Published online May 16, 2012. doi: 10.4253/wjge.v4.i5.185
Revised: February 22, 2012
Accepted: April 27, 2012
Published online: May 16, 2012
AIM: To investigate the role of capsule endoscopy in patients with persistent perianal disease and negative conventional work up for Crohn’s disease (CD).
METHODS: Patients with perianal disease (abscesses, fistulas, recurrent fissures) were evaluated for underlying CD. Patients who had a negative work up, defined as a negative colonoscopy with a normal ileoscopy or a normal small bowel series or a normal CT/MR enterography, underwent a Pillcam study of the small bowel after signing informed consent. Patients using nonsteroidal anti-inflammatory drugs or who had a history of inflammatory bowel disease or rheumatic disease were excluded.
RESULTS: We recruited 26 patients aged 21-61 years (average 35.6 years), 17 males and 9 females. One case could not be evaluated since the capsule did not leave the stomach. In 6 of 25 (24%) patients with a negative standard work up for Crohn's disease, capsule endoscopy (CE) findings were consistent with Crohn's disease of the small bowel. Family history of CD, white blood cell, hemoglobin, erythrocyte sedimentation rate or C-reactive protein did not predict a diagnosis of CD. Capsule endoscopy findings led to a change in treatment.
CONCLUSION: In patients with perianal disease and a negative conventional work up to exclude CD, CE leads to incremental diagnostic yield of 24%.
- Citation: Adler SN, Yoav M, Eitan S, Yehuda C, Eliakim R. Does capsule endoscopy have an added value in patients with perianal disease and a negative work up for Crohn's disease? World J Gastrointest Endosc 2012; 4(5): 185-188
- URL: https://www.wjgnet.com/1948-5190/full/v4/i5/185.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i5.185
Crohn’s disease (CD) is a chronic inflammatory disease that may manifest itself throughout the gastrointestinal tract or by extra intestinal symptoms. The primary presentation of CD may be perianal disease (PD). This presentation is not infrequent. The prevalence of PD in CD ranges between 4% and 80%[1,2]. The large discrepancies in prevalence may be due to the variable defining criteria. In up to 36% of patients, PD precedes the overt intestinal disease, but in the majority of patients, PD occurs either concurrently or after the diagnosis of small bowel CD[1,3]. Mild manifestations of PD include fissures, skin tags and hemorrhoids, whereas perianal abscesses, rectocutaneous or rectovaginal fistulas, cavitating ulcers and/or anorectal dense strictures are defined as severe PD. Severe PD usually carries a poor prognosis. Many of these patients ultimately will require a proctectomy and a permanent stoma[4]. For many years, PD was considered to be a variant of penetrating CD. According to the Vienna Classification of CD[5], PD at any time in the course of the disease is defined as penetrating disease. This categorization has been challenged and is defined separately in the Montreal classification[6].
Recent studies have shown that perianal CD may be a distinct phenotype, possibly associated with specific susceptibility genes and/or environmental factors, and not related to the “classic” penetrating disease[7-9].
Capsule endoscopy (CE) was introduced in early 2000 and became a very powerful and patient friendly tool to investigate the small bowel. Meta analysis has shown that CE has a significantly higher diagnostic yield for small bowel lesions compared to small bowel follow through exams or CT enterography in patients with either suspected or known CD[10].
The aim of our 3 center study was to investigate whether CE provided any additional diagnostic benefit to patients with PD and a negative standard work-up for CD.
We recruited patients with “severe” PD, i.e., perianal fistula or rectal abscess, aged 10-80 years in our study. All of these patients had to have had a normal gastrointestinal investigation within the prior 3 mo to qualify for inclusion in this study. A normal gastrointestinal investigation was defined as a normal ileo-colonoscopy, normal colonoscopy and normal small bowel follow through examination or a normal colonoscopy and a normal CT enterography. We excluded patients with a history of established CD or those with nonsteroidal anti-inflammatory drugs (NSAIDs) usage. All patients signed a written informed consent and the study was approved by the local IRBs of the participating hospitals.
The patients were on a clear liquid diet for 24 h and a 12 h fast prior to capsule ingestion (PillCam SB2, Given Imaging, Yoqneam, Israel). They were allowed to drink clear liquids 2 h after ingestion and to eat a light meal 4 h post ingestion. The data recorder 2 was removed when the capsule ceased to transmit images and data was processed by Rapid 6 software and read by 3 investigators (SA, RE, ES). Complete blood count, sedimentation rate, C reactive protein and inflammatory bowel disease (IBD) serology were recorded from patients’ files.
Twenty six patients aged 21-61 years were recruited to this study (mean 35.6 years), 17 males and 9 females. One patient was excluded from the study because of gastric capsule retention.
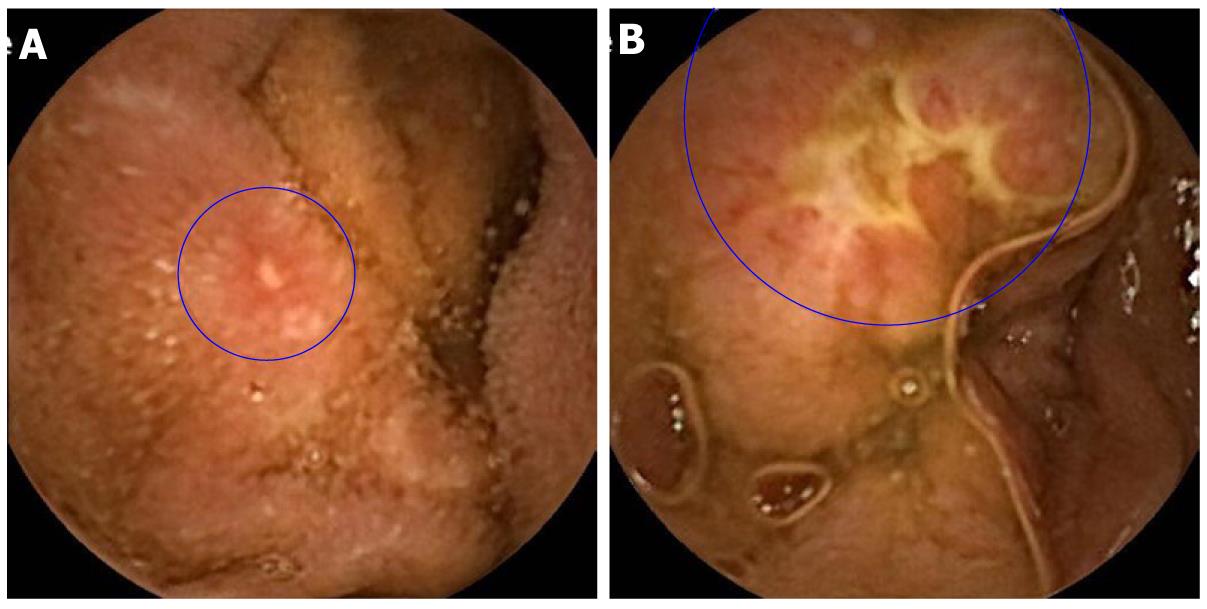
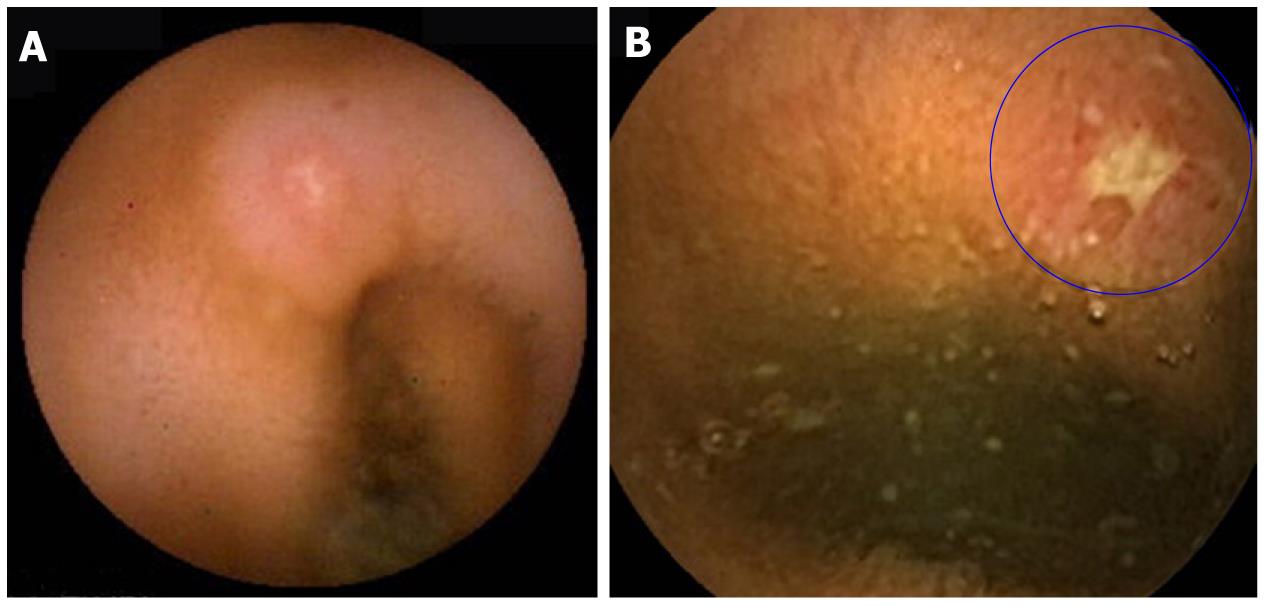
Capsule examination of the small bowel revealed findings compatible with small bowel CD (apthous ulcerations, linear ulcers, circumferential ulcers) in six of the remaining 25 patients (24%) (Figures 1 and 2).
No serious adverse events and no small bowel capsule retention occurred. The observed findings at CE led to change in treatment in all six patients. Statistical analysis of laboratory findings such as CBC, CRP, ESR or family history of IBD, did not reveal any association with CE findings (Table 1).
| Normal SB | SB CD | |
| Age (yr) | 36.05 ± 10.62 | 34.5 ± 12.07 |
| ESR (mm/h) | 16.18 ± 16.19 | 21.5 ± 15.32 |
| CRP (X upper limit) | 3.18 ± 4.11 | 3.616 ± 1.63 |
| Hemoglobin (mg %) | 14.25 ± 1.82 | 13.96 ± 0.99 |
| WBC (x 103/µL) | 10.46 ± 8.42 | 8.11 ± 1.57 |
| Family history of IBD | 10.52% | 16.60% |
Perianal disease may be the primary manifestation of small bowel CD in up to 36% of cases, or be associated with clinically active small bowel CD[1-3]. The spectrum of PD is wide. The mild form of PD includes fissures, skin tags and hemorrhoids, whereas perianal abscesses, external or rectovaginal fistulas, and/or anorectal strictures are the severe manifestations of PD. Physicians treating patients with PD often order a small bowel follow through examination, an entero-CT or a magnetic resonance enterography or perform an ileo-colonoscopy for the investigation of possible small bowel CD.
CE has gained an important role in the investigation of small bowel disease in general and in suspected Crohn’s disease specifically[11]. Many studies comparing CE to other radiographic procedures have been performed over the past ten years. A recent meta-analysis found CE to have a significantly higher diagnostic yield for small bowel pathologies compatible with CD than small bowel follow through examinations, entero CT and even ileo-colonoscopy[10]. Moreover, many studies using CE have found proximal small bowel lesions in up to 50% of patients, lesions that were not detected by other modalities[12,13]. Thus, it is logical to assume that in patients with PD and negative conventional investigations, the addition of a CE examination would increase the diagnostic yield, as demonstrated in the present study. The specificity of the capsule findings can be challenged. To avoid the most frequent imitators, we excluded patients who were on NSAIDs or patients who had taken NSAIDs in the 2 mo prior to capsule ingestion. In fact, studies in patients who were not taking NSAIDs for longer than 1 mo did not show any small bowel pathology[14]. Thus, we think our finding truly reflect small bowel CD.
In summary, CE can make the diagnosis of small bowel CD in an additional one quarter of patients presenting with severe PD and who had a negative conventional work up to exclude CD. Future studies are needed to determine whether in fact CE should be the primary investigational tool in such patients.
Perianal disease such fissure, fistula and abscesses in the ano rectal area can be a manifestation of Crohn’s Disease. The diagnosis of Crohn’s Disease traditionally has been made using barium follow through studies, computed tomographic (CT) examinations and colonoscopy with ileoscopy. In the past ten years, capsule endoscopy of the small bowel has become available. Capsule endoscopy has been shown to have a higher diagnostic yield in the diagnosis of Crohn’s disease compared to barium or CT studies. The question of interest is whether capsule endoscopy is more sensitive in diagnosing Crohn’s disease in patients with perianal disease than established traditional methods.
Capsule endoscopy is a miniature camera that is swallowed and travels through the esophagus, stomach, small intestine and is excreted after passing through the colon. This miniature camera transmits high quality color images from the small bowel to an outside recorder. These images of the surface of the small bowel reveal even minor inflammatory changes such as apthous ulceration and mucosal hemorrhages, which methods such as barium studies and CT examinations cannot appreciate. Capsule endoscopy has proven to be more sensitive in the diagnosis of Crohn’s disease.
With the advent of capsule endoscopy of the small bowel, it has become easy to assess the surface of the small bowel. This, in turn, has given doctors and researchers a more sensitive tool to diagnose Crohn’s disease of the small bowel. Conventional methods have relied on barium studies of the small bowel, CT examinations of the small bowel and ileo-colonoscopy. The diagnostic sensitivity of these methods is inferior to the diagnostic sensitivity of capsule endoscopy for luminal disease.
The study results suggests that capsule endoscopy diagnoses Crohn’s disease in 24% of patients with perianal disease who were thought not to have Crohn’s disease after a conventional work up for Crohn’s disease, which included ileo-colonoscopy, CT exam of the small bowel or barium studies of the small bowel.
Capsule endoscopy is a capsule containing a miniature camera and a transmission system that sends color images from the intestines of the examinee to an outside recorder. Crohn’s disease: chronic inflammatory disease of unknown origin affecting mainly the small bowel and the right colon. Crohn’s disease can lead to inflammatory disease of the perianal area with abscesses, fistula formation and severe fissuring.
The manuscript is perfectly written, the methodology is correct and the discussion is also informative.
Peer reviewer: István Rácz, MD, PhD, Professor, Head of Internal Medicine, Department of Gastroenterology, Petz Aladár County and Teaching Hospital, Győr, Hungary
S- Editor Yang XC L- Editor Roemmele A E- Editor Yang XC
| 1. | Sangwan YP, Schoetz DJ, Murray JJ, Roberts PL, Coller JA. Perianal Crohn's disease. Results of local surgical treatment. Dis Colon Rectum. 1996;39:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | McClane SJ, Rombeau JL. Anorectal Crohn's disease. Surg Clin North Am. 2001;81:169-83, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Williams DR, Coller JA, Corman ML, Nugent FW, Veidenheimer MC. Anal complications in Crohn's disease. Dis Colon Rectum. 1981;24:22-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Alexander-Williams J, Buchmann P. Perianal Crohn's disease. World J Surg. 1980;4:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 6. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5-36. [PubMed] |
| 7. | Smith BR, Arnott ID, Drummond HE, Nimmo ER, Satsangi J. Disease location, anti-Saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn's disease. Inflamm Bowel Dis. 2004;10:521-528. [PubMed] [DOI] [Full Text] |
| 8. | Sachar DB, Bodian CA, Goldstein ES, Present DH, Bayless TM, Picco M, van Hogezand RA, Annese V, Schneider J, Korelitz BI. Is perianal Crohn's disease associated with intestinal fistulization? Am J Gastroenterol. 2005;100:1547-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, Targan SR. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-128; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (36)] |
| 11. | Eliakim R, Suissa A, Yassin K, Katz D, Fischer D. Wireless capsule video endoscopy compared to barium follow-through and computerised tomography in patients with suspected Crohn's disease--final report. Dig Liver Dis. 2004;36:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Mehdizadeh S, Chen GC, Barkodar L, Enayati PJ, Pirouz S, Yadegari M, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Capsule endoscopy in patients with Crohn's disease: diagnostic yield and safety. Gastrointest Endosc. 2010;71:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Petruzziello C, Onali S, Calabrese E, Zorzi F, Ascolani M, Condino G, Lolli E, Naccarato P, Pallone F, Biancone L. Wireless capsule endoscopy and proximal small bowel lesions in Crohn's disease. World J Gastroenterol. 2010;16:3299-3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |