Published online Jul 16, 2012. doi: 10.4253/wjge.v4.i7.296
Revised: April 21, 2012
Accepted: July 1, 2012
Published online: July 16, 2012
Endoscopic submucosal dissection (ESD) enables direct submucosal dissection so that even large early-stage gastrointestinal tumors can be resected en bloc. ESD has recently been applied to the colorectum since it was originally developed for use in the stomach. However, colorectal ESD is technically more difficult with an increased risk of perforation compared with gastric ESD. In addition, this procedure is seldom performed in Western countries. Consequently, further technical advances and the availability of a suitable clinical training system are required for the extensive use of colorectal ESD. In this topic highlight, we review the most recent developments in colorectal ESD.
- Citation: Uraoka T, Saito Y, Yahagi N. What are the latest developments in colorectal endoscopic submucosal dissection? World J Gastrointest Endosc 2012; 4(7): 296-300
- URL: https://www.wjgnet.com/1948-5190/full/v4/i7/296.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i7.296
Endoscopic submucosal dissection (ESD) was developed in Japan in the late 1990s to resect early gastric cancer en bloc[1-3]. ESD enables submucosal dissection with direct visualization of the cutting line using special electrosurgical knives, so that even large early-stage gastrointestinal tumors, with severe scarring and/or in difficult locations can be resected en bloc[4,5]. The primary advantages associated with en-bloc resection are enhanced curability and more accurate histological assessment. Accurate histological assessment is essential for predicting the risk of lymph-node metastasis following endoscopic resection of a lesion, which makes it possible to decide on the most suitable treatment strategy for each individual patient[6].
There are several anatomical features of the colorectum, including its longer length, narrower lumen, extensive angulation and thinner walls, which make the colorectal ESD technically more difficult than gastric ESD[7-14]. As a result, colorectal ESDs are not widely performed even by Japanese endoscopists because of the greater level of technical difficulty, longer time of operation and increased risk of immediate or delayed perforations compared with colorectal endoscopic mucosal resection (EMR).
The perforation rate during the early stages of ESD development was more than 10%[11-12]. However, with advances and refinements in various instruments and devices used in colorectal ESDs and accumulated experience of the endoscopists have resulted in decreased perforation rates despite the fact that a systematic educational and clinical training system has yet to be established in Japan.
Given the extent of these ESD limitations, it should come as little or no surprise that colorectal ESDs are seldom performed in Western countries[15] except by a relatively small group of endoscopists most of whom have received specialized training in Japan.
Continued improvement by individual endoscopists in their technical skills, further advance and refinement of instruments and devices such as electricosurgical knives along with the development of even more effective submucosal injection agents and the introduction of improved traction systems should facilitate easier, faster and safer colorectal ESD procedures in the relatively near future. Establishment of a suitable clinical training system will be necessary, however, to encourage the use of colorectal ESD in Japan and elsewhere on a long-term basis.
Colorectal ESD has been proven safe and effective when performed by highly experienced endoscopists although this procedure is not widespread even in Japan and is seldom performed in Western countries[15,16]. The main reasons for this are that colorectal ESD is extremely challenging technically, the operation time is substantially longer than EMR, particularly for less experienced endoscopists in the initial stages of the learning curve, and the risk of perforation is considerably higher than with EMR. Unfortunately, there are no formal educational and clinical training programs for colorectal ESD in Japan at the present time. Likewise, there are no guidelines concerning the most effective training strategy for colorectal ESD with few published reports on this specific subject.
It is necessary to establish a learning curve so as to decrease the colorectal ESD complication rate. We previously reported that the experience of performing at least 50 colorectal ESDs at a number of specialized medical facilities significantly decreased the risk of complications at those facilities with an odds ratio of 0.4[8].
We recommend that a minimum of 20 gastric ESDs should be performed before first attempting colorectal ESD[10], but there is an important distinction between Japan and Western countries that should be noted here. The incidence and detection rates for early stage gastric cancer are much lower in Western countries. It is advisable, therefore, that initial colorectal ESDs undertaken by Western endoscopists should be performed in the rectum because endoscopic treatment of rectal lesions are technically less difficult with a lower risk of perforation. During such rectal ESD procedures, the use of an upper gastrointestinal endoscope is recommended because it is easier to manipulate than a conventional colonoscope. In addition, we suggest that endoscopists begin by performing colorectal ESDs on smaller lesions and less-experienced endoscopists should not attempt to perform colorectal ESDs in more challenging cases including those with larger lesions particularly lesions that are 50 mm or more in size[8] and lesions with ulceration scarring.
Appropriate professional guidance in learning to perform ESD is an important consideration in terms of the learning curve at least in the early phases of such endoscopic training[17,18]. Gastric ESDs performed in Japan under the supervision of experienced endoscopists on 30 lesions by resident endoscopists, who had already learned the basic techniques, were shown to be safe and feasible with equivalent complete resection rates and acceptable complication rates compared with gastric ESDs performed by more experienced endoscopists[17]. Sakamoto et al[18] showed that colorectal ESD can be performed without serious complications by trainee endoscopists under the guidance of experienced specialists. In addition, they suggested that trainee endoscopists can perform colorectal ESDs safely and independently after preparatory training and experience with 30 cases based on retrospective analysis.
The standard needle knife and an insulation-tipped electrosurgical knife (IT knife) (KD-610L; Olympus Co., Tokyo, Japan) were initially used in performing early gastric ESDs, but safer electrosurgical knives intended for use in the esophagus and colorectum have been developed by several Japanese endoscopists during the past decade including: Flex knife (KD-630L; Olympus)[7,14], Hook knife (KD-260R; Olympus)[19], Flush knife (DK2618JN; FUJIFILM, Saitama, Japan)[20], B-Knife™ (Zeon Medical, Tokyo, Japan)[8,9] and Mucosectom® (Pentax, Tokyo, Japan)[10]. All of these knives have been used in colorectal ESDs with varying degrees of success.
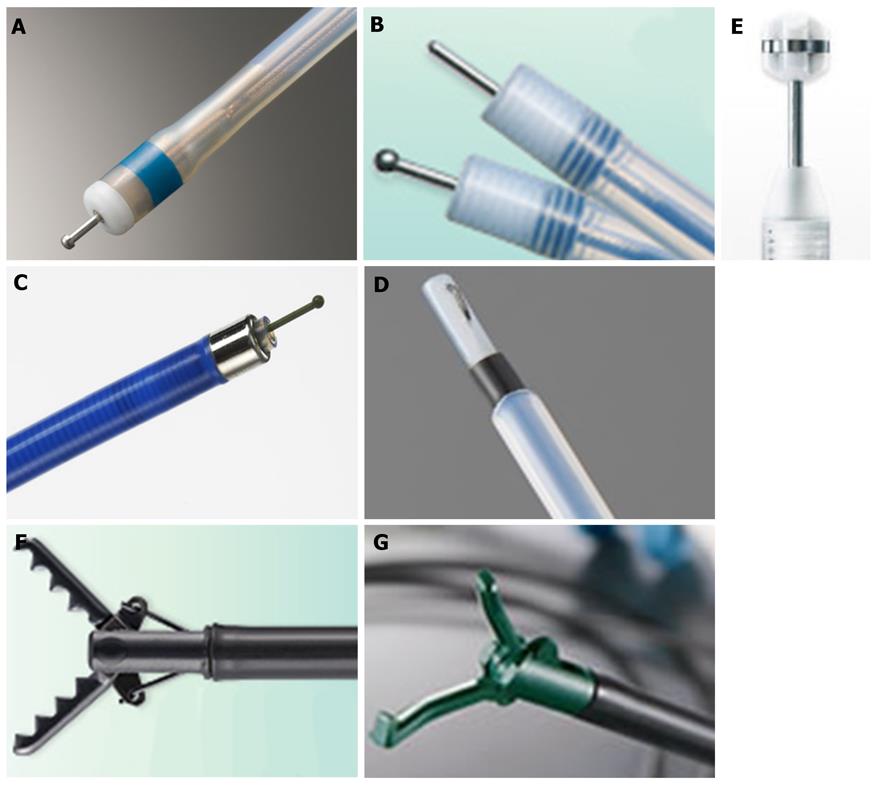
In addition to being considerably safer to use in comparison to earlier instruments, the latest electrosurgical knives feature highly functional points. In fact, several more unique electrosurgical knives have been introduced since late 2010 (Figure 1).
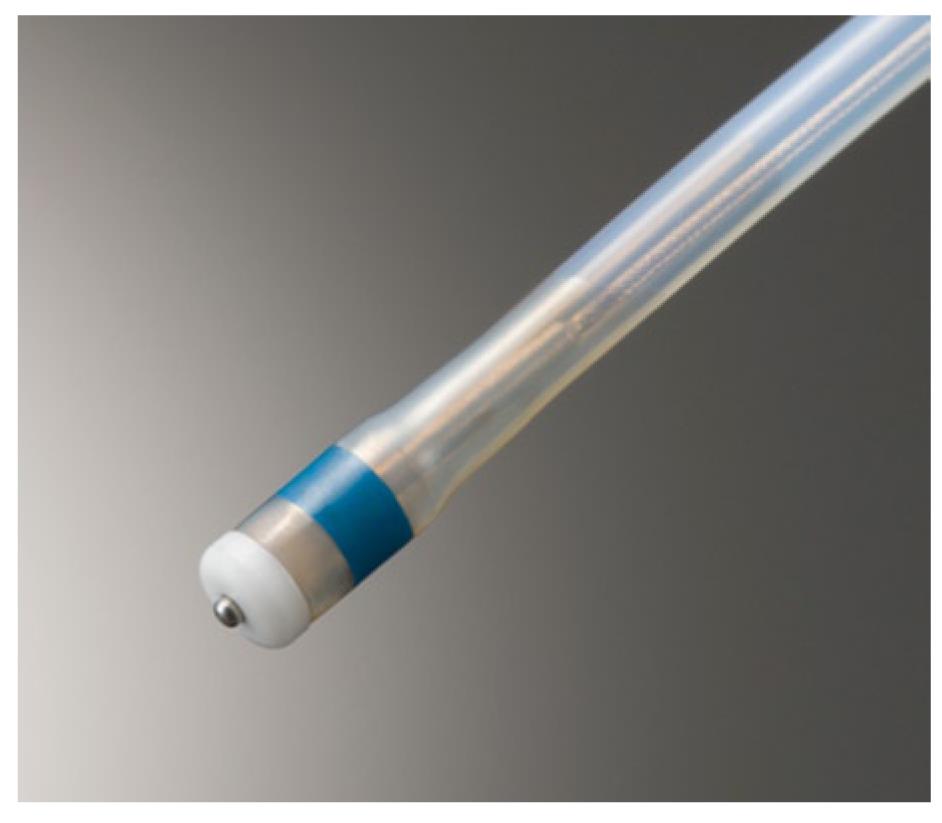
The first of these is a shorter, thinner needle knife with a small apical disk at the tip the Dual knife (KD-650Q: Olympus), which is an improved version of the Flex knife (Figure 1A)[21]. The latest design overcomes some of the previous problems with the Flex knife such as difficulty in adjusting knife length, frequent accumulation of debris at the knife tip during ESD and slippage of the knife tip away from the endoscopic operating field especially in cases involving scarring or loose tissues. The small disk is useful for marking and conducting hemostasis in the closed position (Figure 2) and stabilization of knife movement in the open position (Figure 1A) even in scarring and loose tissue cases. The Dual knife has two different fixed knife lengths: 2 mm for gastric ESD and 1.5 mm for esophageal and colorectal ESDs.
The Flush knife is another kind of needle knife that has the added advantage of allowing local injection. A new Flush knife with a ball-shaped tip [Flush knife ball-tipped (BT) type; DK2618JB; FUJIFILM][22] has recently been developed to improve the hemostatic function of the standard model. In addition, the ball-tip reduces the procedure time in both upper and lower gastrointestinal ESDs compared with the standard Flush knife because it facilitates scooping up incision and dissection tissues.
Finally, the B-Knife is a bipolar current knife that results in safer procedures by reducing the risk of perforations occurring during ESD. Although a ball-tipped type B-Knife had previously been developed, two tongue-type electrosurgical knives (Figure 1E and F) have also been reported as being safer to use recently[23,24]. The basic cutting technique of these knives involves grasping the mucosal or submucosal tissues and pulling back with coagulation resulting in a safer procedure although cutting speed is reduced. A report on using these knives in more difficult colorectal ESD cases is expected reasonably soon.
Submucosal injection solutions are used to lift lesions, but the lengthier ESD procedure requires a longer-lasting elevation to provide direct visualization of the cutting line during dissection of the submucosal layer. Japanese endoscopists generally use glycerol, which consists of 10% glycerol and 5% fructose in normal saline solution[25], along with a small amount of indigo-carmine dye and sodium hyaluronate acid injected into the submucosal layer[26] as submucosal injection agents for colorectal ESDs. The use of these agents has resulted in safer, easier and more effective ESDs than using just normal saline.
We also have successfully demonstrated the efficacy of using CO2 as a satisfactory submucosal injection agent during ESD procedures in preliminary animal studies[27]. An important advantage of CO2 injection is that the increased pressure from the CO2 produces a partial physical dissection of the fibrous submucosal connective tissues thereby making it easier to dissect the submucosal layer. Other important advantages besides its overall effectiveness are: CO2 does not cause tissue damage, is non-allergenic, safer for patients, relatively inexpensive and commonly available worldwide. The next stage of our investigation on the effectiveness of CO2 as a submucosal injection agent will involve a larger number of porcine models and practical clinical demonstrations.
In conclusion, the use of colorectal ESD has been proven to be both safe and highly effective in Japan when performed primarily by a selected group of highly skilled and experienced endoscopists. With further technical advances and refinements and the establishment of a suitable clinical training system required, however, before performing colorectal ESDs, colorectal ESD will become more common in clinical practice not only in Japan, but throughout the rest of the world as well.
Peer reviewer: Somchai Amornyotin, Associate Professor, Department of Anesthesiology, Siriraj gastrointestinal Endoscopy Center, Faculty of Medicine Siriraj Hospital, Mahdol University, Bangkok 10700, Thailand
S- Editor Yang XC L- Editor Ma JY E- Editor Yang XC
| 1. | Hosokawa K, Yoshida S. [Recent advances in endoscopic mucosal resection for early gastric cancer]. Gan To Kagaku Ryoho. 1998;25:476-483. [PubMed] |
| 2. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 3. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 4. | Yoshinaga S, Gotoda T, Kusano C, Oda I, Nakamura K, Takayanagi R. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202-209. [PubMed] |
| 5. | Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 378] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 592] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 9. | Uraoka T, Kato J, Ishikawa S, Harada K, Kuriyama M, Takemoto K, Kawahara Y, Saito Y, Okada H. Thin endoscope-assisted endoscopic submucosal dissection for large colorectal tumors (with videos). Gastrointest Endosc. 2007;66:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Uraoka T, Kawahara Y, Kato J, Saito Y, Yamamoto K. Endoscopic submucosal dissection in the colorectum: present status and future prospects. Dig Endosc. 2009;21 Suppl 1:S13-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S. Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Uraoka T, Higashi R, Kato J, Kaji E, Suzuki H, Ishikawa S, Akita M, Hirakawa T, Saito S, Hori K. Colorectal endoscopic submucosal dissection for elderly patients at least 80 years of age. Surg Endosc. 2011;25:3000-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Yahagi N, Fujishiro M, Imagawa A, Kakushima N, Naomi Kakushima N, Mikitaka Iguchi M, Omata M. Endoscopic submucosal dissection for the reliable en bloc resection of colorectal mucosal tumors. Digest Endosc. 2004;16 Suppl: S89-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Parra-Blanco A, Arnau MR, Nicolás-Pérez D, Gimeno-García AZ, González N, Díaz-Acosta JA, Jiménez A, Quintero E. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol. 2010;16:2895-2900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Bourke M. Current status of colonic endoscopic mucosal resection in the west and the interface with endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S22-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum. 2011;54:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 20. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Yahagi N, Uraoka T, Ida Y, Hosoe N, Nakamura R, Kitagaw Y, Ogata H, Hibi T. Endoscopic submucosal dissection using the Flex and the Dual knives. Tech Gastrointest Endosc. 2011;13:74-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [PubMed] |
| 23. | Honma K, Kobayashi M, Watanabe H, Suga T, Tominaga K, Yamagata M, Hiraishi H. Endoscopic submucosal dissection for colorectal neoplasia. Dig Endosc. 2010;22:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Akahoshi K, Okamoto R, Akahane H, Motomura Y, Kubokawa M, Osoegawa T, Nakama N, Chaen T, Oya M, Nakamura K. Endoscopic submucosal dissection of early colorectal tumors using a grasping-type scissors forceps: a preliminary clinical study. Endoscopy. 2010;42:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Uraoka T, Kawahara Y, Ohara N, Kato J, Hori K, Okada H, Yamamoto K. Carbon dioxide submucosal injection cushion: an innovative technique in endoscopic submucosal dissection. Dig Endosc. 2011;23:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |