Published online May 16, 2013. doi: 10.4253/wjge.v5.i5.226
Revised: March 5, 2013
Accepted: March 15, 2013
Published online: May 16, 2013
Processing time: 242 Days and 21.9 Hours
AIM: To describe colon anatomy with colonoscopy and computed tomography (CT) to develop a rat model for future studies of therapeutic colonoscopy.
METHODS: Eighteen male Sprague-Dawley rats, on average 400-420 g, underwent total colonoscopy, CT and histological examination. Colonoscopy was performed after bowel preparation with a baby upper gastrointestinal endoscopy with an outer diameter of 6.7 mm. CT obtained a 3D image of total colon after a rectal enema with radiological contrast. Macroscopic and microscopic examinations were examined with a conventional technique (hematoxylin and eosin). Colonic wall thickness, length and diameter measurements were taken from the anus, 3, 7, 14 and 20 cm from the anal margin.
RESULTS: The median colonoscope depth was 24 cm (range 20-28 cm). Endoscopic and tomographic study of colon morphology showed an easy access with tubular morphology in the entire left colon (proximal left colon and rectum). Transverse colon was unapparent on colonoscopy. Right colon, proximal to the splenic flexure, was the largest part of the colon and assumed saccular morphology with tangential trabecula. Radiological measurements of the colonic length and diameter substantiate a subdivision of the right colon into two parts, the cecum and distal right colon. In addition, histological measurement of the colonic wall thickness confirmed a progressive decrease from rectum to cecum. The muscular layer was thinner in the proximal left colon.
CONCLUSION: The combination of colonoscopy, tomography and histology leads to a better characterization of the entire colon. These data are important for deciding when to perform endoscopic resections or when to induce perforations to apply endoscopic treatments.
Core tip: There is a need for a solid colonoscopy animal model, complemented with digital radiology. Our subdivision of the rat colon constitutes a simplification of subdivisions presented by others who have emphasized the theoretical anatomical data. Our proposed subdivision of the colon is practical and justified by the importance of endoscopic access and the thickness of various portions of the colon wall. This study identified that the muscular layer was thinner in the proximal left colon. These findings are important for deciding when to perform endoscopic resections or when to induce perforations to apply endoscopic treatments.
- Citation: Bartolí R, Boix J, Òdena G, De la Ossa ND, de Vega VM, Lorenzo-Zúñiga V. Colonoscopy in rats: An endoscopic, histological and tomographic study. World J Gastrointest Endosc 2013; 5(5): 226-230
- URL: https://www.wjgnet.com/1948-5190/full/v5/i5/226.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i5.226
The rat is widely used as a laboratory animal for medical biological and molecular research. The anatomy and topography of the rat colon have been described on the basis of macroscopic and conventional radiological observations on the whole animal[1]. Radiology is useful for studying normal arterial and mucosal anatomy of the explanted rat colon. In contrast, in clinical practice, endoscopy is one of the keystone diagnostic techniques allowing follow-up and management of gastrointestinal inflammation[2-9]. Interestingly, there are no detailed endoscopic descriptions of the gross anatomy of the colon by total colonoscopy (TC)[10-19]. Significant progress in endoscopic techniques has been made in the last years. There is a need for a solid colonoscopy animal model, complemented with digital radiology. The aim of the present study was to describe the colon anatomy with high-definition colonoscopy and computed tomography (CT) to develop a rat model for future studies of therapeutic colonoscopy.
Eighteen male Sprague-Dawley, on average 400-420 g, were used in this study. Rats were acclimatized for a minimum of 7 d preoperatively. Rats were kept at constant room temperature (20-22 °C) with a relative humidity (27%-31%) with aeration under an alternating 12 h cycle of fluorescent light and darkness. The rats were housed individually in polycarbonate box cages with free access to water and food (Teklad Global 2014, Harlan Laboratories Models SL, Barcelona, Spain). Rats are the smallest and lowest among the species suitable for TC. The rats suffered minimal pain and distress due to the use of anesthesia. The protocol was approved by the Institutional Animal Care and Use Committee of Hospital Universitari Germans Trias i Pujol.
The animals had free access to water but food was withdrawn 8 h prior to the initiation of bowel preparation. A rectal enema with saline solution was performed immediately prior to TC[20].
Colonoscopy was performed with a baby upper gastrointestinal Olympus GIF-XP160 video endoscope with an outer diameter of 6.7 mm and a 2.3 mm working channel (Olympus, Tokyo). After a 24 h fasting period with free access to drinking water, the rats were anesthetised by isofluorane inhalation (1.5% with 98% O2) and placed in a supine position. Remaining feces were flushed away by injecting water through the anus. A drop of lubricating jelly (Aquagel®, Ecolab, Leeds, England) was applied on the anal sphincter to facilitate insertion of the scope. The endoscope was then gently passed through the anus and under endoscopic vision further introduced. Water was injected through the scope’s working channel to visualize the lumen of the colon. Occasionally the colon was inflated with air for better visualisation of the lumen. The tip of the endoscope was introduced to the cecum, about 24 cm proximal from the anus. Pictures were captured in each procedure. Rats were placed under surveillance during recovery and were returned to their cages when regaining consciousness.
In vivo X-ray Microtomograph (SkyScan 2002, Aartselaar, Belgium) was used in order to obtain a 3D image of total colon. Briefly, animals were anesthesized with isofluorane, 20 mL of radiological contrast (Plenigraf®, Juste, Madrid, Spain) was administered through a rectal enema and then the animals were placed in the scanning area. Acquisition images for 3D reconstruction of the whole colon lasted over 40 min with a resolution of 32 μm.
Rats were sacrificed 48 h after colonoscopy by anesthetic overdose (60 mg pentobarbital, ip). After sacrifice, the colon was collected and rinsed with ice-cold Krebs solution. The colon was opened longitudinally and pinned out on a Petri dish to examine the colonic mucosa. The mucosal surface of the distal colon was inspected with a binocular microscope (Harvard Apparatus, Panlab, Barcelona, Spain).
Full-thickness samples of approximately 1 cm were taken from anus, 3, 7, 14 and 20 cm from the anal margin. Segments were fixed in 4% formaldehyde for 24 h, embedded in paraffin and cross sections of 5 μm were stained with hematoxylin and eosin. Histological sections were examined using a conventional microscope (Olympus, Shinjuku-ku, Tokyo, Japan).
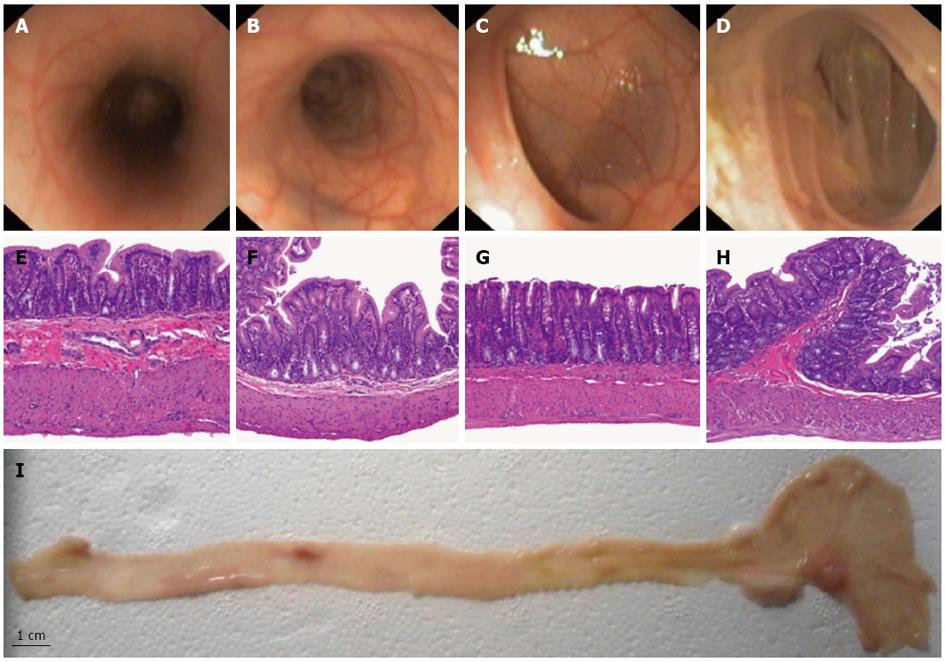
Bowel preparation resulted in complete evacuation of stools in the left colon. In the right colon we found one solid stool and liquid feces that were flushed away. The median colonoscope depth was 24 cm (range 20-28 cm). Confirmation of cecal intubation during colonoscopy was achieved by transillumination through the abdominal wall. The appendiceal orifice was not identified in any case. The scope reached the splenic flexure easily and the tube went straight up from the anus so that the entire left colon (proximal left colon and rectum) assumed a tubular morphology. The transverse colon was unapparent on colonoscopy. The right colon, proximal to the splenic flexure including the cecum, was the largest part of the colon and assumed saccular morphology with tangential trabecula. Splenic and liver impronta were evident in both flexures. Mucosal and vascular pattern were similar in the left and distal right colon. In the cecum, the mucosal surface of the insufflated colon presents folds (Figure 1).
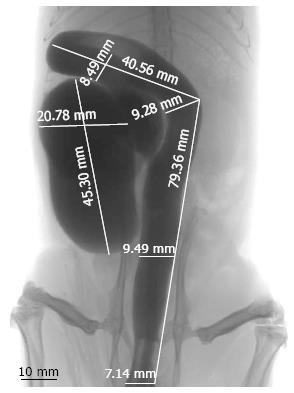
Radiological measurements of the colonic length and diameter substantiate a subdivision of the right colon into two parts, the cecum and distal right colon (Figure 2). The cecum of the rat is 54.6 ± 22.1 mm long, is the most prominent part of the colon and assumes a sack form with a major diameter measurement of 20.78 ± 1.88 mm. The cecum does not have a vermiform appendix. Straight down from the cecum, the colon has a tubular morphology, curved in the distal right colon and linear in the left colon. The distal right colon is 89.3 ± 17.6 mm long with a diameter of 8.49 ± 0.29 mm. The left colon in the rat is 95.4 ± 13.5 mm long, with a diameter of 9.28 ± 0.37 mm in the proximal part and 8.05 ± 0.39 mm in the distal part (Table 1).
| Measurements | Left colon (distal to splenic flexure) | Right colon | ||
| Rectum | Proximal left colon | Distal right colon | Cecum | |
| Distance to anal margin (mm) | 0-45.6 ± 8.4 | 45.6 ± 8.4 - 95.4 ± 13.5 | 95.4 ± 13.5 - 184.7 ± 20.1 | 184.7 ± 20.1-244.3 ± 25.8 |
| Length (mm) | 45.6 ± 8.4 | 49.8 ± 10.5 | 89.3 ± 17.6 | 54.6 ± 22.1 |
| Macroscopic diameter (mm) | 7.79 ± 0.46 | 8.18 ± 0.28 | 8.01 ± 0.32 | 20.11 ± 2.31 |
| Radiological diameter (mm) | 8.05 ± 0.39 | 9.28 ± 0.37 | 8.49 ± 0.29 | 20.78 ± 1.88 |
| Full-thickness samples to anal margin (cm) | 3 | 7 | 14 | 20 |
| Wall thickness (μm) | 658.3 ± 50.7 | 600.0 ± 58.6 | 562.5 ± 21.6 | 550.0 ± 49.2 |
| Muscular thickness (μm) | 229 ± 42.9 | 118.8 ± 11.3 | 170.0 ± 33.6 | 140.0 ± 24.3 |
| Mucosal thickness (μm) | 283.0 ± 25.6 | 289.0 ± 23.4 | 256.3 ± 16.0 | 260.0 ± 22.8 |
Histological measurement of the colonic wall thickness and description of the mucosal pattern substantiates a subdivision of the colon into 4 parts (cecum, distal right colon, proximal left colon and rectum). The wall thickness progressively decreases from the rectum to cecum, whereas the muscular layer was thinner in the proximal left colon (Table 1 and Figure 1).
The present study successfully described the rat colon anatomy to develop a rat model for future studies of therapeutic colonoscopy. Endoscopic and tomographic study of colon morphology showed an easy access with tubular morphology in the entire left colon. The right colon does not assume a linear morphology, the cecum being the most prominent part. In addition, histological measurement of the colonic wall thickness confirmed a progressive decrease from rectum to cecum. The muscular layer was thinner in the proximal left colon. These findings are important for deciding when to perform endoscopic resections or when to induce perforations to apply endoscopic treatments because of the effects of thermal injury and coagulation necrosis of the muscularis propria and serosa. Colonoscopic perforation is a potentially life-threatening complication with an incidence rate ranging from 0.07% to 0.1% in diagnostic and therapeutic colonoscopies, respectively[21,22].
Our anatomical description differs from that of others[23] because in colonoscopy the colonic inflexions are much less evident. Currently available animal models in rats for endoscopy research contain inherent flaws, fail to meet the criteria for extrapolation to humans, and therefore are unlikely to be valid. The description of the normal anatomy is an indispensable premise in experimental therapeutic endoscopy. Proposals have been made to distinguish subdivisions of the rat colon resembling those used for human anatomy with conventional radiology[1,24]. A solution was presented 15 years ago when Hull et al[10] showed that it was feasible to perform bowel preparation and TC on rats. The authors successfully performed TC with a pediatric bronchoscope. Colonoscopy limited to the splenic flexure in rats has been previously reported[10]. Confirmation that the cecum was reached was done by visualizing liquid stool which was present only in the cecum in all rats[25,26].
Our subdivision of the rat colon constitutes a simplification of subdivisions presented by others[11] who have emphasized the theoretical anatomical data. Our proposed subdivision of the colon is practical and justified by the importance of endoscopic access and the thickness of various portions of the colon wall.
In conclusion, a reproducible rat model has been achieved. These data are important for deciding when to perform endoscopic resections or when to induce perforations to apply endoscopic treatments.
Significant progress in endoscopic techniques has been made in the last years. There is a need for a solid colonoscopy animal model, complemented with digital radiology.
The present study successfully described the rat colon anatomy to develop a rat model for future studies of therapeutic colonoscopy.
The subdivision of the rat colon constitutes a simplification of subdivisions presented by others who have emphasized the theoretical anatomical data. The proposed subdivision of the colon is practical and justified by the importance of endoscopic access and the thickness of various portions of the colon wall. This study identified that the muscular layer was thinner in the proximal left colon.
These findings are important for deciding when to perform endoscopic resections or when to induce perforations to apply endoscopic treatments.
Colonoscopy is an invasive technique that permits a direct visualization of the colon mucosa. Computed tomography is a radiology technique that allows obtaining 3D images.
The authors described the rat colon anatomy to develop a rat model for future studies of therapeutic colonoscopy. Endoscopic and tomographic study of colon morphology showed an easy access with tubular morphology in the complete left colon. Otherwise, the results identified that the muscular layer was thinner in the proximal left colon.
P- Reviewers ChoYS, Lohsiriwat V, Teramoto-Matsubara OT S- Editor Song XX L- Editor Roemmele A E- Editor Zhang DN
| 1. | Pomerri F, Gasparini G, Martin A, Fries W, Pagiaro E, Merigliano S. Microradiographic anatomy of the explanted rat colon. Acta Radiol. 1995;36:210-214. |
| 2. | Daperno M, Sostegni R, Lavagna A, Crocellà L, Ercole E, Rigazio C, Rocca R, Pera A. The role of endoscopy in inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2004;8:209-214. |
| 3. | Mittal A, Saha MM, Pandey KK. Peroral pneumo colon--a double contrast technique to evaluate distal ileum and proximal colon. Australas Radiol. 1990;34:72-74. |
| 4. | Desaga JF. Visualization of the mucosal villi on double-contrast barium studies of the small intestine by using a high molecular fraction of guaran. Gastrointest Radiol. 1989;14:25-30. |
| 5. | Fischer HW. The need for more study of gastrointestinal contrast media. Invest Radiol. 1980;15:S148-S150. |
| 6. | Lindström CG, Rosengren JE, Fork FT. Colon of the rat. An anatomic, histologic and radiographic investigation. Acta Radiol Diagn (Stockh). 1979;20:523-536. |
| 7. | Rubesin SE, Levine MS, Laufer I, Herlinger H. Double-contrast barium enema examination technique. Radiology. 2000;215:642-650. |
| 8. | Gelfand DW, Ott DJ, Chen YM. Optimizing single- and double-contrast colon examinations. Crit Rev Diagn Imaging. 1987;27:167-201. |
| 9. | Charpentier C, Marion-Letellier R, Savoye G, Nicol L, Mulder P, Aziz M, Vera P, Déchelotte P, Savoye-Collet C. Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm Bowel Dis. 2012;18:1940-1949. |
| 10. | Hull CC, Stellato TA, Ament AA, Gordon N, Galloway P. Endoscopic and radiographic evaluation of the murine colon. Cancer. 1990;66:2528-2532. |
| 11. | Haughn C, Uchal M, Raftopoulos Y, Rossi S, Santucci T, Torpey M, Pollice A, Yavuz Y, Marvik R, Bergamaschi R. Development of a total colonoscopy rat model with endoscopic submucosal injection of the cecal wall. Surg Endosc. 2006;20:270-273. |
| 12. | Hull CC, Ament AA, Gordon N, Galloway P, Hawkins N, Stellato TA. Endoscopic and roentgenographic evaluation of the colons of rats. Curr Surg. 1988;45:465-468. |
| 13. | Nauss KM, Locniskar M, Pavlina T, Newberne PM. Morphology and distribution of 1,2-dimethylhydrazine dihydrochloride-induced colon tumors and their relationship to gut-associated lymphoid tissue in the rat. J Natl Cancer Inst. 1984;73:915-924. |
| 14. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. |
| 15. | Ellis KK, Fennerty MB. Marking and identifying colon lesions. Tattoos, clips, and radiology in imaging the colon. Gastrointest Endosc Clin N Am. 1997;7:401-411. |
| 16. | Karas JR, Essani R, Haughn C, Uchal M, Bishawi MM, Bergamaschi R. Colonoscopic injection for murine solid cecal cancer model. Surg Endosc. 2011;25:2956-2959. |
| 17. | Mukai M, Tajima T, Oida Y, Kishima K, Nakamura M, Makuuchi H. Experimental model of two-colonoscope surgery for superficially spreading colonic tumors larger than 3 cm in the right colon. Oncol Rep. 2007;18:629-632. |
| 18. | Hurlstone DP, Sanders DS, Thomson M, Cross SS. “Salvage” endoscopic mucosal resection in the colon using a retroflexion gastroscope dissection technique: a prospective analysis. Endoscopy. 2006;38:902-906. |
| 19. | Allendorf JD, Bessler M, Whelan RL. A murine model of laparoscopic-assisted intervention. Surg Endosc. 1997;11:622-624. |
| 20. | Bartolí R, Boix J, Odena G, de Vega Moreno V, Lorenzo-Zúñiga V. Determination of the ideal preparation for colonoscopy in a rat model. Surg Laparosc Endosc Percutan Tech. 2012;22:542-545. |
| 21. | Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418-3422. |
| 22. | Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230-236. |
| 23. | Lorenzo-Zúñiga V, Moreno de Vega V, Doménech E, Mañosa M, Planas R, Boix J. Endoscopist experience as a risk factor for colonoscopic complications. Colorectal Dis. 2010;12:e273-e277. |
| 24. | DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39:209-228. |
| 25. | Hamilton SR, Zhang SZ, O’Ceallaigh D, McAvinchey D. Growth characteristics of autochthonous experimental colonic tumors as assessed by serial colonoscopic measurement in rats. Gastroenterology. 1986;91:1511-1520. |
| 26. | Narisawa T, Wong CQ, Weisburger JH. Evaluation of endoscopic examination of colon tumors in rats. Am J Dig Dis. 1975;20:928-934. |