Published online Jan 16, 2013. doi: 10.4253/wjge.v5.i1.19
Revised: September 23, 2012
Accepted: December 1, 2012
Published online: January 16, 2013
Chronic pancreatitis (CP) is a common gastrointestinal illness, which affects the quality of life with substantial morbidity and mortality. The management includes medical, endoscopic and surgical approaches with the need for interaction between various specialties, calling for a concerted multidisciplinary approach. However, at the time of this publication, guidelines to establish care of these patients are lacking. This review provides the reader with a comprehensive overview of the studies summarizing the various treatment options available, including medical, surgical and endoscopic options. In addition, technological advances such as endoscopic retrograde cholangiopancreatogrophy, endoscopic shock wave lithotripsy and endoscopic ultrasound can now be offered with reasonable success for pancreatic decompression, stricture dilatation with stent placement, stone fragmentation, pseudocyst drainage, and other endoscopic interventions such as celiac plexus block for pain relief. We emphasize the endoscopic options in this review, and attempt to extract the most up to date information from the current literature. The treatment of CP and its complications are discussed extensively. Complications such as biliary strictures. pancreatic pseudocysts, and chronic pain are common issues that arise as long-term complications of CP. These often require endoscopic or surgical management and possibly a combination of approaches, however choosing amongst the various therapeutic and palliative modalities while weighing the risks and benefits, makes the management of CP challenging. Treatment goals should be not just to control symptoms but also to prevent disease progression. Our aim in this paper is to advocate and emphasize an evidence based approach for the management of CP and associated long term complications.
- Citation: Oza VM, Kahaleh M. Endoscopic management of chronic pancreatitis. World J Gastrointest Endosc 2013; 5(1): 19-28
- URL: https://www.wjgnet.com/1948-5190/full/v5/i1/19.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i1.19
Chronic pancreatitis (CP) is debilitating illnesses, with a prevalence estimated between 4% to 5%[1]. The chronicity of CP and the frequent acute exacerbations significantly impact patients’ quality of life. Alcohol is the most common etiology of CP in the western world. Sarles et al[2] reported that 60% to 70% of patients with CP have a 6 to 12 year history of alcohol abuse. Other common etiologies of CP include autoimmune pancreatitis, hypercalcemia, as well as idiopathic CP[3].
CP is characterized by irreversible damage that leads to fibrosis and necrosis of the pancreatic tissue[4]. This destruction of the pancreatic tissue manifests as abdominal pain, the most common presenting symptom of CP[5-9]. Steatorrhea and diabetes are other common presenting symptoms seen with the loss of endocrine and exocrine function of the pancreas[10]. Medical, endoscopic and surgical methods are available for management of CP. Medical management revolves around pain medications, fluid hydration and pancreatic enzyme supplementation surgery seem to be efficacious, at least in the short and mid term but is associated with high morbidity and mortality[11-13]. Technological advances such as endoscopic retrograde cholangiopancreatogrophy (ERCP), endoscopic shock wave lithotripsy (ESWL) and endoscopic ultrasound (EUS) can now be offered with reasonable success for pseudocyst drainage, stricture dilatation with stent placement, and other endoscopic interventions such as celiac plexus block or neurolysis for pain relief[14]. However, choosing amongst the various therapeutic and palliative modalities while weighing the risks and benefits, makes the management of CP challenging.
This review is focused on the current management of CP with emphasis on pain control and treatment of complications. We aim to provide the reader with the most up-to-date evidence on endoscopic modalities available for CP.
Pain is the most common presenting symptom of CP, and ranges from mild discomfort to severe pain that often requires hospitalization. The origin of pain is much debated; and the consensus at this time is that the etiology of pain is multifactorial[4,15-17]. It can be caused by pancreatic duct obstruction, which subsequently leads to ductal hypertension[9]. Pancreatic duct obstruction can be frequently caused by complications of CP such as pancreatic duct strictures, pseudocysts, intraductal stones, and sphincter stenosis[9].
Alcohol abuse is the most common cause of CP in the United States, and the association of binge drinking with acute exacerbation of abdominal pain in CP is well known. Therefore emphasis on alcohol cessation with offering resources on alcohol cessation such as support groups is the first step to manage CP. In addition to alcohol, smoking has also been shown to be an independent risk factor for both acute and CP[18], and smoking cessation is equally important in patients with CP. If the avoidance of exacerbating factors fails to control flare-up of abdominal pain, pain medications should be considered for symptom relief.
Acetaminophen and non-steroidal anti-inflammatory agents should be used for pain relief, if there are no contraindications. Narcotics should never be the first line for control of pain and offering narcotics as first line of pain medication poses a real risk of addiction[16]. Pancreatic enzymes and antioxidants have also been shown to relieve pain in CP. Isakson and co-workers showed a 30% reduction in pain after treatment with oral enzyme preparations in a small number of patients with CP[19]. The mechanism through which enzymatic preparations work is presumed to be via a negative feedback pathway involving the pancreas, specifically involving the cholecystokinin pathway[20]. In recent years, this theory has been challenged by conflicting evidence[21].
It is well documented that in CP there is a decreased absorption of vitamins and minerals[22]. Deficiencies lead to increase in oxygen free radicals. There is some data to suggest that removal of oxygen free radicals may have an increased therapeutic effect in controlling pain[23].
Advances in understanding the pathogenesis of CP combined with progress in technology have led to an emerging role of endoscopy in the management of CP. Experts believe that endoscopic management has an important role in patients[24] as a primary therapeutic measure in poor surgical candidates where medical management fails. Recent evidence by Díte et al[25] suggests that surgical outcomes were more durable than endoscopic therapy in patients with a dilated pancreatic duct (PD), stones and/or strictures[25]. Cahen et al[26] recently reported better outcomes in pain control after surgery than with endoscopic intervention. Although these studies indicate surgery might be a better intervention than endoscopy, it needs to be pointed out that neither one of those studies came from centers using routinely ESWL, which is now incorporated into the management of patients with pancreatic stones[27]. Finally, endoscopy remains a highly effective intervention in patients with severe comorbidities and can also serve as a bridge to surgery[28].
Pancreatic strictures can be caused by prior stones, recurrent inflammation or fibrosis[29]. In cases of pancreatic stricture, where malignancy is suspected it is crucial to obtain cross sectional imaging followed by endoscopic ultrasound with fine needle aspiration (EUS-FNA) of any pancreatic masses. In the absence of a definitive mass, pancreatic brushing should be performed, keeping in mind that the threshold for referral to surgery in those cases should be low[30-32].
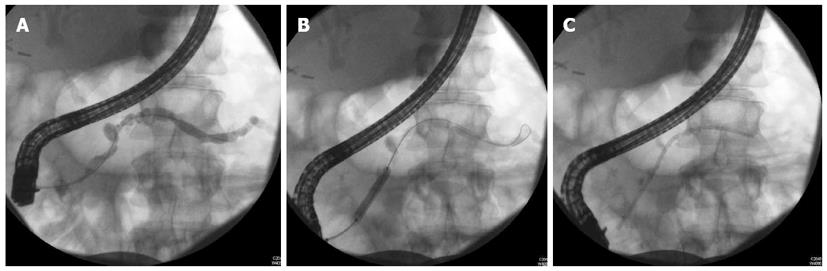
The management of benign strictures includes dilation and stenting (Figure 1). The number of strictures, the location of the strictures and the length of the stricture play key roles in determining the efficacy of endotherapy.
Symptomatic patients with a single stricture in the main PD in the head of the pancreas are the best candidates for ERCP with stenting[33]. It is generally accepted that patients with multiple strictures along the main PD, the so-called “chain of lakes” appearance, are not good candidates for endotherapy[33].
Table 1 summarizes the results of endotherapy in reference to pancreatic strictures. Wilcox[34] summarized the available studies on this topic. The 15 series analysis has a total of 1500 patients. Among the 1500 patients, benefit was seen in 31%-100% of patients with a wide follow-up time period from 8-72 mo. An important finding from these studies was that complete stricture resolution is not needed for the resolution of pain.
The technique of stenting the PD in the event of strictures involves dilation prior to stenting. Dilation can be performed by wire guided balloons (4-6 mm), bougie or with a Soehendra stent retriever. Polyethylene pancreatic stents are then deployed for main pancreatic duct MPD stricture as large as possible to mimic a “pancreatico-duodenostomy”[12,27,33,35,36].
Pain relief is seen in 70%-94% of patients after stent placement (Table 1). Ductal decompression is indicated if the main PD above the stricture is significantly dilated (large duct disease). The strategy remains that the stents are prophylactically exchanged every three months[37]. In some cases, the stent can get clogged, however it will continue to remain effective by what is known as the “wick” effect[38].
It is important to note, however, that after stent removal the rate of recurrence of a main PD stricture is high. In fact, Eleftherladis et al[39] reported the stricture relapse rate after a 2 year follow up period was as high as 38%, with these patients require repeat stenting.
Although the approach of multiple stents for PD strictures seems promising[40-42], to our knowledge, at the time of writing, there have been no studies comparing single and multiple stenting procedures for PD strictures caused by CP.
For patient in whom conventional ERCP is not feasible or fails, access and decompression of the main pancreatic duct using EUS-guided pancreatography has increased the success for PD drainage[30-32,43-45]. This constitutes a minimally invasive alternative to surgery in patient with altered anatomy or severe stone burden not responding to ESWL.
Obstruction of the PD by calcified stones leads to increased pressure upstream from the stone causing increased intraductal hypertension. The data surrounding pancreatic stone removal is clear. Endoscopic therapy alone was found to be successful in 72% of patients with a 68% symptomatic improvement[35,46,47]. ESWL can relieve the elevated intraductal pressure by fragmentation of intraductal stone.
Upon fragmentation the stones can pass spontaneously[48,49], therefore ERCP is not obligatory unless there is an associated stricture. The primary limitation of ESWL is that it cannot be used to fragment larger stones. In such cases, laser lithotripsy might be more effective[50-53].
In 2007, Dumonceau et al[54] compared ESWL alone with ESWL in conjunction with endoscopic drainage of the main PD for pain relief. Two years after intervention, they noted a similar decrease in the number of pain episodes per year. As such, it was concluded that ESWL alone was a safe and effective modality of treatment in reducing pain in CP with stone only disease and addition of endoscopic measures added costs to patient care, with no significant reduction in pain relief[54]. Endotherapy in conjunction with ESWL has been shown to increase stone clearance rates and to improve long-term outcomes[36,49,55-60] in patients with stone and stricture disease. In one study Kozarek et al[36] were able to show that surgery was avoided in 80% of patients who underwent ESWL. with decrease in narcotic use and reduction in hospitalizations (Table 2).
| Study | Total patients | No. of patients in any amount of pain at follow up | Duct clearance | Mean follow up time (mo) |
| Sauerbruch et al[107] | 8 | 8 | 8 | 11 |
| Den Toom et al[108] | 8 | 8 (7 pain relief) | 8 | 17 |
| Sauerbruch et al[109] | 24 | 24 | 24 | 24 |
| Delhaye et al[60] | 123 | 88 | 123 | |
| Schneider et al[110] | 50 | 39 | 48 | 20 |
| Van der Hul et al[111] | 17 | 17 | 17 | 30 |
| Wolf et al[112] | 12 | 9 | 12 | 19-22 |
| Schreiber et al[113] | 10 | 7 | 10 | 12 |
| Johanns et al[114] | 35 | 23 | 16 | NA |
| Ohara et al[115] | 32 | 7 | 24 | 44 |
| Matthews et al[116] | 19 | 13 | 19 | 6 mo-6 yr |
| 1Costamagna et al[117] | 35 | 32 | 35 | 6 |
| Adamek et al[49] | 80 | 80 | NA | NA |
| Brand et al[55] | 48 | 17 | 48 | 7 |
| Karasawa et al[118] | 24 | 12 | 24 | 12 |
| Kozarek et al[36] | 40 | 28 | NA | 2.4 yr |
| Rubenstein et al[119] | 23 | NA | 23 | NA |
A total of 20%-40% of patients with CP can develop this complication[61]. Intraductal hypertension within the main PD, or the rupture of a branching duct can lead to formation of pseudocysts. Pseudocysts[62] who fail to resolve spontaneously and are symptomatic require drainage. Drainage is indicated if there is pain, infection or evidence of obstruction[61,63,64].
The modality employed for drainage is also important. There are two major routes of endoscopic drainage-transmural and transpapillary. The route chosen depends on the size, possible communication between the pseudocyst and the pancreatic duct. There appears to be a trend in the literature for transmural drainage versus transpapillary[65] with an attempt to seal any possible leak or draining a proximal duct by crossing a stricture[65]. Several studies place the technical success of transmural drainage of pseudocyst at 85%-100%. The recurrence rate range from 10%-15% with complications between 10%-34%[63,66-68].
In recent years, EUS-guided pseudocyst (EGPD) drainage has gained in popularity since it allow to avoid intervening vessels and target more challenging collections safely when compared to conventional transmural drainage techniques (CTDT)[69-71]. Our team[64] and others[72] have demonstrated that EUS-guided drainage and conventional transmural drainage techniques have fairly comparable rates of success and similar rates of complications if non bulging collection and patient at higher risk of bleeding are selectively drained using EGPD.
Benign strictures can also form within the biliary ductal system in CP, and if left untreated can lead to jaundice, cholangitis and biliary cirrhosis[41,73]. Traditionally benign biliary strictures in CP are treated by surgery, but as with all surgeries the procedure is invasive and can involve significant morbidity especially if patients have other accompanying co-morbidities such as CP and/or liver disease. Morbidity and mortality of surgical treatment of post-operative biliary strictures is low, with mortality rates ranging from 0%-2.2%, whereas post-operative morbidity rates approaching almost 43% in some studies[74-76]. The multiple stent placement technique was initially popularized by Costamagna et al[40] for the treatment of postoperative strictures. In their study, stricture resolution was observed in 95% of patients at stent removal, and at follow up (average time of 38 mo after stent removal) 84% of patients were pain free and only 10.5% (2 patients) had recurrence of stricture.
They reported good long term results in treatment of post-operative biliary strictures by insertion of plastic stents after greater than a ten year follow up. While, success is dependant on the number of sessions and the number of stents placed, it appears that this maybe a reasonable first-line option[42]. Several groups have studied biliary strictures and endoscopic approach to treatment, and in all cases average stricture resolution was reported between 10%-33% (Table 3)[57,77-83].
| Study | Total patients | Success rate (%)-short term | Stricture resolution (%) | Stent occlusion (%) | Stent migration (%) | Follow up time (mo) |
| Deviere et al[85] | 25 | 100 | 3 (12) | 32 | 40 | 14 |
| Barthet et al[83] | 19 | 100 | 2 (11) | 0 | 5 | 18 |
| Smits et al[82] | 58 | 100 | 16 (28) | 62 | 7 | 49 |
| Kiehne et al[81] | 14 | 100 | 2 (16) | 36 | NA | NA |
| Vitale et al[80] | 25 | 100 | 20 (80) | 12 | 8 | 32 |
| Farnbacher et al[79] | 31 | 100 | 10 (32) | 29 | 23 | 24 |
| Eickhoff et al[78] | 39 | 100 | 12 (31) | 33 | 10 | 58 |
| Average | 30 | 100 | 30 | 29.14 | 17.16 | 32.5 |
Uncovered metal stents have also been evaluated. Since biliary strictures related to CP can be difficult to treat with plastic stents, there have been several studies that examined the use of uncovered self-expanding metal stents (USEMS) in patients with primarily CP[84-87]. Deviere et al[85] deployed USEMS in patients (n = 20) with CP, and initially demonstrated relief of cholestasis for up to 33 mo for 18 patients. Repeat ERCP 3 mo later demonstrated that the stent was embedded within the bile duct wall. All subsequent studies confirmed that uncovered metal stents proved to be problematic due to epithelial hyperplasia, occlusion, and the inability to easily remove the stent without overwhelming evidence of improved patency or stricture resolution[88]. This lack of removability also predisposes the patient to chronic inflammation and a potential for cholangiocarcinoma.
Covered metal stent, partially or fully covered have been used, with stricture resolution for partially covered metal stent[89] noted to be about 77% in CP, whereas fully covered metal stents provided a success rate of 83%[90]. Given the limitations noted with uncovered stents, and in an effort to improve patency, partially covered self-expanding metal stents (PCMS) were assessed in this biliary stricture related to CP. They were noted to be easier to remove, offering the option of temporary placement[91-93]. Cantù et al[94] placed PCMS in patients with CP and associated common duct stricture who failed prior plastic stent therapy. All the patients responded initially but with a median follow up of 22 mo (range 12-33 mo), 7 patients developed stent dysfunction, requiring re-intervention. Stent patency, however, decreased over time, from 100% at 12 mo to 37.5% at 36 mo and none of the PCMS were removed during the study period, demonstrating that PCMS left in place over time decrease in patency, requiring additional endoscopic interventions[94]. Another similar study deployed PCMS in 6 patients with limited patency (2/6) at 35 mo (range 33-37 mo) follow up. In addition, this study compared uncovered (n = 18) to PCMS and found longer patency with uncovered stents (mean 46 mo vs 20 mo, P = 0.002), although overall follow up was much longer for uncovered stents (mean 61 mo), which could account for the significant difference[86].
Kahaleh et al[95] performed the largest series of patients (n = 79) with partially covered metal stents coated with Permalume (Wallstent, Boston Scientific, Natick, MA). Sixty five patients had stent left in place for a median of 4 mo (range 1-28 mo) and removed once successful treatment was confirmed. Follow up after stent removal was a median of 12 mo (range 3-26 mo). Three patients developed a stricture at uncovered proximal portion, 3 failed primary therapy and 2 developed duodenal edema preventing SEMS insertion, resulting in 90% success (59/65). Successful resolution of the stricture was noted to be lowest with strictures related to CP (17/22, 77%)[95]. As a follow up to this study, Sauer et al[96] further analyzed long term response of those patients. Notably, migration occurred with 15 stents, as well as intimal hyperplasia and stent embedment into the mucosa in 7 patients each respectively[96].
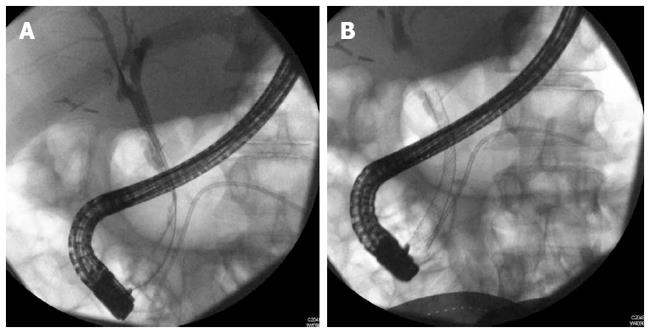
With limitations related to partially covered metal stents namely epithelial hyperplasia at the uncovered portions and migration, fully covered metal stents (FCSEMS) were then tried in this indication (Figure 2). Cahen et al[97] published a series of 6 patients with strictures resulting from CP receiving FCSEMS (Hanaro; M.I.Tech Co., Ltd., Seoul, South Korea), with 66% resolution, however 2 stents were unable to be removed requiring plastic stents placement through the other metal stent. More recently, Mahajan et al[90] analyzed a FCSEMS with anchoring fins (Viabil, Conmed, Utica, NY) to treat benign biliary strictures. A total of 44 patients (28 men, median age 53.5 years) were included. Etiologies included 19 CP. Complications were observed in 6/44 (14%) patients after placement, and 4/44 (9%) patients after removal, mainly pain and post ERCP pancreatitis. Lower rate of resolution was seen with CP (58%) and moderate difficulty in deploying and removing the stent due to its anchoring fins proved to be limitations in its widespread use. The anchoring fins also caused ulceration and bleeding with stent extraction[90].
A follow up study came from the same group with 55 patients and subsequent mean stent time of 126 ± 74 d and follow up of 524.2 ± 297.7 d. The success rate was 67% for those with CP and 71% for other etiologies[96].
The data that we are seeing in literature on FCSEMS are promising, but larger randomized control trials are needed to evaluate this treatment modality. It is conclusive however, that endotherapy in treatment of biliary strictures is a good option for high risk surgical patients and for those who prefer a less invasive approach.
Celiac plexus block (CPB) is performed via a gastric approach using EUS-guidance and has high success rates and relatively low complication rates. EUS-guided CPB is preferred over CT-guided CPB not only because there are fewer side effects[98] but also because of clarity obtained via EUS. CPB can be performed by injection of anesthetics and/or steroids. Celiac plexus neurolysis, used for pain secondary to malignancy, is similar but involves injection of pure ethanol which results in complete destruction of the celiac plexus. EUS allows for live imaging of the celiac space which improves visualization. EUS guided celiac plexus block improves pain in about 50% of patients for a period of 3-6 mo[98]. In a prospective randomized study, Gress et al[98,99] compared EUS to CT-guided CPB for the treatment of CP pain and discovered that about 50% of patients in the EUS group had significant pain reduction. In addition, about 40% (8 wk group) and 30% (24 wk group) of the EUS-guided CPB had continued benefit. This, when compared to 12% (12 wk) in the CT-guided CPB, clearly suggest superiority of the EUS method.
Several retrospective and prospective studies have put the success rate was as high as 95%[98-101]. While technical success has been high, long term pain relief are disappointing. Short-term pain improvement was approximately 50%, whereas long term pain relief at 24 wk was only 10%. A similar number has been achieved for short-term pain relief by Kaufman et al[102].
Given the low long-term success rates, EUS-guided celiac block should be considered as a temporary measure. It should be considered in acute flares of chronic pain in those patients with limited options.
Advances in understanding the pathogenesis of CP combined with progress in technology have led to an emerging role of endoscopy in the management of CP. Experts believe that endoscopic management has an important role in patients[24] as a primary therapeutic measure in poor surgical candidates where medical management fails. Recent evidence by Díte et al[25] suggests that surgical outcomes were more durable than endoscopic therapy in patients with a dilated PD, stones and/or strictures. Cahen et al[26] recently reported better outcomes in pain control after surgery than with endoscopic intervention. Although these recent studies that indicate surgery as a better intervention than endoscopy, endoscopy is a highly effective intervention especially in patients who are high-risk surgical candidates especially if combined to ESWL. Delhaye et al[28], concluded that endotherapy can also serve as a bridge to surgery.
Díte et al[25] analyzed patients with CP secondary to large duct CP and compared endoscopic therapy to lateral pancreatojejunostomy procedure, and found that in the randomized and the non-randomized groups the results were similar. Moreover, on a five year follow up, patients in the surgery group were more likely to be pain free than in the endoscopic group. Cahen et al[26] also reported similar results. The primary difference between the two studies was that in the former study, endoscopic techniques were not optimized. Specifically, it did not involve patients undergoing cumulative stenting, or repeat treatment after recurrence, and it did not include ESWL.
CP is a disabling disease with serious complications affecting quality of life. There have been significant advances particularly on the endoscopic front with advent of endoscopic techniques such as pancreatic stenting, ESWL, pseudocyst drainage and EUS-guided access and therapy. A multidisciplinary team approach with judicious and appropriate utilization of the medical, endoscopic and surgical treatment options holds promise to revolutionize patient care. Given the variability in the presentation and patient preferences, treatment should be tailored on a case-to-case basis.
P- Reviewers Zhu JF, Oruc N S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2009;25:454-459. [PubMed] |
| 2. | Sarles H, Cros RC, Bidart JM. A multicenter inquiry into the etiology of pancreatic diseases. Digestion. 1979;19:110-125. [PubMed] |
| 3. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [PubMed] |
| 5. | Karanjia ND, Widdison AL, Leung F, Alvarez C, Lutrin FJ, Reber HA. Compartment syndrome in experimental chronic obstructive pancreatitis: effect of decompressing the main pancreatic duct. Br J Surg. 1994;81:259-264. [PubMed] |
| 6. | Ebbehøj N, Borly L, Bülow J, Rasmussen SG, Madsen P. Evaluation of pancreatic tissue fluid pressure and pain in chronic pancreatitis. A longitudinal study. Scand J Gastroenterol. 1990;25:462-466. [PubMed] |
| 7. | Ebbehøj N, Borly L, Bülow J, Rasmussen SG, Madsen P, Matzen P, Owre A. Pancreatic tissue fluid pressure in chronic pancreatitis. Relation to pain, morphology, and function. Scand J Gastroenterol. 1990;25:1046-1051. [PubMed] |
| 8. | Ebbehøj N, Borly L, Madsen P, Matzen P. Pancreatic tissue fluid pressure during drainage operations for chronic pancreatitis. Scand J Gastroenterol. 1990;25:1041-1045. [PubMed] |
| 9. | Jalleh RP, Aslam M, Williamson RC. Pancreatic tissue and ductal pressures in chronic pancreatitis. Br J Surg. 1991;78:1235-1237. [PubMed] |
| 10. | Clain JE, Pearson RK. Diagnosis of chronic pancreatitis. Is a gold standard necessary? Surg Clin North Am. 1999;79:829-845. [PubMed] |
| 11. | Kalady MF, Broome AH, Meyers WC, Pappas TN. Immediate and long-term outcomes after lateral pancreaticojejunostomy for chronic pancreatitis. Am Surg. 2001;67:478-483. [PubMed] |
| 12. | Laugier R, Renou C. Endoscopic treatment in chronic pancreatitis. Ital J Gastroenterol Hepatol. 1998;30:566-570. [PubMed] |
| 13. | Laugier R, Renou C. Endoscopic ductal drainage may avoid resective surgery in painful chronic pancreatitis without large ductal dilatation. Int J Pancreatol. 1998;23:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575-3580. [PubMed] |
| 15. | Ammann RW, Heitz PU, Klöppel G. The “two-hit” pathogenetic concept of chronic pancreatitis. Int J Pancreatol. 1999;25:251. [PubMed] |
| 16. | Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132-1140. [PubMed] |
| 17. | Di Sebastiano P, di Mola FF, Bockman DE, Friess H, Büchler MW. Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut. 2003;52:907-911. [PubMed] |
| 18. | Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035-1045. [PubMed] |
| 19. | Isaksson G, Ihse I. Pain reduction by an oral pancreatic enzyme preparation in chronic pancreatitis. Dig Dis Sci. 1983;28:97-102. [PubMed] |
| 20. | Gachago C, Draganov PV. Pain management in chronic pancreatitis. World J Gastroenterol. 2008;14:3137-3148. [PubMed] |
| 21. | Brown A, Hughes M, Tenner S, Banks PA. Does pancreatic enzyme supplementation reduce pain in patients with chronic pancreatitis: a meta-analysis. Am J Gastroenterol. 1997;92:2032-2035. [PubMed] |
| 22. | Bhardwaj P, Thareja S, Prakash S, Saraya A. Micronutrient antioxidant intake in patients with chronic pancreatitis. Trop Gastroenterol. 2004;25:69-72. [PubMed] |
| 23. | McCloy R. Chronic pancreatitis at Manchester, UK. Focus on antioxidant therapy. Digestion. 1998;59 Suppl 4:36-48. [PubMed] |
| 24. | Elta GH. Is there a role for the endoscopic treatment of pain from chronic pancreatitis? N Engl J Med. 2007;356:727-729. [PubMed] |
| 25. | Díte P, Ruzicka M, Zboril V, Novotný I. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy. 2003;35:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Laméris JS, Dijkgraaf MG, Huibregtse K. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684. [PubMed] |
| 27. | Hirota M, Asakura T, Kanno A, Shimosegawa T. Endoscopic treatment for chronic pancreatitis: indications, technique, results. J Hepatobiliary Pancreat Sci. 2010;17:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Delhaye M, Arvanitakis M, Bali M, Matos C, Devière J. Endoscopic therapy for chronic pancreatitis. Scand J Surg. 2005;94:143-153. [PubMed] |
| 29. | Cremer M, Devière J, Delhaye M, Baize M, Vandermeeren A. Stenting in severe chronic pancreatitis: results of medium-term follow-up in seventy-six patients. Endoscopy. 1991;23:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 195] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567-570. [PubMed] |
| 31. | Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound-guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Dig Dis. 2008;26:356-363. [PubMed] |
| 32. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-36; quiz 751, 753. [PubMed] |
| 33. | Tringali A, Boskoski I, Costamagna G. The role of endoscopy in the therapy of chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2008;22:145-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Wilcox CM. Endoscopic therapy for pain in chronic pancreatitis: is it time for the naysayers to throw in the towel? Gastrointest Endosc. 2005;61:582-586. [PubMed] |
| 35. | Rösch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Kozarek RA. Endoscopic treatment of chronic pancreatitis. Indian J Gastroenterol. 2004;21:67-73. [PubMed] |
| 37. | Delhaye M, Matos C, Devière J. Endoscopic technique for the management of pancreatitis and its complications. Best Pract Res Clin Gastroenterol. 2004;18:155-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Binmoeller KF, Jue P, Seifert H, Nam WC, Izbicki J, Soehendra N. Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: long-term results. Endoscopy. 1995;27:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 174] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Eleftherladis N, Dinu F, Delhaye M, Le Moine O, Baize M, Vandermeeren A, Hookey L, Devière J. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005;37:223-230. [PubMed] |
| 40. | Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, Petruzziello L, Familiari P, Mutignani M. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Costamagna G, Familiari P, Tringali A, Mutignani M. Multidisciplinary approach to benign biliary strictures. Curr Treat Options Gastroenterol. 2007;10:90-101. [PubMed] |
| 42. | Costamagna G, Tringali A, Mutignani M, Perri V, Spada C, Pandolfi M, Galasso D. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, Meyer F. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. [PubMed] |
| 45. | Kahaleh M, Yoshida C, Yeaton P. EUS antegrade pancreatography with gastropancreatic duct stent placement: Review of two cases. Gastrointest Endosc. 2003;58:919-923. [PubMed] |
| 46. | Sherman S, Ruffolo TA, Hawes RH, Lehman GA. Complications of endoscopic sphincterotomy. A prospective series with emphasis on the increased risk associated with sphincter of Oddi dysfunction and nondilated bile ducts. Gastroenterology. 1991;101:1068-1075. [PubMed] |
| 47. | Sherman S, Lehman GA, Hawes RH, Ponich T, Miller LS, Cohen LB, Kortan P, Haber GB. Pancreatic ductal stones: frequency of successful endoscopic removal and improvement in symptoms. Gastrointest Endosc. 1991;37:511-517. [PubMed] |
| 48. | Dumonceau JM. Endoscopic versus surgical treatment for chronic pancreatitis. N Engl J Med. 2007;356:2102; author reply 2103-2104. [PubMed] |
| 49. | Adamek HE, Jakobs R, Buttmann A, Adamek MU, Schneider AR, Riemann JF. Long term follow up of patients with chronic pancreatitis and pancreatic stones treated with extracorporeal shock wave lithotripsy. Gut. 1999;45:402-405. [PubMed] |
| 50. | Howell DA, Dy RM, Hanson BL, Nezhad SF, Broaddus SB. Endoscopic treatment of pancreatic duct stones using a 10F pancreatoscope and electrohydraulic lithotripsy. Gastrointest Endosc. 1999;50:829-833. [PubMed] |
| 51. | Shim CS. How Should Biliary Stones be Managed? Gut Liver. 2010;4:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Yoo KS, Lehman GA. Endoscopic management of biliary ductal stones. Gastroenterol Clin North Am. 2010;39:209-27, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Kow AW, Wang B, Wong D, Sundeep PJ, Chan CY, Ho CK, Liau KH. Using percutaneous transhepatic cholangioscopic lithotripsy for intrahepatic calculus in hostile abdomen. Surgeon. 2011;9:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Dumonceau JM, Costamagna G, Tringali A, Vahedi K, Delhaye M, Hittelet A, Spera G, Giostra E, Mutignani M, De Maertelaer V. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomised controlled trial. Gut. 2007;56:545-552. [PubMed] |
| 55. | Brand B, Kahl M, Sidhu S, Nam VC, Sriram PV, Jaeckle S, Thonke F, Soehendra N. Prospective evaluation of morphology, function, and quality of life after extracorporeal shockwave lithotripsy and endoscopic treatment of chronic calcific pancreatitis. Am J Gastroenterol. 2000;95:3428-3438. [PubMed] |
| 56. | Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher-Ravens A, Knöfel WT, Jäckle S, Soehendra N. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35:1221-1228. [PubMed] |
| 57. | Devière J, Devaere S, Baize M, Cremer M. Endoscopic biliary drainage in chronic pancreatitis. Gastrointest Endosc. 1990;36:96-100. [PubMed] |
| 58. | Deviere J, Baize M, Vandermeeren A, Buset M, Delhaye M, Cremer M. Endoscopic stenting for biliary strictures. Acta Gastroenterol Belg. 1990;55:295-305. [PubMed] |
| 59. | Dumonceau JM, Devière J, Le Moine O, Delhaye M, Vandermeeren A, Baize M, Van Gansbeke D, Cremer M. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547-555. [PubMed] |
| 60. | Delhaye M, Vandermeeren A, Baize M, Cremer M. Extracorporeal shock-wave lithotripsy of pancreatic calculi. Gastroenterology. 1992;102:610-620. [PubMed] |
| 61. | Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Endoscopic management of pancreatic pseudocysts. Br J Surg. 1997;84:1638-1645. [PubMed] |
| 62. | Avula H, Sherman S. What is the role of endotherapy in chronic pancreatitis. Ther Adv in Gastroenterol. 2010;3:1-16. |
| 63. | Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7-17. [PubMed] |
| 64. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Avula H, Sherman S. What is the role of endotherapy in chronic pancreatitis? Therap Adv Gastroenterol. 2010;3:367-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Smits ME, Badiga SM, Rauws EA, Tytgat GN, Huibregtse K. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc. 1995;42:461-467. [PubMed] |
| 67. | Smits ME, Rauws EA, Tytgat GN, Huibregtse K. The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:202-207. [PubMed] |
| 68. | Grimm H, Meyer WH, Nam VC, Soehendra N. New modalities for treating chronic pancreatitis. Endoscopy. 1989;21:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 70. | Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video). Gastrointest Endosc. 2008;68:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 73. | Köcher M, Cerná M, Havlík R, Král V, Gryga A, Duda M. Percutaneous treatment of benign bile duct strictures. Eur J Radiol. 2007;62:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Walsh RM, Henderson JM, Vogt DP, Brown N. Long-term outcome of biliary reconstruction for bile duct injuries from laparoscopic cholecystectomies. Surgery. 2007;142:450-46; discussion 450-46;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Chapman WC, Halevy A, Blumgart LH, Benjamin IS. Postcholecystectomy bile duct strictures. Management and outcome in 130 patients. Arch Surg. 1995;130:597-602; discussion 602-4. [PubMed] |
| 76. | Nuzzo G, Giuliante F, Giovannini I, Murazio M, D’Acapito F, Ardito F, Vellone M, Gauzolino R, Costamagna G, Di Stasi C. Advantages of multidisciplinary management of bile duct injuries occurring during cholecystectomy. Am J Surg. 2008;195:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Cahen DL, van Berkel AM, Oskam D, Rauws EA, Weverling GJ, Huibregtse K, Bruno MJ. Long-term results of endoscopic drainage of common bile duct strictures in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2005;17:103-108. [PubMed] |
| 78. | Eickhoff A, Jakobs R, Leonhardt A, Eickhoff JC, Riemann JF. Endoscopic stenting for common bile duct stenoses in chronic pancreatitis: results and impact on long-term outcome. Eur J Gastroenterol Hepatol. 2001;13:1161-1167. [PubMed] |
| 79. | Farnbacher MJ, Rabenstein T, Ell C, Hahn EG, Schneider HT. Is endoscopic drainage of common bile duct stenoses in chronic pancreatitis up-to-date? Am J Gastroenterol. 2000;95:1466-1471. [PubMed] |
| 80. | Vitale GC, Reed DN, Nguyen CT, Lawhon JC, Larson GM. Endoscopic treatment of distal bile duct stricture from chronic pancreatitis. Surg Endosc. 2000;14:227-231. [PubMed] |
| 81. | Kiehne K, Fölsch UR, Nitsche R. High complication rate of bile duct stents in patients with chronic alcoholic pancreatitis due to noncompliance. Endoscopy. 2000;32:377-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Smits ME, Rauws EA, van Gulik TM, Gouma DJ, Tytgat GN, Huibregtse K. Long-term results of endoscopic stenting and surgical drainage for biliary stricture due to chronic pancreatitis. Br J Surg. 1996;83:764-768. [PubMed] |
| 83. | Barthet M, Bernard JP, Duval JL, Affriat C, Sahel J. Biliary stenting in benign biliary stenosis complicating chronic calcifying pancreatitis. Endoscopy. 1994;26:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Dumonceau JM, Devière J. Self-expandable metal stents. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:109-130. [PubMed] |
| 85. | Deviere J, Cremer M, Baize M, Love J, Sugai B, Vandermeeren A. Management of common bile duct stricture caused by chronic pancreatitis with metal mesh self expandable stents. Gut. 1994;35:122-126. [PubMed] |
| 86. | Eickhoff A, Jakobs R, Leonhardt A, Eickhoff JC, Riemann JF. Self-expandable metal mesh stents for common bile duct stenosis in chronic pancreatitis: retrospective evaluation of long-term follow-up and clinical outcome pilot study. Z Gastroenterol. 2003;41:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | van Berkel AM, Cahen DL, van Westerloo DJ, Rauws EA, Huibregtse K, Bruno MJ. Self-expanding metal stents in benign biliary strictures due to chronic pancreatitis. Endoscopy. 2004;36:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Silvis SE, Sievert CE, Vennes JA, Abeyta BK, Brennecke LH. Comparison of covered versus uncovered wire mesh stents in the canine biliary tract. Gastrointest Endosc. 1994;40:17-21. [PubMed] |
| 89. | Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, Sundaram V, Tokar J, Adams RB, Yeaton P. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc. 2008;67:446-454. [PubMed] |
| 90. | Mahajan A, Ho H, Sauer B, Phillips MS, Shami VM, Ellen K, Rehan M, Schmitt TM, Kahaleh M. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc. 2009;70:303-309. [PubMed] |
| 91. | Kahaleh M, Tokar J, Le T, Yeaton P. Removal of self-expandable metallic Wallstents. Gastrointest Endosc. 2004;60:640-644. [PubMed] |
| 92. | Familiari P, Bulajic M, Mutignani M, Lee LS, Spera G, Spada C, Tringali A, Costamagna G. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest Endosc. 2005;62:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 93. | Trentino P, Falasco G, d’orta C, Coda S. Endoscopic removal of a metallic biliary stent: case report. Gastrointest Endosc. 2004;59:321-323. [PubMed] |
| 94. | Cantù P, Hookey LC, Morales A, Le Moine O, Devière J. The treatment of patients with symptomatic common bile duct stenosis secondary to chronic pancreatitis using partially covered metal stents: a pilot study. Endoscopy. 2005;37:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Kahaleh M, Tokar J, Conaway MR, Brock A, Le T, Adams RB, Yeaton P. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc. 2005;61:528-533. [PubMed] |
| 96. | Sauer B, Kahaleh M. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts: a need for a large randomized study. Gastrointest Endosc. 2010;71:432; author reply 432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008;40:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-905. [PubMed] |
| 99. | Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409-416. [PubMed] |
| 100. | Faigel DO, Kochman ML. The role of endoscopic ultrasound in the preoperative staging of pancreatic malignancies. Gastrointest Endosc. 1996;43:626-628. [PubMed] |
| 101. | Faigel DO, Veloso KM, Long WB, Kochman ML. Endosonography-guided celiac plexus injection for abdominal pain due to chronic pancreatitis. Am J Gastroenterol. 1996;91:1675. [PubMed] |
| 102. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 103. | Weber A, Schneider J, Neu B, Meining A, Born P, Schmid RM, Prinz C. Endoscopic stent therapy for patients with chronic pancreatitis: results from a prospective follow-up study. Pancreas. 2007;34:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Eisendrath P, Devière J. Expandable metal stents for benign pancreatic duct obstruction. Gastrointest Endosc Clin N Am. 1999;9:547-554. [PubMed] |
| 105. | Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481-1487. [PubMed] |
| 106. | Cremer M, Deviere J, Delhaye M, Vandermeeren A, Baize M. Non-surgical management of severe chronic pancreatitis. Scand J Gastroenterol Suppl. 1990;175:77-84. [PubMed] |
| 107. | Sauerbruch T, Holl J, Sackmann M, Paumgartner G. Extracorporeal shock wave lithotripsy of pancreatic stones. Gut. 1989;30:1406-1411. [PubMed] |
| 108. | den Toom R, Nijs HG, van Blankenstein M, Schröder FH, Jeekel J, Terpstra OT. Extracorporeal shock wave lithotripsy of pancreatic duct stones. Am J Gastroenterol. 1991;86:1033-1036. [PubMed] |
| 109. | Sauerbruch T, Holl J, Sackmann M, Paumgartner G. Extracorporeal lithotripsy of pancreatic stones in patients with chronic pancreatitis and pain: a prospective follow up study. Gut. 1992;33:969-972. [PubMed] |
| 110. | Schneider HT, May A, Benninger J, Rabenstein T, Hahn EG, Katalinic A, Ell C. Piezoelectric shock wave lithotripsy of pancreatic duct stones. Am J Gastroenterol. 1994;89:2042-2048. [PubMed] |
| 111. | van der Hul R, Plaisier P, Jeekel J, Terpstra O, den Toom R, Bruining H. Extracorporeal shock-wave lithotripsy of pancreatic duct stones: immediate and long-term results. Endoscopy. 1994;26:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Wolf JS, Nakada SY, Aliperti G, Edmundowicz SA, Clayman RV. Washington University experience with extracorporeal shock-wave lithotripsy of pancreatic duct calculi. Urology. 1995;46:638-642. [PubMed] |
| 113. | Schreiber F, Steindorfer P, Pristautz H, Gurakuqi GC, Schnedl W, Trauner M. Complications and surgical interventions during 4 years of biliary extracorporeal shockwave lithotripsy. Hepatogastroenterology. 1996;43:1124-1128. [PubMed] |
| 114. | Johanns W, Jakobeit C, Greiner L, Janssen J. Ultrasound-guided extracorporeal shock wave lithotripsy of pancreatic ductal stones: six years’ experience. Can J Gastroenterol. 1996;10:471-475. [PubMed] |
| 115. | Ohara H, Hoshino M, Hayakawa T, Kamiya Y, Miyaji M, Takeuchi T, Okayama Y, Gotoh K. Single application extracorporeal shock wave lithotripsy is the first choice for patients with pancreatic duct stones. Am J Gastroenterol. 1996;91:1388-1394. [PubMed] |
| 116. | Matthews K, Correa RJ, Gibbons RP, Weissman RM, Kozarek RA. Extracorporeal shock wave lithotripsy for obstructing pancreatic duct calculi. J Urol. 1997;158:522-525. [PubMed] |
| 117. | Costamagna G, Gabbrielli A, Mutignani M, Perri V, Pandolfi M, Boscaini M, Crucitti F. Extracorporeal shock wave lithotripsy of pancreatic stones in chronic pancreatitis: immediate and medium-term results. Gastrointest Endosc. 1997;46:231-236. [PubMed] |
| 118. | Karasawa Y, Kawa S, Aoki Y, Ochi Y, Unno H, Kiyosawa K, Watanabe T. Extracorporeal shock wave lithotripsy of pancreatic duct stones and patient factors related to stone disintegration. J Gastroenterol. 2002;37:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |