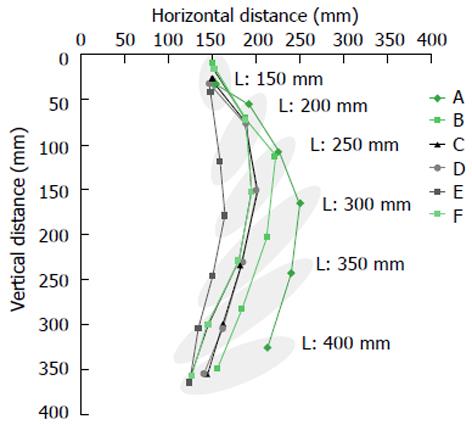
The flexibility of each endoscope was evaluated as follows. We fixed the middle portion of the endoscope to a flat surface, and allowed the tip of the endoscope to bend freely under the influence of gravity. After adjusting the length of endoscope from 150 to 400 mm allowed free movement under the influence of gravity, we mapped the position of the tip of the endoscope on a two dimensional grid. Continuous two-dimensional horizontal and vertical distances were plotted, as shown in Figure 1. The mean horizontal distances at the fixed points of 200, 250, 300, 350 and 400 mm were utilized as an endoscopic flexibility index (EFI) to provide a surrogate value of flexibility for each endoscope. Measurements of EFI for each endoscope were performed at room temperature.
The combination of endoscopes changed depending on the day of the week. Consequently, the patients were randomly allocated to six endoscope groups.
All examinations were performed by two operators who had been certified by the Japanese Gastroenterological Endoscopy Society. For transoral insertion, the endoscope was equipped with a thin-type mouthpiece and tongue depressor (Endo-leader; Top Corp.; Tokyo, Japan)[23]. In cases where transnasal insertion failed due to narrowness of nasal cavity or intolerable discomfort, transoral insertion was performed continuously after confirmation with the patient. After completion of the examination, EGD-associated discomfort was evaluated using a visual analogue scale (VAS) by patients themselves in another room from 0 to 10, which were minimum and maximum of discomfort respectively.
Parameters analyzed in this study were examination time and VAS score. Moreover, the insertion success rate and nasal bleeding rate were evaluated for each endoscope for transnasal insertion. Patients with a past history of surgical resection in the upper gastrointestinal tract and those in whom biopsy or another procedure had been performed were excluded from the analyses to avoid effects of these factors on examination time or VAS scores.
Statistical analyses were performed using Student’s t-test, χ2 test, and Fisher’s exact test. For multivariate analysis, the least-squares method was employed using dummy variables for nominal variables. All analyses were performed using JMP software (SAS Institute Inc., Cary, NC, United States). P < 0.05 was considered significant.
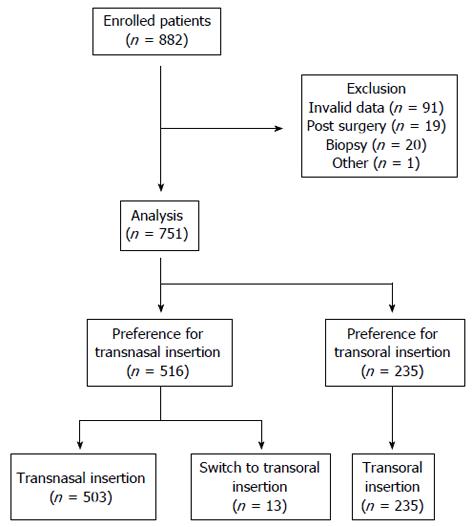
Among the 882 patients, 91 patients were excluded because of invalid responses or missing data. Thirty-nine patients were excluded because of past history of surgery in the upper gastrointestinal tract (n = 19) and biopsy during the examination (n = 20). One asymptomatic patient in whom anisakiasis was coincidentally discovered and who underwent endoscopy for removal of this parasite was also excluded from the analysis. In total, data of 751 patients were analyzed, as shown in Figure 2. Among them, 516 patients (68.7%) preferred transnasal insertion and 235 patients (31.3%) preferred transoral insertion. Thirteen patients who preferred transnasal insertion were switched to transoral insertion after failure of transnasal insertion. EGD was performed more than once in 665 patients (88.5%).
Characteristics of patients and outcomes are shown in Table 2. Rates of preference for transnasal insertion were significantly higher in male patients (male/female 299/204 vs 118/117 for transnasal vs transoral insertion, respectively; P < 0.05) and younger patients (56.8 ± 11.2 years vs 61.3 ± 13.0 years; P < 0.05). Examination time for transnasal insertion was significantly longer than that for transoral insertion, although no significant difference was found between VAS scores for transnasal and transoral insertion (3.9 ± 2.3 vs 4.1 ± 2.5; NS).
| Transnasal insertion(n = 503) | Transoral insertion(n = 235) | P value | |
| Gender M/F | 299/204 | 118/117 | < 0.05 |
| Age (yr) | 56.8 ± 11.2 (25-84) | 61.3 ± 13.0 (27-88) | < 0.05 |
| 1st examination Y/N | 54/449 | 19/216 | 0.29 |
| Operator A/B | 326/177 | 143/92 | 0.32 |
| Endoscope | 0.36 | ||
| A | 58 | 31 | |
| B | 110 | 52 | |
| C | 119 | 58 | |
| D | 112 | 61 | |
| E | 47 | 17 | |
| F | 57 | 16 | |
| Examination time (s) | 343.4 ± 59.4 (210-630) | 324.5 ± 59.8 (196-600) | < 0.05 |
| VAS | 3.9 ± 2.3 (0-10) | 4.1 ± 2.5 (0-10) | 0.90 |
For multivariate analysis of VAS scores, six parameters were employed: gender, age, experience of previous EGD, operator, type of endoscope, and examination time. Results of multivariate analysis of VAS scores for transnasal and transoral insertion are shown in Tables 3 and 4, respectively. For transnasal insertion, gender (positive correlation with female gender), age, operator, and endoscope (negative correlation with endoscope E) were independent significant predictors of VAS scores. On the other hand, gender (positive correlation with female gender), age, and endoscope (positive correlation with endoscope C) were independent significant predictors of VAS scores for transoral insertion.
| Parameter estimate ± SE | P value | |
| Gender (F) | 0.780 ± 0.100 | < 0.05 |
| Age | -0.0193 ± 0.00886 | < 0.05 |
| 1st examination (N) | 0.252 ± 0.160 | 0.12 |
| Operator (A) | -0.341 ± 0.110 | < 0.05 |
| Scope (E) | -0.719 ± 0.281 | < 0.05 |
| Examination time | 0.00270 ± 0.00180 | 0.134 |
| Parameter estimate ± SE | P value | |
| Gender (F) | 0.575 ± 0.156 | < 0.05 |
| Age | -0.0343 ± 0.0125 | < 0.05 |
| 1st examination (N) | -0.00289 ± 0.294 | 0.99 |
| Operator (A) | -0.297 ± 0.177 | 0.10 |
| Scope (C) | 0.634 ± 0.313 | < 0.05 |
| Examination time | -0.00159 ± 0.00291 | 0.59 |
Multivariate analysis was also performed using EFI and tip diameter as alternative features of the endoscopes. Although both EFI and tip diameter were independent significant predictors of VAS scores for transoral insertion, only EFI was an independent significant predictor of VAS scores for transnasal insertion as shown in Table 5.
| Transnasal insertion | Transoral insertion | |
| EFI | 0.0125 ± 0.00563 (P < 0.05) | 0.0212 ± 0.00966 (P < 0.05) |
| Tip diameter | 0.450 ± 0.338 (P = 0.18) | 1.33 ± 0.561 (P < 0.05) |
The appropriate usage of ultrathin endoscopes in the transoral and transnasal insertion techniques remains controversial[24]. In addition, although various ultrathin endoscopes are presently available, predictors of EGD-associated discomfort are unclear. This study demonstrated that both tip diameter and flexibility of ultrathin endoscopes can be predictors in reducing EGD-associated discomfort, especially for transnasal insertion.
Greater flexibility of the endoscope may lead to poorer handleability, resulting in prolonged examination time, which may in turn increase the discomfort accompanying EGD. However, although the most flexible endoscope (endoscope E) in this study required the longest examination time among the six endoscopes, VAS scores were lowest for EGD using this endoscope for transnasal insertion. This result indicates that prolonging the examination for a certain amount of time may be acceptable in terms of the level of tolerable discomfort.
In a high proportion of regular patients in this study, EGD had been periodically performed in the past. Almost all patients selected the insertion route based on their experience with discomfort in previous examinations. Consequently, although no significant difference in VAS scores was observed between transoral and transnasal insertion, patient characteristics and preferences showed their propensity for discomfort with either one technique or the other. Table 2 shows the trend toward preference for transnasal insertion among males and younger patients. We speculate that younger patients preferred transnasal insertion to suppress a stronger gagging reflex that is reported by Enomoto et al[25]. By contrast, smaller female patients may have preferred transoral insertion because of their narrower nasal cavities, which are more prone to discomfort caused by transnasal insertion. However, VAS scores are reported to be affected by gender[26]. Additionally, there might be a gender deference in diminishing of gagging reflex or nasal pain by aging. We need further accumulation of data for appropriate insertion route in each gender or age-groups.
One limitation of this study is its unequal allocation of patients to each endoscope because of the system utilized in our institute. Moreover, the objectivity and reproducibility of VAS and EFI are questionable. EFI is affected by the weight of the endoscope, whose mass/length is not homogenous. However, this parameter can be a surrogate marker that can be evaluated simply and non-destructively.
In summary, this study demonstrated that flexibility of the ultrathin endoscope can be a reliable predictor of reduction in transnasal EGD-associated discomfort. Although further analysis of details concerning appropriate location and degree of flexibility is required, patient compliance can be improved for follow-up and surveillance EGD by utilizing less uncomfortable tools.
As gastrointestinal endoscopic technology has advanced considerably with improvements in resolution and image enhancement, importance of surveillance esophagogastroduodenoscopy (EGD) to detect superficial neoplasms in early stage has been emphasized more than ever to achieve curative resection.
Although EGD using an ultrathin endoscope has been accepted as a minimally invasive modality, the effects of choice of insertion route and ultrathin endoscope types have not been evaluated.
The authors’ study using six types of ultrathin endoscopes demonstrated that flexibility of the ultrathin endoscope can be a reliable predictor of reduction in transnasal EGD-associated discomfort.
To decrease unsedated EGD-associated discomfort, transnasal insertion of ultrathin endoscopy should be chosen for younger males. A flexible ultrathin endoscope can reduce transnasal EGD-associated discomfort for the other people.
Endoscopic flexibility index is a surrogate marker that can be evaluated simply and non-destructively.
This is the first report of comparison of the difference between several models of ultrathin endoscope. The conclusion that the discomfort is associated with the flexibility of the endoscope is a novel and unique.
P- Reviewers Nishiwaki S, Deutsch JC, Herrerias-Gutierrez JM S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288-295. |
| 2. | Kuraoka K, Hoshino E, Tsuchida T, Fujisaki J, Takahashi H, Fujita R. Early esophageal cancer can be detected by screening endoscopy assisted with narrow-band imaging (NBI). Hepatogastroenterology. 2009;56:63-66. |
| 3. | Yoshizawa M, Osawa H, Yamamoto H, Kita H, Nakano H, Satoh K, Shigemori M, Tsukui M, Sugano K. Diagnosis of elevated-type early gastric cancers by the optimal band imaging system. Gastrointest Endosc. 2009;69:19-28. |
| 4. | Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010;16:1043-1049. |
| 5. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. |
| 6. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. |
| 7. | Yahagi N, Fujishiro M, Kakushima N, KobayashiK , Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Dig Endosc. 2004;16:34-38. |
| 8. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. |
| 9. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. |
| 10. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688-694. |
| 11. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. |
| 12. | Mukoubayashi C, Yanaoka K, Ohata H, Arii K, Tamai H, Oka M, Ichinose M. Serum pepsinogen and gastric cancer screening. Intern Med. 2007;46:261-266. |
| 13. | Yokoyama A, Kumagai Y, Yokoyama T, Omori T, Kato H, Igaki H, Tsujinaka T, Muto M, Yokoyama M, Watanabe H. Health risk appraisal models for mass screening for esophageal and pharyngeal cancer: an endoscopic follow-up study of cancer-free Japanese men. Cancer Epidemiol Biomarkers Prev. 2009;18:651-655. |
| 14. | Freeman ML. Sedation and monitoring for gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 1994;4:475-499. |
| 15. | Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317-322. |
| 16. | Trevisani L, Sartori S, Gaudenzi P, Gilli G, Matarese G, Gullini S, Abbasciano V. Upper gastrointestinal endoscopy: are preparatory interventions or conscious sedation effective A randomized trial. World J Gastroenterol. 2004;10:3313-3317. |
| 17. | Yi SY, Shin JE. Midazolam for patients undergoing upper gastrointestinal endoscopy: a prospective, single-blind and randomized study to determine the appropriate amount and time of initiation of endoscopy. J Gastroenterol Hepatol. 2005;20:1873-1879. |
| 18. | Yagi J, Adachi K, Arima N, Tanaka S, Ose T, Azumi T, Sasaki H, Sato M, Kinoshita Y. A prospective randomized comparative study on the safety and tolerability of transnasal esophagogastroduodenoscopy. Endoscopy. 2005;37:1226-1231. |
| 19. | Hayashi Y, Yamamoto Y, Suganuma T, Okada K, Nego M, Imada S, Imai M, Yoshimoto K, Ueki N, Hirasawa T. Comparison of the diagnostic utility of the ultrathin endoscope and the conventional endoscope in early gastric cancer screening. Dig Endosc. 2009;21:116-121. |
| 20. | Toyoizumi H, Kaise M, Arakawa H, Yonezawa J, Yoshida Y, Kato M, Yoshimura N, Goda K, Tajiri H. Ultrathin endoscopy versus high-resolution endoscopy for diagnosing superficial gastric neoplasia. Gastrointest Endosc. 2009;70:240-245. |
| 21. | Yuki M, Amano Y, Komazawa Y, Fukuhara H, Shizuku T, Yamamoto S, Kinoshita Y. Unsedated transnasal small-caliber esophagogastroduodenoscopy in elderly and bedridden patients. World J Gastroenterol. 2009;15:5586-5591. |
| 22. | Abe K, Miyaoka M. Trial of transnasal esophagogastroduodenoscopy. Dig Endosc. 2006;18:212-217. |
| 23. | Kataoka H, Hayano J, Mizushima T, Tanaka M, Kubota E, Shimura T, Mizoshita T, Tanida S, Kamiya T, Nojiri S. Cardiovascular tolerance and autonomic nervous responses in unsedated upper gastrointestinal small-caliber endoscopy: a comparison between transnasal and peroral procedures with newly developed mouthpiece. Dig Endosc. 2011;23:78-85. |
| 24. | Miyake K, Kusunoki M, Ueki N, Yamada A, Nagoya H, Kodaka Y, Shindo T, Kawagoe T, Gudis K, Futagami S. Classification of patients who experience a higher distress level to transoral esophagogastroduodenoscopy than to transnasal esophagogastroduodenoscopy. Dig Endosc. 2013;25:397-405. |
| 25. | Enomoto S, Watanabe M, Yoshida T, Mukoubayashi C, Moribata K, Muraki Y, Shingaki N, Deguchi H, Ueda K, Inoue I. Relationship between vomiting reflex during esophagogastroduodenoscopy and dyspepsia symptoms. Dig Endosc. 2012;24:325-330. |
| 26. | Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156-160. |