Published online Jan 16, 2013. doi: 10.4253/wjge.v5.i1.29
Revised: October 2, 2012
Accepted: November 2, 2012
Published online: January 16, 2013
Processing time: 164 Days and 16.4 Hours
AIM: To determine if surgical knotting performed via endoscopy is an effective closure method for natural orifice translumenal endoscopic surgery.
METHODS: The proposed method was tested on an in vitro pig stomach model using standard endoscopy suite materials. A single use laparoscopy trocar (Versaport Plus manufactured by Tyco Healthcare) was fixed onto a plastic rectangular box in a horizontal position. A fresh pig stomach was tightly attached via its esophageal end to the trocar opening on the inner side of the box. The stomach cavity was closed at the duodenal end with Kocher forceps. A standard upper gastrointestinal endoscope fitted at its tip with a transparent plastic cap was introduced into the stomach through the outer trocar opening, so that the passage of the surgical trocar would mimic the passage of an esophagus. The stomach was subsequently inflated, followed by irrigation and washing. A neutral electrode of an electrocautery unit was placed inside the plastic box, underneath the pig stomach. The stomach’s outer surface was kept moist using normal saline in order to maintain the natural elasticity and to ensure good contact with the electrode.
RESULTS: The submucosal space on the anterior face of the stomach was accessed using the technique of endoscopic submucosal dissection. First, a site on the anterior face of the stomach was chosen, near the angle. Then, saline was injected into the submucosa with a standard endoscopic needle, so as to create a 20 mm diameter elevation. A linear 15 mm vertical incision was created at its center using a Dual Knife (KD650U manufactured by Olympus). This incision was used to access the submucosal space, and about 10 mm was dissected on both sides of the incision. The endoscope was then pushed through to the outside of the stomach after dilating a small puncture made by the Dual Knife in the muscularis propria, which simulated the peritoneoscopy procedure. Then, a 0.025” guidewire (Jagwire/450 cm manufactured by Boston Scientific) was inserted into the puncture, followed by a dilating balloon (Quantum TT manufactured by Cook Medical) that was used to enlarge the aperture orifice. After withdrawing the scope back into the stomach, the procedure continued with guidewires being passed from the submucosal space into the gastric lumen through small orifices on the left and right sides of the mucosal opening. These orifices were made with the Dual Knife, and the guidewires were inserted via a guiding catheter (HGC-6 manufactured by Cook Medical). As the guidewires were pulled outside of the stomach, they were replaced with a single surgical suture that had been initially attached to their tip and was now untied. Finally, one loop of this surgical suture was formed on the exterior. One loop end was fixed while the opposite suture end was pulled by biopsy forceps through the endoscope channel as the scope was inserted into the stomach. The loop was advanced until it approached and fixed the two mucosal incision margins. Three alternating loops were made in this manner to create a genuine tight surgical knot.
CONCLUSION: Endoscopic knotting of the gastric wall is feasible, but an in vitro survival study is necessary to validate clinical significance.
-
Citation: Ciocirlan M, Ionescu ME, Diculescu MM. Endoscopic knot tying:
In vitro assessment in a porcine stomach model. World J Gastrointest Endosc 2013; 5(1): 29-33 - URL: https://www.wjgnet.com/1948-5190/full/v5/i1/29.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i1.29
The concept of natural orifice translumenal endoscopic surgery (NOTES) was introduced in 2004, when Kaloo et al[1] reported a successful transgastric peritoneoscopy performed in an in vivo porcine model. Since then, the variety of NOTES interventions using the porcine survival model has expanded to include splenectomy[2], gastrojejunostomy[3], hysterectomy[4], ligation of fallopian tubes[5], oophorectomy[6,7], cholecystectomy[8], appendectomy[9], hernia repair[10], pancreatectomy[11], and lymphadenectomy[12]. Human trials are currently under way[13].
From the beginning, two of the main scientific endoscopic societies have been involved in assessing and promoting research related to the NOTES procedures, namely the North American Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR) group[14] and the European EURO-NOTES group[15]. In 2006, NOSCAR published a White Paper outlining twelve critical features that can impact the safety of NOTES to guide its appropriate usage and highlighted the need for increased research and analysis of data[16]. Gastric (intestinal) closure was designated as a very important area of research, and the group mandated a strict objective of the NOTES procedure to achieve closure with absolutely no leaks.
To date, the reported closure methods for the various NOTES interventions have used dedicated suture and anchor tools[17], such as T tags[18], purse string-modified T tags[19], Eagle Claw VIII[20], flexible endoscopic stapler[21], purse string suturing device[22], and flexible Endostitch[17]. All of these devices are cumbersome and have not yet received approval for use in clinical settings.
Therefore, this study was designed to investigate the feasibility of performing a surgical suture of a stomach opening by using common endoscopy devices.
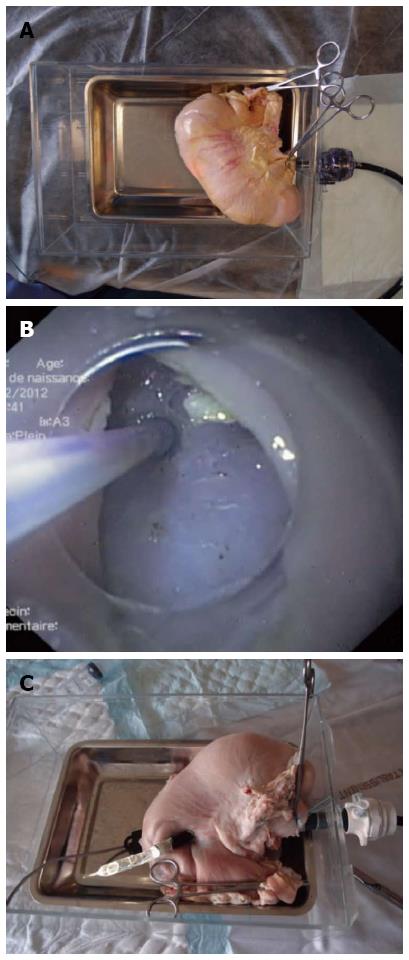
A modified version of the in vitro porcine stomach model described by Hon et al[23] was used. Briefly, a trocar with radiolucent sleeve and 10-15 mm seal (Versaport Plus; Tyco Healthcare, Gosport, United Kingdom) was fixed onto a plastic rectangular box. A fresh pig stomach was tightly attached to the trocar on the inner side of the box via the esophageal opening. The duodenum was closed with a pair of Kocher forceps (Figure 1A). A standard gastroscope (GIF 160; Olympus, Rungis, France) fitted with a transparent straight plastic cap was inserted through the trocar (emulating passage through the esophagus) into the lumen of the stomach. The lumen was inflated and the procedure was performed as detailed in the Results.
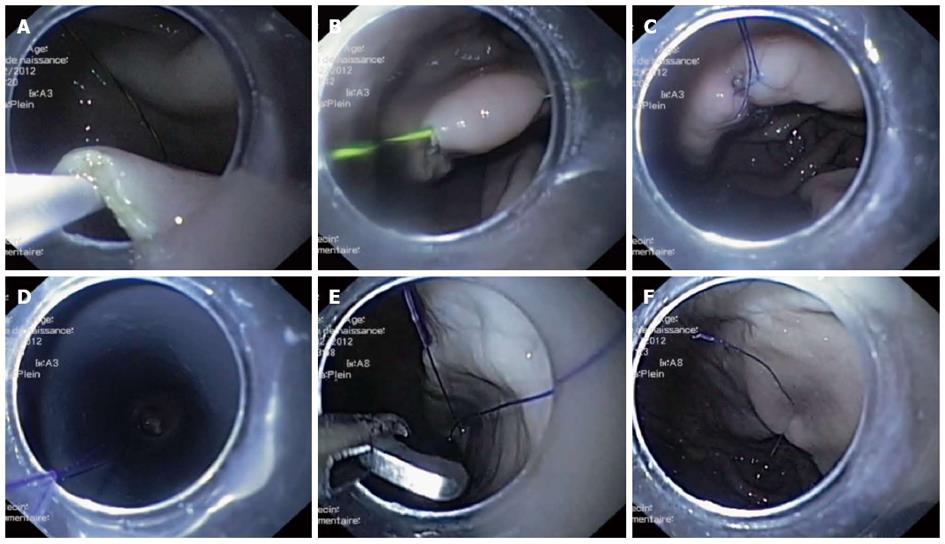
The gastroscope-assisted knotting procedure was carried out with the following nine steps: (1) A 20 mm gastric submucosal bleb was created by injecting saline (25G 1-JectS; ABS Bolton Medical, Saint Michel/Meurthe, France) into the anterior inner face of the stomach, near the angle. A 15 mm linear incision was then made at the top of the submucosal elevation using a Dual Knife (KD650U; Olympus) coupled with a standard electrosurgical unit (Erbotom ICC200; ERBE, Tübingen, Germany); (2) The submucosal space was dissected at about 10 mm on both sides of the incision by introducing the cap-fitted endoscope inside the submucosal space (Figure 1B); (3) Peritoneoscopy was performed by the standard technique[1]. First, the Dual Knife was used to puncture the muscular layer from the submucosal space into the middle of the initial incision. A 0.025” guidewire (Jagwire/450 cm; Boston Scientific, Nanterre, France) was introduced into this orifice, followed by a 10 mm dilating balloon (Quantum TT; Cook Medical, Charenton le Pont, France) that was inflated to facilitate the scope’s passage out of the stomach (Figure 1C). Finally, the balloon was deflated and the scope was retracted into the stomach; (4) On one incision side, a puncture was made in the mucosa from the submucosal space towards the lumen. A guiding catheter (HGC-6; Cook Medical) was introduced into this puncture to facilitate introduction of a 0.025” guidewire on the luminal side of the mucosa, traversing into the gastric lumen (Figure 2A); (5) After creating several loops in the stomach with the guidewire from Step (4), the endoscope was withdrawn, leaving the guidewire in place, and then reintroduced near it. The guidewire’s distal end was captured with forceps (Radial Jaw; Boston Scientific) and pulled outside of the stomach (Figure 2B). Both ends of the guidewire were now outside the stomach, with the guidewire passing through an orifice from the submucosal space into the gastric lumen; (6) A 120 cm 4-0 surgical suture (Prolene; Ethicon, Issy les Moulineaux, France) was tied to one end of the guidewire. The other end of the guidewire was then pulled out, effectively dragging the surgical wire into the previously occupied position. The extracted guidewire was detached from the in-place surgical wire; (7) Steps (4), (5), and (6) were repeated on the second incision side, with minor modification. At step (6), the submucosal end of the guidewire was tied outside the stomach, with the submucosal end of the surgical wire remaining in place on the first incision side. The guidewire was again pulled out, so that the surgical wire passed through both sides of the submucosal incision (Figure 2C); (8) A single loop had formed on the outside, and one wire end was fixed into place. Biopsy forceps were used to pull the other end through the working channel of the endoscope, simultaneously introducing the endoscope into the stomach and pushing the loop with the endoscope tip towards the incision line (Figure 2D). In this manner, the incision mucosal sides were brought towards one another as the loop was tightened. Three alternating loops were made to form the final surgical knot; and (9) The wire ends were cut with a reusable loop cutter (FS-5Q-1; Olympus) (Figure 2E). A photograph of the completed surgical knot is shown in Figure 2F.
The aim of this study was purely theoretical, by which we sought to prove that a surgical suture may be created using only commonplace endoscopy suite materials, without metallic clips, to close a hole in the wall of a hollow digestive organ. As such, the study has several important limitations.
Since the study was based on an in vitro model, neither the strength of the suture, its resistance nor tightness was evaluated. Moreover, other treatment-related quality parameters, such as infection rate and histological response, were not evaluated. Although infectious complications may be prevented in the in vivo model by antibiotic lavage of the stomach before gastric NOTES procedures[24]. Another limitation is that only a single knot was used to close a 15 mm incision, which would be insufficient for a surgical closure. We speculate that two or more suture wires may be passed through both incision sides and tightened at the end, so as to form two or more surgical knots and increase the fidelity of the closure. However, this may prove unfeasible since surgical wires could tangle or form spontaneous knots inside the stomach, beyond the operator’s control.
Nonetheless, the endoscopic method does have an important safety advantage. The endoscopic surgical suturing reduces the risk of injury to organs adjacent to the stomach, which is a significant concern when using T-tags[25]. The method itself may also prove useful as a feasibility model for future development of safer suturing devices that work within a previously dissected submucosal space. In fact, some researchers have already attempted to investigate the utility and safety of an artificially generated submucosal tunnel, but the mucosal incision site had been closed with metallic clips[26]. Testing of this method in an in vivo animal model is necessary to better understand not only its clinical significance with NOTES interventions but also to help realize its potential for other applications.
The authors would like to thank the endoscopy nurses Virginie Dispard, Alice Gâla and Cezarina Vasile.
Traditionally, surgery has been the only method available for removing pathological tissue from the inner abdomen. Laparoscopic surgery and digestive endoscopy have made diagnostic and therapeutic procedures less invasive. Laparoscopic surgery requires creation of orifices in the abdominal wall to access the peritoneal space, while digestive endoscopy travels along and is confined to the digestive tract. In the last 10 years, however, the natural orifice translumenal endoscopic surgery (NOTES) approach passed the endoscope into the peritoneal cavity through a created orifice in the wall of the digestive tract.
The NOTES approach has not yet been fully developed. Questions remain about how to prevent peritoneal infection, how to accurately stabilize the endoscope in the peritoneal cavity and obtain a good grip and orientation (triangulation), and how to finally close the parietal access point. The simplest way to close the orifice is to use endoscopic metallic clips, which are already used for closing accidental perforations, for hemostasis, or for marking. More elaborate methods have been proposed, including endoscopic suture machines and staplers, or trans-parietal metallic tags tightened together. Yet, these methods are complicated, costly, high risk, and not approved for clinical practice.
The authors have described a method to close the digestive wall orifice with a surgical knot using only common endoscopy suite materials. This approach avoids the use of additional devices and reproduces the gold standard surgical closure method-the surgical knot.
The method may be used as a model for creating simple suturing devices that work within the submucosal space. It must first be validated by in vivo survival animal experiments.
NOTES: Natural orifice translumenal endoscopic surgery, a method to perform abdominal surgery by entering the peritoneal space through small orifices made into hollow organs (i.e., stomach, colon, vagina, urinary bladder).
The case is interesting and extremely rare. It is well written and is describing a new method of endoscopic suture. It can be accepted for publication an intra operative image during laparotomy would be of added value.
P- Reviewers Rotondano G, Akyuz F, Iglesias-Garcia J S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 903] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 2. | Kantsevoy SV, Hu B, Jagannath SB, Vaughn CA, Beitler DM, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ. Transgastric endoscopic splenectomy: is it possible? Surg Endosc. 2006;20:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Kantsevoy SV, Jagannath SB, Niiyama H, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Vaughn CA. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Merrifield BF, Wagh MS, Thompson CC. Peroral transgastric organ resection: a feasibility study in pigs. Gastrointest Endosc. 2006;63:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Jagannath SB, Kantsevoy SV, Vaughn CA, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Scorpio DG, Magee CA. Peroral transgastric endoscopic ligation of fallopian tubes with long-term survival in a porcine model. Gastrointest Endosc. 2005;61:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Wagh MS, Merrifield BF, Thompson CC. Endoscopic transgastric abdominal exploration and organ resection: initial experience in a porcine model. Clin Gastroenterol Hepatol. 2005;3:892-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Wagh MS, Merrifield BF, Thompson CC. Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointest Endosc. 2006;63:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Park PO, Bergström M, Ikeda K, Fritscher-Ravens A, Swain P. Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis (videos). Gastrointest Endosc. 2005;61:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA, Hawes RH, Kalloo AN, Pasricha PJ, Chung S. Pilot study of the porcine uterine horn as an in vivo appendicitis model for development of endoscopic transgastric appendectomy. Gastrointest Endosc. 2006;64:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Hu B, Kalloo AN, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Isakovich NV, Nakajima Y, Kawashima K. Peroral transgastric endoscopic primary repair of a ventral hernia in a porcine model. Endoscopy. 2007;39:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Matthes K, Yusuf TE, Willingham FF, Mino-Kenudson M, Rattner DW, Brugge WR. Feasibility of endoscopic transgastric distal pancreatectomy in a porcine animal model. Gastrointest Endosc. 2007;66:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Fritscher-Ravens A, Mosse CA, Ikeda K, Swain P. Endoscopic transgastric lymphadenectomy by using EUS for selection and guidance. Gastrointest Endosc. 2006;63:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Moris DN, Bramis KJ, Mantonakis EI, Papalampros EL, Petrou AS, Papalampros AE. Surgery via natural orifices in human beings: yesterday, today, tomorrow. Am J Surg. 2012;204:93-102. [PubMed] |
| 14. | Available from: http//www.noscar.org. |
| 15. | Available from: http//www.euro-notes.org. |
| 16. | Rattner D, Kalloo A. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. October 2005. Surg Endosc. 2006;20:329-333. [PubMed] |
| 17. | Voermans RP, Worm AM, van Berge Henegouwen MI, Breedveld P, Bemelman WA, Fockens P. In vitro comparison and evaluation of seven gastric closure modalities for natural orifice transluminal endoscopic surgery (NOTES). Endoscopy. 2008;40:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA. Endoscopic full-thickness closure of large gastric perforations by use of tissue anchors. Gastrointest Endosc. 2007;65:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Desilets DJ, Romanelli J, Surti VC, Earle DB, Cody JS, Romero RV. The ties that bind: durable, transmural, purse−string−like gastrotomy closure using a novel device [abstract]. Gastrointest Endosc. 2007;65:AB292. |
| 20. | Pham BV, Raju GS, Ahmed I, Brining D, Chung S, Cotton P, Gostout CJ, Hawes RH, Kalloo AN, Kantsevoy SV. Immediate endoscopic closure of colon perforation by using a prototype endoscopic suturing device: feasibility and outcome in a porcine model (with video). Gastrointest Endosc. 2006;64:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Magno P, Giday SA, Dray X, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Kalloo AN, Pasricha PJ, White JJ. A new stapler-based full-thickness transgastric access closure: results from an animal pilot trial. Endoscopy. 2007;39:876-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Ryou M, Pai RD, Sauer JS, Rattner DW, Thompson CC. Evaluating an optimal gastric closure method for transgastric surgery. Surg Endosc. 2007;21:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Hon SS, Ng SS, Lee JF, Li JC, Lo AW. In vitro porcine training model for colonic endoscopic submucosal dissection: an inexpensive and safe way to acquire a complex endoscopic technique. Surg Endosc. 2010;24:2439-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Yang QY, Zhang GY, Wang L, Wang ZG, Li F, Li YQ, Ding XJ, Hu SY. Infection during transgastric and transvaginal natural orifice transluminal endoscopic surgery in a live porcine model. Chin Med J (Engl). 2011;124:556-561. [PubMed] |
| 25. | Raju GS, Malhotra A, Ahmed I. Colonoscopic full-thickness resection of the colon in a porcine model as a prelude to endoscopic surgery of difficult colon polyps: a novel technique (with videos). Gastrointest Endosc. 2009;70:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Yoshizumi F, Yasuda K, Kawaguchi K, Suzuki K, Shiraishi N, Kitano S. Submucosal tunneling using endoscopic submucosal dissection for peritoneal access and closure in natural orifice transluminal endoscopic surgery: a porcine survival study. Endoscopy. 2009;41:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |