Published online Dec 16, 2012. doi: 10.4253/wjge.v4.i12.565
Revised: July 4, 2012
Accepted: October 20, 2012
Published online: December 16, 2012
AIM: To investigate the feasibility and safety of the treatment of an upper gastrointestinal (GI) submucosal tumor with endoscopic submucosal dissection (ESD).
METHODS: A total of 20 patients with esophageal and gastric submucosal tumors emerged from the muscular layer identified by endoscopic ultrasonography were collected from January 2009 to June 2010. Extramural or dumbbell-like lesions were excluded by an enhanced computerized tomography (CT) scan. All patients had intravenous anesthesia with propofol and then underwent the ESD procedure to resect these submucosal tumors. The incision was closed by clips as much as possible to decrease complications, such as bleeding or perforation, after resection of the tumor. All the specimens were collected and evaluated by hematoxylin, eosin and immunohistochemical staining, with antibodies against CD117, CD34, desmin, α-smooth muscle actin and vimentin to identify the characteristics of the tumors. Fletch’s criteria was used to evaluate the risk of gastrointestinal stromal tumors (GISTs). All patients underwent a follow-up endoscopy at 3, 6 and 12 mo and CT scan at 6 and 12 mo.
RESULTS: The study group consisted of 5 men and 15 women aged 45-73 years, with a mean age of 60.2 years. Three tumors were located in the esophagus, 9 in the gastric corpus, 4 in the gastric fundus, 3 lesions in the gastric antrum and 1 in the gastric angulus. Apart from the one case in the gastric angulus which was abandoned due to being deeply located in the serosa, 94.7% (18/19) achieved complete gross dissection by ESD with operation duration of 60.52 ± 30.32 min. The average maximum diameter of tumor was 14.8 ± 7.6 mm, with a range of 6 to 30 mm, and another lesion was ligated by an endoscopic ligator after most of the lesion was dissected. After pathological and immunohistochemical analysis, 12 tumors were identified as a GI stromal tumor and 6 were leiomyoma. Mitotic count of all 12 GIST lesions was fewer than 5 per 50 HPF and all lesions were at very low (9/12, 75.0%) or low risk (3/12, 25.0%) according to Fletch’s criteria. Procedure complications mainly included perforation and GI bleeding; perforation occurred in 1 patient and conservative treatment succeeded by a suturing clip and no post-operative GI bleeding occurred. All patients were followed up for 6.5 ± 1.8 mo (range, 3-12 mo) by endoscopy and abdominal CT. Local recurrence and metastasis did not occur in any patient.
CONCLUSION: ESD shows promise as a safe and feasible technique to resect esophageal and gastric submucosal tumors and the incidence of complications was very low. Clinical studies with more subjects and longer follow-up are needed to confirm its treatment value.
- Citation: Huang ZG, Zhang XS, Huang SL, Yuan XG. Endoscopy dissection of small stromal tumors emerged from the muscularis propria in the upper gastrointestinal tract: Preliminary study. World J Gastrointest Endosc 2012; 4(12): 565-570
- URL: https://www.wjgnet.com/1948-5190/full/v4/i12/565.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i12.565
Gastrointestinal stromal tumors (GIST) are the most common submucosal tumor in the gastrointestinal (GI) tract, accounting for 80% of GI mesenchymal neoplasms, although they are rare, representing only 0.1% to 3% of all GI malignancies[1,2]. It is estimated that there are about 5000 new cases per year in the United States[3]. As more of these lesions are found by virtue of the greater availability of endoscopy, a strategy to manage these lesions will need to be developed.
GISTs show a wide variety of clinical behavior, from benign to frankly malignant, and outcome in individual patients remains difficult to predict[4]. Important parameters of malignancy and prognosis are thought to include tumor size, mitotic index, necrosis, cellularity and proliferative index[5,6]. However, these parameters need en bloc specimens and limit their utility in screening and follow-up. To date, total surgical resection still constitutes the only standard treatment for non-metastatic GISTs[7,8] and a wide surgical margin is not necessary for total resection as long as the premise of negative margins is respected in comparison with that of other GI malignant tumors[3].
With more and more submucosal tumors smaller than 2 cm being found, the management of these lesions is a dilemma in clinic practice as they comprise a range of diverse diagnoses (GIST, leiomyoma, ectopic pancreas, neuroendocrine tumor and lipoma)[9]. The diagnosis of a GIST must always be investigated since every GIST is potentially malignant[10,11]. The recent rapid advances in endoscopic intervention therapy provide a potential method for en bloc resection of GISTs.
The aim of our study is to investigate the clinical outcomes of endoscopic dissection of small stromal tumors in the upper GI and evaluate the feasibility and safety of endoscopic dissection of the smaller, low risk submucosal stromal tumors.
From January 2009 to June 2010, all 20 patients diagnosed with submucosal tumors emerged from the muscular layer by endoscopic ultrasound (EUS) underwent endoscopic dissection. Extramural or dumbbell-like lesions were excluded by enhanced computerized tomography (CT) scan. Blood routine and prothrombin time were both normal. Nonsteroidal anti-inflammatory drugs such as aspirin and clopidogrel were discontinued on the third preoperative day. All participants signed informed consent before endoscopic dissection.
Prior to an endoscopic dissection, all lesions were examined with EUS (GF-UC240P-AL5, Olympus). All patients had intravenous anesthesia with propofol. Endoscopic submucosal dissection (ESD) procedures were performed with the following steps. The margins of the lesion were marked with electrocautery (40W soft coagulation) to determine the resection border. A salt solution containing 0.005 mg/mL epinephrine and 0.1% indigo carmine was injected into the submucosa. After sufficient lifting, a hook knife (KD 620LR, Olympus) was used to create a circumferential incision around the lesion extending into the submucosa. After the circumferential incision, a submucosal dissection with IT knife-2 (KD 611L, Olympus) was made carefully by using the endocut mode of electrosurgical accessories (ICC300; Erbe Co). To avoid bleeding, small vessels were coagulated directly by knives; large vessels with high bleeding risk were coagulated with hemostatic forceps (Olympus). The body of the tumor was gradually exposed and bulged out when the incision was wide enough and the submucosal tumor was snared or continued to be dissected by the IT knife after complete exposure of the root of the tumor. After resection of the tumor, the incision was closed by clips as much as possible to decrease complications, such as bleeding or perforation, and then the specimen was collected with three-claw forceps or a stone basket.
Immunohistochemical staining with antibodies against CD117, CD34, desmin, α-smooth muscle actin (α-SMA) and vimentin was used to identify the tumors. Fletch’s criteria[12] was used to evaluate the risk of GISTs.
All the specimens were evaluated by hematoxylin and eosin stain and immunohistochemistry. Complete gross dissection was defined as the removal of the lesion with the complete capsule, the so-called margin of the lesion was unavailable in the ESD procedure. Patients underwent follow-up endoscopy at 3, 6 and 12 mo and CT scan at 6 and 12 mo.
The series consisted of 5 men and 15 women aged 45-73 years, with a mean age of 60.2 years. 19 patients (95%) underwent endoscopy because of epigastric discomfort or other dyspeptic symptoms and 1 patient with a 3 cm submucosal lesion presented with upper GI bleeding.
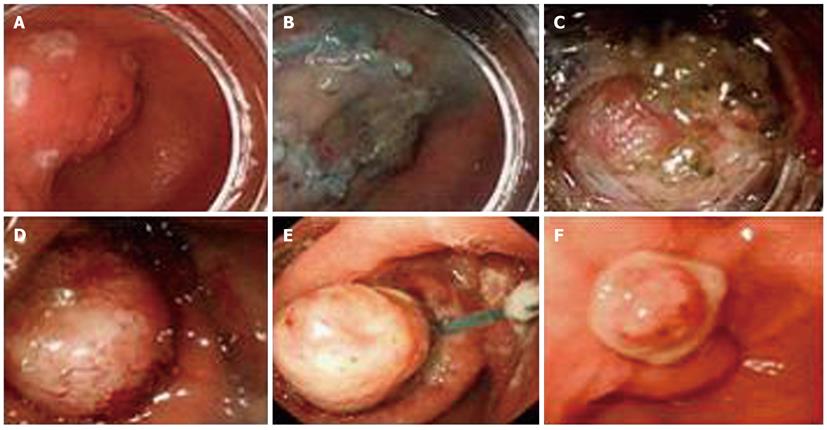
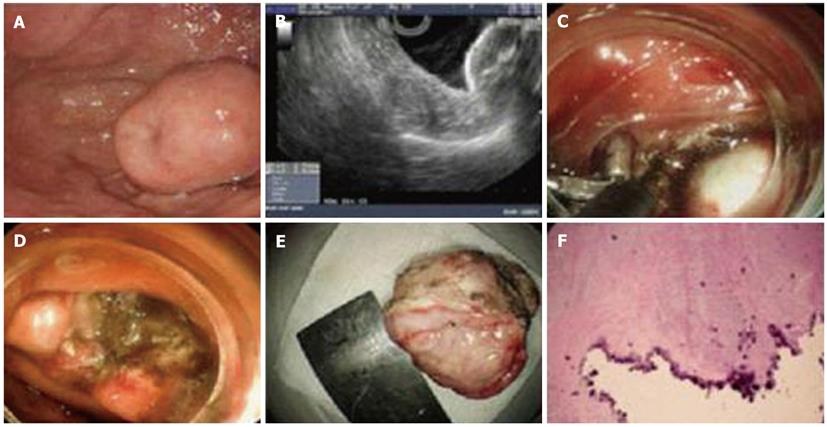
Almost all of the patients underwent successful endoscopic dissection, except for one case in the gastric angulus which was abandoned due to being deeply located in serosa. Operation duration was 60.52 ± 30.32 min and the average blood loss was estimated to be less than 50 mL during operation. Furthermore, complete gross dissection was achieved in 94.7% (18/19) of patients without tumor rupture occurring. One lesion located in the antrum, 2.5 × 2.0 cm in size, was ligated by an Olympus HX-20-1 endoscopic ligator with a detachable “endoloop” after most of the lesion was dissected due to conglutination with the muscularis propria and the lesion was found to have completely disappeared in the follow-up after 1 mo (Figure 1). Perforation occurred in one case; the incision was sutured by metal clips (HX-610-090, Olympus), the gastric tube was detained for 3 d and then the patient discharged after 3 d without any complications. All patients were followed up for 6.5 ± 1.8 mo (range, 3-12 mo) by endoscopy and abdominal CT; local recurrence and metastasis did not occur in any patient.
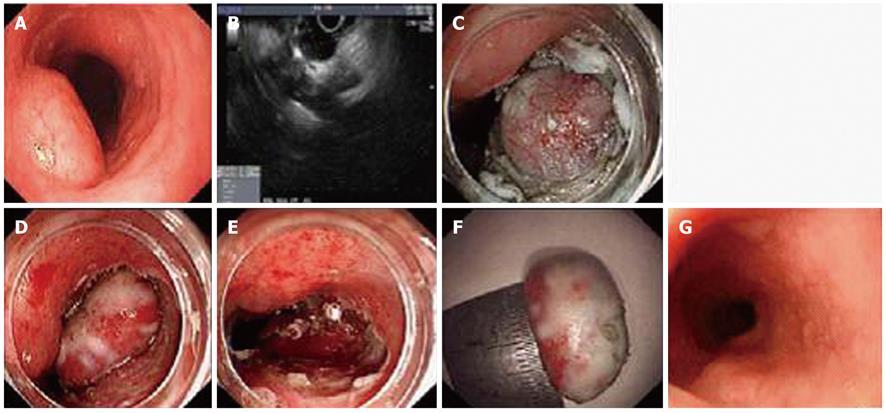
Three tumors were located in the esophagus (Figure 2), 9 in the gastric corpus, 4 in the gastric fundus (Figure 3), 3 lesions in the gastric antrum and 1 in the gastric angulus. The average size of tumors was 14.8 ± 7.5 mm, with a range of 6 to 30 mm. Pathological and immunostaining results showed that the 12 submucosal tumors were GISTs and were all located in stomach. 6 lesions were leiomyoma, of which 3 lesions were located in esophagus and the others in the gastric fundus. All the results are shown in Table 1. The mitotic count of 12 GIST lesions was all fewer than 5 per 50 HPF and all lesions were at very low (9/12, 75.0%) or low risk (3/12, 25.0%) according to Fletch’s criteria. Among the CD117-positive lesions, CD34 staining was positive in 91.7% (11/12) lesions, vimentin was positive in all lesions and SMA coexisted in 25.0% (3/12) lesions.
| Tumor size | |
| < 1 cm | 10 (50) |
| 1-2 cm | 6 (30) |
| 2-3 cm | 4 (20) |
| Tumor location | |
| Esophagus | 3 (15) |
| Stomach | |
| Fundus | 4 (20) |
| Body | 9 (45) |
| Antrum | 3 (15) |
| Angulus | 1 (5) |
| Pathological results | |
| Leiomyoma | 6 (33) |
| GIST | 12 (67) |
Generally, all GISTs are considered to be potentially malignant whatever their size, small or large[13,14], and surgery remains the primary treatment for patients with resectable, localized GIST > 2 cm in size. For submucosal tumors smaller than 2 cm in the upper GI tract, the management of these lesions is difficult to handle and the diagnosis of GIST always needs to be identified[15]. Although there are prognostic factors that can stratify GISTs according to their biological behavior, reports exist about the development of metastasis, even in low risk lesions (lesions between 2 cm and 5 cm with fewer than 5 mitoses/50HPF)[16,17], and this means that, if a GIST is suspected, the lesion needs to be resected completely so as to allow risk stratification and reduce the likelihood of metastasis or tumor growth, even when it is small lesion. So, patients with very low or low risk GISTs face two choices: follow-up and bear a heavy psychological burden for a lifetime or undergo surgery. Most patients decline the follow-up option if they know that there is a minimally invasive technology such as endoscopic resection.
As for the endoscopic approach, endoscopic treatment of GISTs has been little reported for a long time but the role of endoscopy has remained controversial owing to the increased risk of procedure-related complications, such as bleeding, perforation or incomplete resection. The modality of endoscopic treatment includes band ligation[18], polypectomy snare[19] and submucosal dissection[20-23]. The main defect of band ligation is that sloughed specimens are not available for pathological confirmation and risk evaluation and direct polypectomy snare for submucosal tumor face a high risk of perforation and bleeding. In 2010, Bai et al[22] reported that submucosal dissection technology for small GISTs < 2 cm in stomach is feasible with a 28% perforation rate, obviously higher than an overall 4% perforation in ESD for early gastric cancer. Marshall et al[24] thought that the high perforation rate in small GISTs dissection cannot be accepted for a very benign condition and the best approach would still be follow-up for small, low risk GISTs unless safer endoscopic technology becomes available. As for full-thickness resection, Zhou et al[23] reported the complete resection rate was 100% in 26 patients with a submucosal tumor at a single endoscopy center; no bleeding, peritonitis, abdominal abscess occurred after full-thickness resection and so this technique brought a promising approach to deep, even extramural lesions, but its feasibility needs large scale clinical trials to confirm and resolution of a full-thickness suture technique might be pivotal for its application.
In our case series, the overall removal rate for a submucosal tumor is 95% (19/20) without incidence of serious complications. Sufficiently lifting submucosal tissue around the lesions and prophylactic hemostasis for small vessels are the key technology and blunt dissection by negative pressure suction using a transparent cap is a very helpful skill for facilitating and speeding up the procedure. The difficulty of the procedure is changed by the location of lesions. Lesions located in the fundus, especially adjacent to the cardiac orifice, are much more difficult to resect and the other difficult places include the posterior and lesser curvature in the corpus, the posterior wall of the antrum and the angular notch. To avoid delayed perforation, we selected an endoloop to ligate the lesion after most of lesion was dissected. The main complication (5%, 1/20) is perforation which occurred in only one patient. The incidence of perforation in our series is similar to that of ESD for early gastric cancer. If perforation occurs, endoscopic suture with a clip is the first-line choice and the case of perforation in our study was rehabilitated successfully without surgery. In addition, we suggested that the size of lesions for ESD is better not to exceed 3 cm, as lesions > 3 cm are very difficult to be taken out of the esophageal upper orifice.
In our study, GISTs consisted of 66.7% (12/18) of submucosal lesion confirmed by histopathology; the others were leiomyoma (33.3%, 6/18). Esophageal leiomyoma is the most common benign mesenchymal tumor, accounting for two-thirds of esophageal benign tumors; esophageal leiomyomas less than 5 cm in size generally cause no symptoms[25]. For those larger than 5 cm, surgery or a thoracoscopic operation is always preferentially considered[26,27]. Here, we have successfully resected 3 cases of esophageal leiomyoma emerged from the muscularis propria by endoscopy without any complications, in which the maximum diameter is 2 cm. In fact, most of small esophageal leiomyomas < 1 cm were located in the submucosal layer and we snared directly or resected these lesions by endoscopic mucosal resection (data not shown).
Follow-up in our patients ranged from 3 to 12 mo (mean, 6.5 ± 1.8 mo), during which no recurrence was observed in any patient by endoscopy or EUS. However, judgment of complete resection of GISTs seems to be a little premature because of the limited cases and relatively short follow-up duration. The superiority of endoscopic dissection is en bloc removal of entire tumors just like surgical resection but surgery can provide a margin of normal tissue to make a clear pathological diagnosis regarding complete removal of the lesion. Fortunately, a wide margin of normal tissue is not needed in GIST resection, so we can perform endoscopic treatment on GISTs. According to suggested guidelines for accessing malignant potential of GISTs, small GISTs ≤ 3 cm are considered to have a low risk of malignant potential. We recommend that every patient should have close follow-up after endoscopic treatment and it is always beneficial for patients to understand the need for long-term follow-up.
In conclusion, ESD for submucosal tumors shows promise as a relatively safe and feasible technique to treat small submucosal tumors ≤ 3 cm in the upper GI tract. In our pilot study, ESD allowed complete resection of lesions, definitive diagnosis and risk stratification for GISTs without serious procedure-related complications. However, further clinical trials involving many more subjects and a longer period of follow-up are needed.
Gastrointestinal stromal tumors (GISTs) are the most common submucosal tumors with potential malignancy in the upper gastrointestinal (GI) tract, no matter if their size is small or large, and it is difficult to predict their properties. Total surgical resection remains the mainstay of treatment for non-metastatic GISTs. The recent rapid advances in endoscopic techniques, such as endoscopic submucosal dissection (ESD), provide potential alternative therapeutical methods. However, the feasibility and safety of ESD for GISTs need further clinical evaluation.
The role of endoscopy for treatment of GISTs has remained controversial because of the risk of procedure-related complications and incomplete resection. However, as a wide negative margin is not needed for GISTs resection, endoscopic management for GISTs is still worth exploring, especially for GISTs no more than 2 cm.
In the previous studies of ESD for submucosal tumors, a high perforation rate was unacceptable for a relative benign condition and the so-called initiative perforation full-thickness resection for GISTs needed further clinical evaluation unless a safer full-thickness suture was developed. The results showed that ESD for small submucosal tumors in the upper GI tract was a relatively safe and feasible optional treatment besides surgery, but endoscopic ultrasonography should be carried out to evaluate the depth and origin of lesions before endoscopic treatment which would help to avoid unnecessary perforation.
The results suggest that the ESD technique for small submucosal tumors emerged from submucosal or muscularis propria layer in the upper GI tract is safe and has clinical benefits, such as diagnostic value and a decrease of psychological burden for patients.
In this manuscript, the authors described how they obtained the complete resection of submucosal tumors under 3 cm in the upper GI tract and they provide a full account of what is necessary to resect the lesions.
Peer reviewers: Naoto Sakamoto, Associate Professor, Department of Gastroenterology, Juntendo University, 2-1-1 Hongo Bunkyo-ku Tokyo, Tokyo 113-8421, Japan; Noriya Uedo, Director, Endoscopic Training and Learning Center, Department of Gastrointestinal Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-0025, Japan
S- Editor Song XX L- Editor Roemmele A E- Editor Zhang DN
| 1. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 3. | Chaudhry UI, DeMatteo RP. Management of resectable gastrointestinal stromal tumor. Hematol Oncol Clin North Am. 2009;23:79-96, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Grotz TE, Donohue JH. Surveillance strategies for gastrointestinal stromal tumors. J Surg Oncol. 2011;104:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Antonescu CR. Targeted therapy of cancer: new roles for pathologists in identifying GISTs and other sarcomas. Mod Pathol. 2008;21 Suppl 2:S31-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 8. | Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176-184. [PubMed] |
| 9. | Gutierrez JC, De Oliveira LO, Perez EA, Rocha-Lima C, Livingstone AS, Koniaris LG. Optimizing diagnosis, staging, and management of gastrointestinal stromal tumors. J Am Coll Surg. 2007;205:479-491 (Quiz 524). [PubMed] |
| 10. | Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301-307. [PubMed] [DOI] [Full Text] |
| 11. | Rodriguez SA, Faigel DO. Endoscopic diagnosis of gastrointestinal stromal cell tumors. Curr Opin Gastroenterol. 2007;23:539-543. [PubMed] |
| 12. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 13. | Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Raut CP, Morgan JA, Ashley SW. Current issues in gastrointestinal stromal tumors: incidence, molecular biology, and contemporary treatment of localized and advanced disease. Curr Opin Gastroenterol. 2007;23:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Valadão M, Linhares E. The role of the surgeon in the management of GIST. Rev Col Bras Cir. 2009;36:261-265. [PubMed] |
| 16. | Kim MY, Park YS, Choi KD, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH, Jung HY, Kim JH. Predictors of recurrence after resection of small gastric gastrointestinal stromal tumors of 5 cm or less. J Clin Gastroenterol. 2012;46:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Sun S, Ge N, Wang C, Wang M, Lü Q. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg Endosc. 2007;21:574-578. [PubMed] |
| 19. | Piccinni G, Marzullo A, Angrisano A, Iacobone D, Nacchiero M. Endoscopic resection of benign very low-risk gastric gastrointestinal stromal tumors. Is it enough? Eur J Gastroenterol Hepatol. 2007;19:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Endoscopic submucosal dissection for gastric submucosal tumor, endoscopic sub-tumoral dissection. Dig Endosc. 2009;21:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Bai J, Wang Y, Guo H, Zhang P, Ling X, Zhao X. Endoscopic resection of small gastrointestinal stromal tumors. Dig Dis Sci. 2010;55:1950-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Marshall CA, Hyatt BJ, Wassef W. Endoscopic removal of small gastrointestinal stromal tumors: can we GIST-ify the risk? Dig Dis Sci. 2010;55:1815-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Punpale A, Rangole A, Bhambhani N, Karimundackal G, Desai N, de Souza A, Pramesh CS, Jambhekar N, Mistry RC. Leiomyoma of esophagus. Ann Thorac Cardiovasc Surg. 2007;13:78-81. [PubMed] |
| 26. | Choi SH, Kim YT, Han KN, Ra YJ, Kang CH, Sung SW, Kim JH. Surgical management of the esophageal leiomyoma: lessons from a retrospective review. Dis Esophagus. 2010;Epub ahead of print. [PubMed] [DOI] [Full Text] |
| 27. | Tay YC, Ng CT, Lomanto D, Ti TK. Leiomyoma of the oesophagus managed by thoracoscopic enucleation. Singapore Med J. 2006;47:901-903. [PubMed] |