Published online Sep 16, 2011. doi: 10.4253/wjge.v3.i9.171
Revised: July 4, 2011
Accepted: August 15, 2011
Published online: September 16, 2011
Esophageal adenocarcinoma is the most rapidly increasing cancer in western countries. High-grade dysplasia (HGD) arising from Barrett’s esophagus (BE) is the most important risk factor for its development, and when it is present the reported incidence is up to 10% per patient-year. Adenocarcinoma in the setting of BE develops through a well known histological sequence, from non-dysplastic Barrett’s to low grade dysplasia and then HGD and cancer. Endoscopic surveillance programs have been established to detect the presence of neoplasia at a potentially curative stage. Newly developed endoscopic treatments have dramatically changed the therapeutic approach of BE. When neoplasia is confined to the mucosal layer the risk for developing lymph node metastasis is negligible and can be successfully eradicated by an endoscopic approach, offering a curative intention treatment with minimal invasiveness. Endoscopic therapies include resection techniques, also known as tissue-acquiring modalities, and ablation therapies or non-tissue acquiring modalities. The aim of endoscopic treatment is to eradicate the whole Barrett’s segment, since the risk of developing synchronous and metachronous lesions due to the persistence of molecular aberrations in the residual epithelium is well established.
- Citation: Ortiz-Fernández-Sordo J, Parra-Blanco A, García-Varona A, Rodríguez-Peláez M, Madrigal-Hoyos E, Waxman I, Rodrigo L. Endoscopic resection techniques and ablative therapies for Barrett’s neoplasia. World J Gastrointest Endosc 2011; 3(9): 171-182
- URL: https://www.wjgnet.com/1948-5190/full/v3/i9/171.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i9.171
Esophageal adenocarcinoma (EAC) is the most rapidly increasing cancer in western countries. Its incidence has increased up to six-fold in the past decade in the United States[1] and it is estimated that about 10 000 new cases were diagnosed last year[2]. Barrett’s esophagus (BE) increases the risk for developing EAC up to 30-40 times and the presence of high-grade dysplasia (HGD) is the most important risk factor[2,3].
The global incidence of EAC arising from BE is 0.5% per year[2,3] and increases to 10% per patient-year when HGD is present[4]. A recently published meta-analysis reports an estimated incidence of 6.3 cases/1000 patient-years of follow-up and a mortality by cancer of 3/1000 patient-years of follow-up[5]. Adenocarcinoma in the setting of BE develops through a well known histological sequence, from non-dysplastic Barrett’s to low grade dysplasia (LGD) and then HGD and cancer[6]. Despite the lack of randomized controlled trials and cost-effective analysis, endoscopic surveillance programs, with targeted biopsies from any visible lesion and random four-quadrant biopsies according to the Seattle protocol[7], have been shown to detect the presence of neoplasia at a potentially curative stage. The widely accepted approach in high-risk selected patients; is further endoscopic surveillance at follow-up intervals which are determined according to the presence and grade of dysplasia[8,9].
A careful examination with high-resolution endoscopy (HRE) is the first step for an appropriate selection of patients who are potential candidates for endoscopic therapy. Newly developed imaging techniques such as narrow band imaging, autofluroescence imaging or confocal endomicroscopy can be helpful for detection of early neoplastic lesions. Surgery has been advocated as the appropriate treatment for HGD due to the high reported rates of occult adenocarcinoma in esophagectomy specimens, up to 40% in some series[10,11]. The current consensus definition of invasive cancer includes lesions involving the submucosal layer (T1sm/T1b). A recent review demonstrated that the true prevalence of cancer invading the submucosal layer in patients with prior diagnosis of HGD was 12.7%[12] although subsequent studies have shown rates of 7%, and even lower (3%) in the absence of visible lesions[13]. These large differences are explained by the use in several studies of an inaccurate definition of invasive cancer that included T1a lesions, and by the low proportion (30%) of patients included in these studies who had been enrolled in an endoscopic surveillance program with an appropriate biopsy protocol[12].
It is also important to keep in mind that esophagectomy is associated with significant morbidity and mortality rates, even in high volume centers[14,15] and has been performed in patients with HGD or intramucosal carcinoma (IMC). These patients have a risk of lymph node (LN) metastasis lower than 1%[16-19] and could be successfully treated by endoscopic therapies. Newly developed endoscopic treatments have dramatically changed the therapeutic approach of BE. The rationale for endoscopic therapy is that lesions confined to the mucosal layer have negligible risk for developing LN metastasis and can be successfully eradicated by an endoscopic approach, offering a curative intention treatment with minimal invasiveness[20]. Risk of LN metastasis[16,21] and tumor differentiation grade[22,23] (G1 well differentiated, G2 moderately differentiated and G3 poorly differentiated) in early Barrett’s adenocarcinoma are clearly related to the depth of tumor infiltration in the esophageal wall. The incidence of LN metastasis is between 0% and 3% for lesions limited to the mucosa (T1m), rising to 30% when the lesion involves the submucosal layer[17-19].
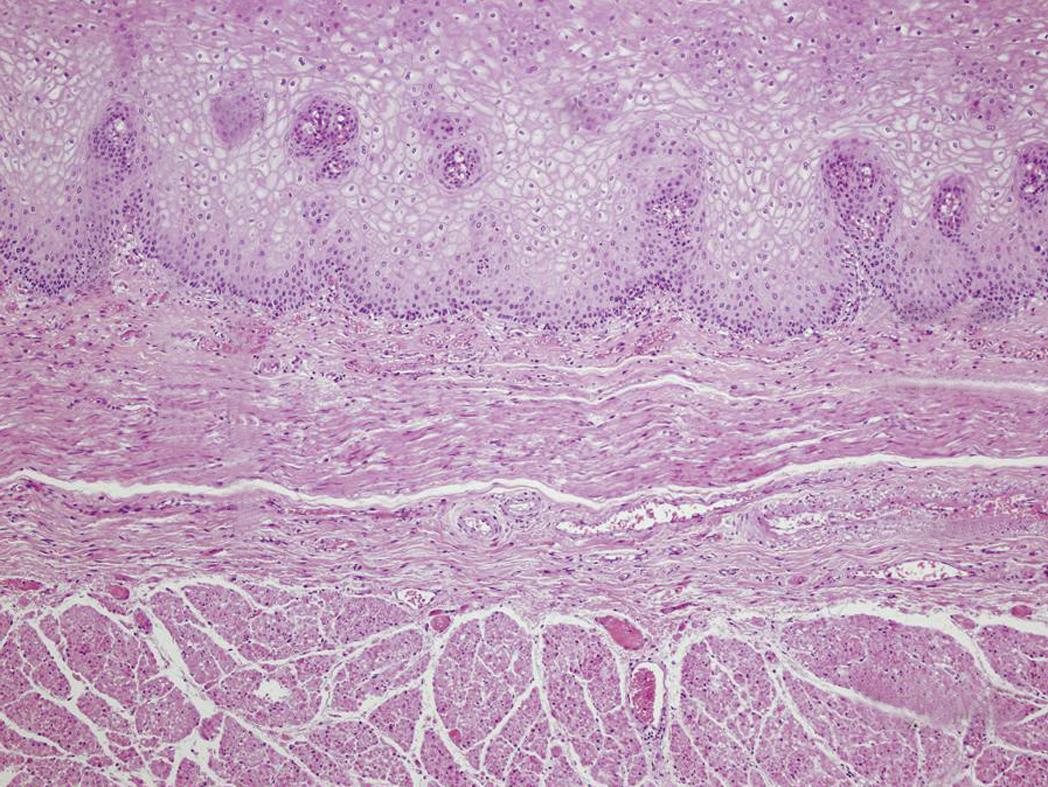
A recently published study, that includes a review of 805 endoscopic resections from 472 patients, showed that the depth of invasion correlates with differentiation grade (G3 0.9% in T1m1 vs 41.4% in T1sm3), lymphatic vessel involvement (0.6% in T1m1 vs 44.8% in T1sm3) and venous involvement (0% in T1m1 vs 13.8% in T1sm3), all well established risk factors for LN metastasis[22]. According to these findings, the endoscopic approach is clearly indicated for IMC and might be extended to lesions with limited invasion into the submucosa (< 200 μm, T1sm1) because of the low risk for LN metastasis reported in some studies[24-27]. Further investigations should be conducted to establish if patients with type I-II lesions, superficial submucosal invasion (T1sm1) and low risk of LN involvement, such as good differentiation grade (G1/G2) and no lymphovascular invasion, could be considered candidates for endoscopic therapy in high volume centers[27]. Figure 1 displays the esophageal layers and shows the subclassification of T1 lesions according to the depth of invasion. The aim of endoscopic therapy is to eradicate the whole Barrett’s segment, since the risk of developing synchronous and metachronous lesions, due to the persistence of molecular aberrations in the residual epithelium, is well established[28]. Endoscopic eradication can be achieved through resection techniques (tissue-acquiring modalities), or through ablation therapies (non-tissue acquiring modalities)[29,30].
Endoscopic resection is the basis of endoscopic therapy for BE and has been advocated not only as a therapeutic approach but also as a staging tool in Barrett’s neoplasia. The major advantage of the tissue-acquiring modalities is their ability to provide resection samples of appropriate size and depth for an accurate histopathological diagnosis. En-bloc resection techniques allow lateral resection margins to be assessed for the need of further treatments[29]. In 1984 Tada et al[31] introduced the use of “strip-off biopsy” for treatment of early gastric cancer. Endoscopic mucosal resection (EMR) of early esophageal neoplasia was first described in 1991 in two different manuscripts by Makuuchi et al[32] and Inoue et al[33], who published their results in four patients, three with squamous cell cancer and one with adenocarcinoma. In all cases complete resection was achieved and no recurrence neither metachronous lesions were observed during follow-up[33].
Several EMR techniques have been developed for excision of mucosal based lesions; the most commonly used are the cap-assisted technique (ER-Cap) and the multi-band ligation assisted technique (MBM). No significant differences in the safety and efficacy profiles have been reported between these two approaches. The only observed difference was the maximum diameter of the resected specimens, where the ER-cap method was favoured[34-36]. In both modalities, after marking 2 mm away from the lesion margins and lifting with saline solution injection, the targeted area is suctioned into the cap and grasped by the snare or by releasing the rubber band to create a pseudopolyp. The lesion is then cut using a snare with blended-current electrocautery. If the MBM technique is performed, the procedure can be carried out safely with no prior submucosal injection and lifting[37-40].
There is extensive experience of performing focal EMR for treatment of macroscopically visible lesions arising in BE. The available data show complete regression of neoplasia in 97%-100% of cases and 5-year survival rates of 98%-100%[41-54] (Table 1). EMR is the only endoscopic technique that has proved increasing the 5-year survival rate in Barrett’s patients in uncontrolled trials[41]. In addition, endoscopic resection has been demonstrated to be a highly safe technique. Alvarez-Herrero and colleagues reporting the outcome of more than 1000 EMR procedures performed in 243 patients, observed an acute bleeding rate was 2.9% and delayed bleeding rate of 2.1%, with no perforations and successfully management of all adverse events by an endoscopic approach[38].
| Author | n | Complete regression of dysplasia/esophageal cancer (%) | Histology | Follow-up (mo) |
| Ell et al[43], 2000 | 64 | 86 | HGD/EC | 12 ± 8 |
| May et al[44], 2002 | 28 | 79 (1001) | HGD/EC | 34 ± 10 |
| May et al[45], 2002 | 70 | 70 (981) | HGD/EC | 34 ± 10 |
| Behrens et al[48], 2005 | 14 | 93 (1001) | HGD | 38 |
| Peters et al[49], 2005 | 33 | 79 (1001) | Barrett’s esophagus | 19 |
| Conio et al[50], 2005 | 39 | 97.5 | HGD/EC | 35 |
| Ell et al[53], 2007 | 100 | 88 (991) | Adenoca. | 36 |
| Pech et al[41], 2008 | 231 | 95.7 | EC | 61 |
| Moss et al[54], 2010 | 35 | 77 (851) | HGD/EC | 31 |
The radical differences between treatments for T1m and T1sm tumors make a definitive histopathological staging essential, in order to identify the patients amenable for curative endoscopic therapy. There are several concerns about the ability of conventional biopsy specimens to provide an accurate histological diagnosis. The sampling error associated with the random biopsy protocol is well known and there are also important doubts about the adequacy of the depth of specimen obtained with conventional biopsy forceps. Published studies have reported a limited reproducibility, particularly for dysplasia, as well as low inter-observer agreement rates. Rates are between 61% and 75% when three categories are evaluated (no dysplasia, indefinite for dysplasia/LGD and HGD/carcinoma), but go down to κ value of 0.49 when HGD is diagnosed separately from carcinoma[55].
A recent study performed in two tertiary referral centers, has demonstrated a higher inter-observer agreement for diagnosis of dysplasia from the analysis of EMR specimens than from conventional biopsies (κ 0.33 vs 0.22, P < 0.001 for LGD; 0.43 vs 0.35, P = 0.018 for HGD). Submucosa was present in up to 88% of EMR specimens but only in 1% of biopsy samples and the presence of muscularis mucosae was observed only in 58% of biopsy specimens[56]. EMR samples permit an accurate evaluation of depth and lateral resection margins and also provide information about the presence of submucosal involvement. The histological examination of EMR pieces can also asses the degree of lymph and blood vessel invasion, important risk factors for the presence of LN metastasis[57-59]. Different studies have shown that final staging by EMR modifies the previous diagnosis in up to 48% of cases[54,59,60] and dramatically changes the clinical management of these patients (Table 2). Similar discrepancy rates have been reported for gastrointestinal neoplasia from other locations[61]. Finally, EMR staging has shown to be consistent with surgical pathology staging. The presence of free of disease margins in EMR samples, directly correlates with the absence of residual tumor at esophagectomy[62].
The major drawback of using focal EMR as the only treatment for Barrett’s neoplasia is the possible development of synchronous, metachronous and recurrent lesions, arising in the residual Barrett’s epithelium. After a mean follow-up period of 3 years, the reported incidence rates range between 11% and 47% and are even higher with longer follow-up. Because of this, complete Barrett’s resection has been proposed as an alternative treatment[41-54].
The rationale for radical endoscopic resection of BE is the proven coexistence of multifocal HGD in Barrett’s mucosa, the aforementioned high rate of synchronous and metachronous lesions when focal EMR is performed as single treatment and the lack of histological correlation of the non-tissue acquiring ablative techniques[29,30,63]. With this approach, the whole Barrett’s segment is eradicated by endoscopic resection in a single or multiple sessions, achieving the treatment of any occult neoplasia and preventing the development of any new lesion during follow-up[60]. It was firstly described by Satodate et al[64], and since then, several studies have been conducted involving a total of 390 patients with HGD or IMC[54,60,63,65-69] and achieving complete eradication of IM in 86% to 100% of cases and eradication of any neoplasia from 75% to 100% of patients (Table 3). The global recurrence rate of neoplasia after a follow-up period of up to 32 mo was 3% (12/390), much lower than the previously reported with focal EMR[54,60,63,65-69].
| Author | n | Complete regression of intestinal metaplasia (%) | Complete regression of dysplasia/esophageal cancer (%) | Sessions (%) | Recurrence (%) | Progression (%) | Follow-up (mo) |
| Seewald et al[65], 2003 | 12 | 100 | 100 | 2.5 | 0 | 0 | 9 |
| Giovannini et al[66], 2004 | 21 | 75 | 86 | 2 | 14 | 0 | 18 |
| Peters et al[67], 2006 | 39 | 89 | 95 | 3 | 0 | 0 | 11 |
| Larghi et al[68], 2007 | 24 | 87 | 100 | 1.8 | 4 | 0 | 28 |
| Lopes et al[69], 2007 | 41 | 76 | 90 | 1.5 | 12 | 0 | 31.6 |
| Chennat et al[60], 2009 | 49 | 97 | 100 | 2.1 | 0 | 0 | 17 |
| Moss et al[54], 2010 | 35 | 97 | 97 | 2 | 0 | 0 | 31 |
| Pouw et al[63], 2010 | 169 | 97.6 | 85.2 | 2 | 1.8 | 0.6 | 27 |
Only one case of disease progression was observed (0.25%) with this approach. In the largest published series, Pouw et al[63] reported one case of progression to T1sm1 tumor after complete removal of a T1m2 cancer, the subsequent surgery showed neither residual tumor nor LN involvement. In the same study, all cases with recurrence of neoplasia [3 patients (1.8%), two HGD and one of T1sm1 tumor] were found distally to the neo-esophagogastric junction. This finding highlights the recommendation of a careful inspection of this area[63]. The complete Barrett’s eradication EMR (CBE-EMR) is a safe procedure when performed by expert endoscopists and complications are successfully treated by an endoscopic approach with no need of additional surgery in most of cases (Table 4).
The major limitation for CBE-EMR is the high incidence of symptomatic stenosis, with rates reaching 50% in some reports. The rate of esophageal stricture was related to the length of Barrett’s resected segment[63] and significant statistical differences were found with regard to the number of EMR procedures between patients who did and did not develop strictures[60]. New strategies to prevent the development of strictures should be evaluated[63]. A recent study reports a decrease in the incidence and severity of stricture after EMR/endoscopic submucosal dissection (ESD) involving more than 75% of circumference when preventive dilation is performed. Endoscopic balloon dilation was carried out 1 wk after treatment and once a week thereafter, until the mucosal defect was healed. No complications related to endoscopic dilation were observed[70]. Despite the relative low number of patients enrolled in these studies and the short follow-up period, CBE-EMR has shown excellent endoscopic and histological short-term results and could be considered as an alternative to esophagectomy in high volume centers for selected patients with short Barrett’s segment (≤ 5 cm)[60,63].
ESD is regarded in Japan as the treatment of choice for intramucosal gastric neoplasias, and when performed by experts the results for esophageal and colonic lesions are encouraging and superior to conventional EMR in terms of curative resection rate and recurrence[71-73]. With this approach, en bloc resection can be achieved regardless of the size of the lesions but it is a challenging technique, time consuming and is associated with a higher rate of adverse events[72,74,75].
The first step is marking the targeted lesion 5 mm away from its limits and perform submucosal injection using any of the available solutions (saline solution, hyaluronic acid, glycerine). The addition of epinephrine (1:100 000-1:300 000) is used for vasoconstriction of small submucosal vessels and indigo-carmine for a better visualization of the stained submucosal layer. Incision at the proximal and distal margins and then circumferential cutting of the surrounding mucosa is performed. Finally, dissection of the tissue beneath the isolated mucosa is carried out to achieve the removal of the lesion in one piece. Many different knives have been designed and developed.
Because of the low incidence of BE and adenocarcinoma in Japan and other eastern countries, the reported experience with early esophageal neoplasia is mainly limited to squamous cancer[75-81] (Table 5). Yoshinaga et al[82] reported a 100% of en bloc resection and a curative resection rate of up to 72% in adenocarcinoma located at the esophagogastric junction. When compared to EMR, ESD shows a better en bloc resection rate and a better curative resection rate (free of disease resection margins) for treatment of superficial tumors in the gastrointestinal tract, leading to a dramatically reduced local recurrence rate[75,83,84]. Perforation and bleeding were significant higher in the ESD group, although most of them were successfully managed by endoscopic intervention. There were no studies from Western countries and no randomized controlled trials included in this analysis[83-87] (Table 6).
| Author | n | En bloc resection rate (%) | Curative resection rate (resection margins free of neoplasia) (%) | Recurrence (%) | Histology | Follow-up (mo) |
| Oyama et al[75], 2005 | 102 | 95 | 95 | - | Squamous | - |
| Fujishiro et al[76], 2006 | 43 | 100 | - | 2.3 | Squamous | 17 |
| Kakushima et al[77], 2006 | 30 | 97 | 70 | 01 | Adenoca. 2 | 15 |
| Motohashi et al[78], 2009 | 9 | 100 | 100 | 0 | N/D | 12 |
| Ono et al[79], 2009 | 84 | 100 | 88 | 3.6 | N/D | 21 |
| Ishii et al[80], 2010 | 35 | 100 | 95 | 01 | Adenoca./Squamous. | 19 |
| Neuhaus et al[81], 2010 | 18 | 83 | 22 | 5.5 | BE (HGIN/IMC) | 1.5 |
| Yoshinaga et al[82], 2008 | 24 | 100 | 72 | 01 | Adenoca.2 | 30 |
| Author | n | En bloc resection rate (%) | Curative resection rate (resection margins free of disease) (%) | Complications1 (%) | ||||
| EMR | ESD | EMR | ESD | EMR | ESD | EMR | ESD | |
| Ishihara et al[84], 2008 | 148 | 29 | 78.5 | 100 | 57.8 | 97 | 0.03 | 0.03 |
| Jung et al[85], 2008 | 69 | 37 | 25 | 97.3 | 53.1 | 86.5 | 12.5 | 16 |
| Teoh et al[86], 2008 | 26 | 11 | 71.4 | 94.4 | - | - | 0.06 | 0.36 |
| Deprez et al[87], 2010 | 25 | 25 | 0 | 96 | 24 | 64 | 52 | 24 |
It is important to keep in mind that ESD is a time-consuming and technically demanding procedure. Learning methods should be standardized, with animal models playing a significant role[88-90] and the technique should be performed in an appropriate stepwise fashion. The minimum training requirements recommended by a panel of experts were recently published: enough previous experience with conventional EMR; knowledge of indications, instruments and complications management; visits to expert centers and observation of at least 15 live procedures performed by the experts; hands-on experience in isolated animal models and live pigs; starting with treatments on less challenging locations such as rectum and then moving to distal stomach, colon, proximal stomach and esophagus[73]. There is no consensus regarding of the minimum case load, but Japanese experts recommend at least 50 ESD procedures in distal stomach before performing the technique in the more challenging locations[91,92]. The role of ESD in the therapeutic algorithm of BE in the western countries is still not established[87,93]. Long-term results with EMR techniques are excellent, as previously shown, and ESD is a challenging technique with an increased risk of perforation compared to EMR and it does not provide a high R0 rate (lateral resection margins free of tumor) in Barrett’s lesions. In these patients, the entire Barrett’s segment must be eradicated after resection of any visible lesion regardless of the negative resection margins. Thus, the potential advantages of ESD compared to conventional EMR could be less relevant in treatment of early Barrett’s neoplasia[93].
The rationale for developing new ablative methods for BE is the well established presence of molecular abnormalities in the remaining Barrett’s epithelium after focal resection of neoplastic lesions[28], making the eradication of the entire Barrett’s segment essential. The current consensus for use of non-tissue-acquiring modalities is in the eradication of all BE after endoscopic resection of all visible lesions for an accurate staging. When no visible lesion is macroscopically detected after a carefully examination with HRE, ablative methods may be the first of choice therapy for HGD[29].
Radiofrequency ablation (RFA) using the HALO® system (BÂRRX Medical Inc., Sunnyvale, California, United States) uniformly delivers thermal energy with a prefixed density (12-15 J/cm2) and power (40 W/cm2). With these settings, the tissue penetration depth of the RF energy is limited to 500-1000 μm, which has been demonstrated as sufficient for the successful ablation of esophageal epithelium with no submucosal injury in animal models and humans[94]. The HALO360® device is a balloon catheter with spindle-shaped electrodes on its surface that allows the ablation of 3 cm long segments in a circumferential fashion. In order to choose the appropriate balloon size (available diameters 18 mm, 22 mm, 25 mm, 28 mm, 31 mm and 34 mm) an inflatable sizing balloon is used to measure the esophageal inner diameter. The catheter is introduced into the esophagus over a guide-wire and the RFA is performed under endoscopic direct view. The HALO90® is a square-shaped catheter with the same electrodes on its external surface, which is attached to the tip of the endoscope. It allows the focal ablation of small areas of residual Barrett’s epithelium[29,95,96].
The available data from prospective trials are summarized in Table 7; they show a complete eradication of dysplasia in 70%-100% of cases and the eradication of IM in 50% to 100%[97-111]. Several trials assessing the efficacy and safety of RFA in BE have been conducted. After the publication of several studies in non dysplastic BE[97-99] Ganz et colleagues in 2008 conducted the first study in patients with HGD. A complete regression of IM, any dysplasia and HGD was achieved respectively in 54%, 80% and 90% of the 142 enrolled patients[100]. In the only multicenter, randomized and sham-controlled trial conducted to date, 127 patients with prior diagnosis of dysplastic BE (63 HGD and 64 LGD) were randomized in a 2:1 ratio to receive either RFA or sham endoscopic procedure (control group). After 1-year follow-up, all measured primary and secondary outcomes showed significant differences favoring the treatment group: progression rate, progression rate to cancer, complete regression of IM, complete regression of LGD and complete regression of HGD[112] (Table 8). Only three relevant adverse events occurred in the treatment group and five patients (6%) developed esophageal stricture (with or without dysphagia), a rate markedly lower than reported with resection therapies[112].
| Author | n | Complete regression of intestinal metaplasia (%) | Complete regression of dysplasia/early cancer (%) | Patients | Study | Follow-up (mo) |
| Roorda et al[97], 2007 | 13 | 46 | 71 | BE | Single-center | 12 |
| Sharma et al[98], 2007 | 70 | 70 | - | Non D-BE | Multic. | 12 |
| Fleischer et al[99], 20081 | 70 | 70-98 | - | Non D-BE | Multic. | 12-30 |
| Ganz et al[100], 2008 | 142 | 54.3 | 80.4-90.2 | HGD | Multic. | 12 |
| Pouw et al[101], 2008 | 44 | 98 | - | BE | Single-center | 21 |
| Gondrie et al[102], 2008 | 11 | 100 | 100 | BE | Single-center | 14 |
| Gondrie et al[103], 2008 | 12 | 100 | 100 | BE | Single-center | 14 |
| Sharma et al[104], 2008 | 10 | 90 | 100 | LGD | Single-center | 24 |
| Hernandez et al[105], 2008 | 10 | 70 | - | BE | Single-center | 12 |
| Sharma et al[106], 2009 | 63 | 79 | 89 | LGD/HGD | Single-center | 24 |
| Velanovich[107], 2009 | 66 | 93 | - | BE | Single-center | 12 |
| Vassiliou et al[108], 2010 | 25 | 78.5 | - | LGD/HGD/IMC | Single-center | 20 |
| Lyday et al[109], 2010 | 429 | 72 | 89 | LGD/HGD | Multic. | 9 |
| Eldaif et al[110], 2010 | 27 | 100 | - | Non D-BE/LGD | Single-center | 2 |
| Fleischer et al[111], 20102 | 50 | 92 | - | Non D-BE | Multic. | 60 |
| Study characteristics | Radiofrequency ablation group | Sham group | P value |
| n = 127 Dysplastic Barrett’s esophagus patients | 84 | 43 | - |
| Complete regression of intestinal metaplasia | 77.4% | 2.3% | < 0.001 |
| Complete regression of low grade dysplasia | 90.5% | 22.7% | < 0.001 |
| Complete regression of high grade dysplasia | 81.0% | 19.0% | < 0.001 |
| Global progression rate | 3.6% | 16.3% | < 0.05 |
| Progression to cancer rate | 1.2% | 9.3% | < 0.05 |
A systematic review of nine observational studies, involving 429 patients, and at least 12 mo of follow-up was recently published[113]. After analysis, complete eradication of IM was achieved in 46%-100% of patients and complete regression of neoplasia in 46%-100%. There were only 6 cases of stenosis after treatment (1.4%) and no major complications were observed. RFA has proved to be a safe procedure. Of all 657 patients involved in the aforementioned trials, only one case of perforation has been reported (0.15%) and only 3 patients required hospitalization for any complication related to ablation. The global rate of stenosis is 2.3%, with all instances successfully treated by endoscopic dilation, and the most frequent adverse event is chest pain, usually controlled with conventional analgesics[97-111].
The Amsterdam group has reported excellent outcomes from the stepwise treatment in patients with HGD and any visible lesion in the index endoscopy exam. This approach consists of the resection of all macroscopic lesions and the subsequent ablation by RFA of the remaining Barrett’s epithelium. Initially, circumferential ablation with HALO360® is performed with a maximum of three sessions; thereafter focal ablation with a HALO90® device is performed in order to eradicate any residual IM, with the same three session limit[102,103,114-116]. Complete eradication of neoplasia is achieved in up to 100% of cases and complete regression of IM in 96%. Escape EMR is performed if any abnormality is seen during follow-up. No recurrence of neoplasia has been observed 22 mo after treatment[114-116] (Table 9).
| Results | End of treatment | Follow-up (22 mo) |
| Pouw et al[115], 2010 | ||
| Complete regression of neoplasia | 20/21 (95%)1 | 24/24 (100%) |
| Complete regression of intestinal metaplasia | 21/24 (88%)2 | 20/24 (83%) |
| Progression | 0% | 0% |
| Buried glands (1201 biopsies) | 0% | 0% |
| Pouw et al[116], 2010 | ||
| Complete regression of neoplasia | 55/55 (100%) | N/A |
| Complete regression of intestinal metaplasia | 53/55 (96%) | N/A |
| Progression | 0% | N/A |
| Buried glands | N/D | N/A |
One of the most relevant concerns about RFA and other ablative therapies is the incidence of buried IM after treatment. The aforementioned review revealed only one case of buried Barrett’s epithelium, after the assessment of more than 8500 biopsy samples obtained during follow up from the 429 patients enrolled in the 9 analyzed trials[113]. No randomized controlled trials comparing RFA vs CBE-EMR have been conducted to date. According to the published data, stepwise treatment should be the treatment of choice for patients with visible lesions arising on HGD[117] and CBE-EMR could be recommended, in high volume centers, for patients with short segment BE (SSBE).
In this technique, ablation is achieved by light activation of a photosensitizer drug, which leads to oxygen radicals formation and thereafter cell death. The photosensitizing agent, usually porfimer sodium, is administered before the procedure and it is selectively accumulated in the malignant esophageal mucosa. Cylindrical or balloon-based diffuser fibers are then placed over the targeted lesion under endoscopic view[20,29]. The published trials have proved the efficacy of photodynamic therapy (PDT) in eradicating Barrett’s dysplasia[118-123] (Table 10). The only randomized and controlled trial reported complete regression of IM in 52% of cases and complete regression of any dysplasia in 59% out of 138 patients with dysplastic BE[122].
| Autor | n | Study | Sessions | Complete regression of intestinal metaplasia (%) | Complete regression of dysplasia/early cancer (%) | Recurrence (%) | Follow-up (mo) |
| Wolfsen et al[118], 2002 | 48 | Case series | 1 | 56 | 98 | N/D | 18.5 |
| Ackroyd et al[119], 2003 | 40 | Case series | 1 | 0 | 100 | 2.5 | 53 |
| Overholt et al[120], 2003 | 94 | Case series | 1-2 | 56 | 80 | N/D | 50 |
| Wolfsen et al[121], 2004 | 102 | Case series | 1 | 56 | N/D | N/D | 19 |
| Overhalt et al [122], 2005 | 138 | Randomized controlled trial | 2 | 52 | 59 | 1.4 | 24 |
| Pech et al[123], 2005 | 66 | Case series | 1.2 | N/D | 98 | 17 | 37 |
The major drawback of PDT is the relatively high rate of reported adverse events, mainly photosensitivity and symptomatic strictures, which have been reported in up to 36% of patients. Number of PDT treatments per session, prior EMR and a previous history of esophageal stenosis are associated with development of strictures[124]. Buried glands under the neo-squamous epithelium after PDT have been described in up to 51% of patients[125] and cases of adenocarcinoma arising from buried Barrett’s glands have also been reported[120]. For all these reasons, PDT has been abandoned in recent years in favour of other ablation techniques. Further investigations aimed to identify biomarkers, which may stratify the patients more likely to respond to this treatment, and the development new photosensitizing agents could improve its safety profile[29].
This is the latest added option to the therapeutical armamentarium of BE and has shown promising results in the available reports. For ablation, a liquid cryogen is focally sprayed onto the targeted lesion and results in freezing of the epithelium, causing intracellular disruption and ischemia. Liquid nitrogen and carbon dioxide have been used as cryogenic agents. The depth of ablation is limited to
2 mm and treatment sessions are performed every 4-6 wk until complete remission of the IM is achieved[126]. Several trials have shown cryotherapy as a safe and effective tool[127-130]. Short-term results are promising with eradication rates of IM in 46%-78% and of dysplasia between 79% and 87% of cases (Table 11). No major complications have been reported except for a gastric perforation in one patient with Marfan syndrome. This therapy is now contraindicated in patients with limited distensibility of the stomach. Multi-center randomized trials are required to confirm these results and determine the long-term response. It is still necessary to establish the optimal treatment protocol, duration and number of cycles per session, and frequency of treatment sessions. Finally, it remains to be determined if there is any clinical relevant difference in safety or efficacy profiles between CO2 and N2[126].
| Author | n | Complete regression of intestinal metaplasia (%) | Complete regression of dysplasia (%) | Complete regression of high grade dysplasia (%) | Patients | Follow-up (mo) |
| Johnston et al[127], 2005 | 9 | 78 | - | - | Non D-BE/LGD/HGD | 6 |
| Canto et al[128], 2009 | 44 | 50 | 84 | 86 | HGD/IMC | 12 |
| Greenwald et al[129], 2010 | 21 | 46 | 79 | 83 | HGD/IMC | 12 |
| Shaheen et al[130], 2010 | 60 | 57 | 87 | 97 | HGD | 10.5 |
Argon plasma coagulation has reported eradication rates of IM in non dysplastic Barrett’s patients of up to 100%[131-134] and about 75% in cases of HGD, although with significant long term recurrence rates[29,135]. Techniques such as multipolar electrocoagulation and laser therapies have been replaced by the ablation modalities discussed in this manuscript[20,29].
According to the results achieved by endoscopic therapies, the reported rates of LN metastasis in lesions limited to the mucosal layer, and true prevalence of occult invasive adenocarcinoma in HGD, esophagectomy should not be routinely considered as a part of therapeutical algorithm for HGD in BE.
Barrett’s patients with any visible superficial lesion should be treated by endoscopic resection for an accurate histopathological staging. In cases with favorable histology, all residual Barrett’s epithelium should be ablated in order to avoid the risk of developing synchronous or metachronous lesions. Of all available ablation modalities, RFA has shown the best efficacy and safety profile.Patients with Barrett segment ≤ 5 cm could be considered for complete eradication by EMR in selected high volume centers[60,63]. The role of endoscopic ablation therapies is well established for HGD. Further investigations should be conducted to establish its role in LGD and non-dysplastic BE.
Peer reviewers: Kazuki Sumiyama, MD, PhD, Department of Endoscopy, The Jikei University School of Medicine, 3-25-8 Nishi Shinbashi, Minato-ku, Tokyo 105-8461, Japan; Fauze Maluf-Filho, MD, Hospital das Clínicas, São Paulo University School of Medicine, 488 Olegario Mariano, São Paulo, SP, Brazil; Seamus Joseph Murphy, MB, BCh, MRCP, PhD, Consultant Gastroenterologist, Department of Medicine, Daisy Hill Hospital, 5 Hospital Road, Newry, Co. Down, N. Ireland BT35 9YE, United Kingdom
S- Editor Zhang SJ L- Editor Hughes D E- Editor Zheng XM
| 1. | Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 962] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 2. | Sharma P. Clinical practice. Barrett's esophagus. N Engl J Med. 2009;361:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Buttar NS, Wang KK, Sebo TJ, Riehle DM, Krishnadath KK, Lutzke LS, Anderson MA, Petterson TM, Burgart LJ. Extent of high-grade dysplasia in Barrett's esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 264] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Weston AP, Sharma P, Topalovski M, Richards R, Cherian R, Dixon A. Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:1888-1893. [PubMed] [DOI] [Full Text] |
| 5. | Ramoner R, Rieser C, Bartsch G, Thurnher M. Nordihydroguaiaretic acid blocks secretory and endocytic pathways in human dendritic cells. J Leukoc Biol. 1998;64:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256. [PubMed] |
| 7. | Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993;105:40-50. [PubMed] |
| 8. | Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788-797. [PubMed] [DOI] [Full Text] |
| 9. | Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 515] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 10. | Ferguson MK, Naunheim KS. Resection for Barrett's mucosa with high-grade dysplasia: implications for prophylactic photodynamic therapy. J Thorac Cardiovasc Surg. 1997;114:824-829. [PubMed] |
| 11. | Pellegrini CA, Pohl D. High-grade dysplasia in Barrett's esophagus: surveillance or operation? J Gastrointest Surg. 2000;4:131-134. [PubMed] |
| 12. | Konda VJ, Ross AS, Ferguson MK, Hart JA, Lin S, Naylor K, Noffsinger A, Posner MC, Dye C, Cislo B. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol. 2008;6:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Wang VS, Hornick JL, Sepulveda JA, Mauer R, Poneros JM. Low prevalence of submucosal invasive carcinoma at esophagectomy for high-grade dysplasia or intramucosal adenocarcinoma in Barrett's esophagus: a 20-year experience. Gastrointest Endosc. 2009;69:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Patti MG, Corvera CU, Glasgow RE, Way LW. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186-192. [PubMed] |
| 15. | Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference?: A follow-up analysis of another decade. Ann Surg. 2009;250:472-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Stolte M, Kirtil T, Oellig F, Vogel C, Mueller H, May A, Ell C, Wittenberg R. The pattern of invasion of early carcinomas in Barrett's esophagus is dependent on the depth of infiltration. Pathol Res Pract. 2010;206:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bergman JJ. Endoscopic treatment of high-grade intraepithelial neoplasia and early cancer in Barrett oesophagus. Best Pract Res Clin Gastroenterol. 2005;19:889-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1318] [Article Influence: 59.9] [Reference Citation Analysis (4)] |
| 19. | Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 642] [Article Influence: 32.1] [Reference Citation Analysis (1)] |
| 20. | Wani S, Sayana H, Sharma P. Endoscopic eradication of Barrett's esophagus. Gastrointest Endosc. 2010;71:147-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Feith M, Stein HJ, Siewert JR. Pattern of lymphatic spread of Barrett's cancer. World J Surg. 2003;27:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Zemler B, May A, Ell C, Stolte M. Early Barrett's carcinoma: the depth of infiltration of the tumour correlates with the degree of differentiation, the incidence of lymphatic vessel and venous invasion. Virchows Arch. 2010;456:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Vieth M, Ell C, Gossner L, May A, Stolte M. Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy. 2004;36:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Manner H, May A, Pech O, Gossner L, Rabenstein T, Günter E, Vieth M, Stolte M, Ell C. Early Barrett's carcinoma with "low-risk" submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103:2589-2597. [PubMed] [DOI] [Full Text] |
| 28. | Hage M, Siersema PD, Vissers KJ, Steyerberg EW, Haringsma J, Kuipers EJ, van Dekken H. Molecular evaluation of ablative therapy of Barrett's oesophagus. J Pathol. 2005;205:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Waxman I, Konda VJ. Mucosal ablation of Barrett esophagus. Nat Rev Gastroenterol Hepatol. 2009;6:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Chennat J, Waxman I. Endoscopic treatment of Barrett's esophagus: From metaplasia to intramucosal carcinoma. World J Gastroenterol. 2010;16:3780-3785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Tada M, Murata M, Murakami F, Shimada M, Okazaki Y, Takemoto T, Iida Y. Development of the strip-off biopsy. Gastroenterol Endosc. 1984;26:833-839. |
| 32. | Makuuchi H, Machimura T, Soh Y Mizutani K, Shimada H, Sugihara T, Tokuda Y, Sasaki T, Tajima T, Mitomi T, Ohmori T. Endoscopic mucosectomy for mucosal carcinomas in the esophagus. Jpn J Surg Gastroenterol. 1991;24:2599-2603. |
| 33. | Inoue H, Endo M, Takeshita K, Kawano T, Goseki N, Takiguchi T, Yoshino K. Endoscopic resection of early-stage esophageal cancer. Surg Endosc. 1991;5:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Abrams JA, Fedi P, Vakiani E, Hatefi D, Remotti HE, Lightdale CJ. Depth of resection using two different endoscopic mucosal resection techniques. Endoscopy. 2008;40:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | May A, Gossner L, Behrens A, Kohnen R, Vieth M, Stolte M, Ell C. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Peters FP, Kara MA, Curvers WL, Rosmolen WD, Fockens P, Krishnadath KK, Ten Kate FJ, Bergman JJ. Multiband mucosectomy for endoscopic resection of Barrett's esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol. 2007;19:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 38. | Alvarez-Herrero L, Pouw RE, Weusten BL, Bergman JJ. Multi-Band Mucosectomy in Barrett’s esophagus: A prospective registration of 1060 resections in 243 procedures. Gastrointest Endosc. 2010;71:AB127. [DOI] [Full Text] |
| 39. | Inoue H, Minami H, Kaga M, Sato Y, Kudo SE. Endoscopic mucosal resection and endoscopic submucosal dissection for esophageal dysplasia and carcinoma. Gastrointest Endosc Clin N Am. 2010;20:25-34, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Wang KK, Prasad G, Tian J. Endoscopic mucosal resection and endoscopic submucosal dissection in esophageal and gastric cancers. Curr Opin Gastroenterol. 2010;26:453-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008;57:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 42. | Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc. 2000;52:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Ell C, May A, Gossner L, Pech O, Günter E, Mayer G, Henrich R, Vieth M, Müller H, Seitz G. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2000;118:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 430] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 44. | May A, Gossner L, Pech O, Fritz A, Günter E, Mayer G, Müller H, Seitz G, Vieth M, Stolte M. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol. 2002;14:1085-1091. [PubMed] |
| 45. | May A, Gossner L, Pech O, Müller H, Vieth M, Stolte M, Ell C. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett's esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy. 2002;34:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Pacifico RJ, Wang KK, Wongkeesong LM, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol. 2003;1:252-257. [PubMed] |
| 47. | Pech O, May A, Gossner L, Ell C. Barrett's esophagus: endoscopic resection. Gastrointest Endosc Clin N Am. 2003;13:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Behrens A, May A, Gossner L, Günter E, Pech O, Vieth M, Stolte M, Seitz G, Ell C. Curative treatment for high-grade intraepithelial neoplasia in Barrett's esophagus. Endoscopy. 2005;37:999-1005. [PubMed] [DOI] [Full Text] |
| 49. | Peters FP, Kara MA, Rosmolen WD, Aalders MC, Ten Kate FJ, Bultje BC, Krishnadath KK, Fockens P, van Lanschot JJ, van Deventer SJ. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett's esophagus. Gastrointest Endosc. 2005;61:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Conio M, Repici A, Cestari R, Blanchi S, Lapertosa G, Missale G, Della Casa D, Villanacci V, Calandri PG, Filiberti R. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett's esophagus: an Italian experience. World J Gastroenterol. 2005;11:6650-6655. [PubMed] |
| 51. | Larghi A, Lightdale CJ, Memeo L, Bhagat G, Okpara N, Rotterdam H. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett's esophagus. Gastrointest Endosc. 2005;62:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 52. | Mino-Kenudson M, Brugge WR, Puricelli WP, Nakatsuka LN, Nishioka NS, Zukerberg LR, Misdraji J, Lauwers GY. Management of superficial Barrett's epithelium-related neoplasms by endoscopic mucosal resection: clinicopathologic analysis of 27 cases. Am J Surg Pathol. 2005;29:680-686. [PubMed] |
| 53. | Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007;65:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 54. | Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 55. | Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H. Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol. 1988;19:166-178. [PubMed] |
| 56. | Wani S, Mathur SC, Curvers WL, Singh V, Herrero LA, Hall SB, Ulusarac O, Cherian R, McGregor DH, Bansal A. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett's dysplasia. Clin Gastroenterol Hepatol. 2010;8:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Mino-Kenudson M, Hull MJ, Brown I, Muzikansky A, Srivastava A, Glickman J, Park DY, Zuckerberg L, Misdraji J, Odze RD. EMR for Barrett's esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660-666; quiz 767, 769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Maish MS, DeMeester SR. Endoscopic mucosal resection as a staging technique to determine the depth of invasion of esophageal adenocarcinoma. Ann Thorac Surg. 2004;78:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Hull MJ, Mino-Kenudson M, Nishioka NS, Ban S, Sepehr A, Puricelli W, Nakatsuka L, Ota S, Shimizu M, Brugge WR. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol. 2006;30:114-118. [PubMed] |
| 60. | Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, Lin S, Ferguson MK, Posner MC, Waxman I. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Lee CK, Chung IK, Lee SH, Kim SP, Lee SH, Lee TH, Kim HS, Park SH, Kim SJ, Lee JH. Is endoscopic forceps biopsy enough for a definitive diagnosis of gastric epithelial neoplasia? J Gastroenterol Hepatol. 2010;25:1507-1513. [PubMed] [DOI] [Full Text] |
| 62. | Prasad GA, Buttar NS, Wongkeesong LM, Lewis JT, Sanderson SO, Lutzke LS, Borkenhagen LS, Wang KK. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett's esophagus. Am J Gastroenterol. 2007;102:2380-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Pouw RE, Seewald S, Gondrie JJ, Deprez PH, Piessevaux H, Pohl H, Rösch T, Soehendra N, Bergman JJ. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 64. | Satodate H, Inoue H, Yoshida T, Usui S, Iwashita M, Fukami N, Shiokawa A, Kudo SE. Circumferential EMR of carcinoma arising in Barrett's esophagus: case report. Gastrointest Endosc. 2003;58:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Seewald S, Akaraviputh T, Seitz U, Brand B, Groth S, Mendoza G, He X, Thonke F, Stolte M, Schroeder S. Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854-859. [PubMed] [DOI] [Full Text] |
| 66. | Giovannini M, Bories E, Pesenti C, Moutardier V, Monges G, Danisi C, Lelong B, Delpero JR. Circumferential endoscopic mucosal resection in Barrett's esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy. 2004;36:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | Peters FP, Kara MA, Rosmolen WD, ten Kate FJ, Krishnadath KK, van Lanschot JJ, Fockens P, Bergman JJ. Stepwise radical endoscopic resection is effective for complete removal of Barrett's esophagus with early neoplasia: a prospective study. Am J Gastroenterol. 2006;101:1449-1457. [PubMed] [DOI] [Full Text] |
| 68. | Larghi A, Lightdale CJ, Ross AS, Fedi P, Hart J, Rotterdam H, Noffsinger A, Memeo L, Bhagat G, Waxman I. Long-term follow-up of complete Barrett's eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Lopes CV, Hela M, Pesenti C, Bories E, Caillol F, Monges G, Giovannini M. Circumferential endoscopic resection of Barrett's esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc. 2007;21:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Ezoe Y, Muto M, Horimatsu T, Morita S, Miyamoto S, Mochizuki S, Minashi K, Yano T, Ohtsu A, Chiba T. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol. 2011;45:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Gotoda T. Endoscopic resection for premalignant and malignant lesions of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am. 2008;18:435-50, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Lawner P, Laurent J, Simeone F, Fink E, Rubin E. Attenuation of ischemic brain edema by pentobarbital after carotid ligation in the gerbil. Stroke. 1979;10:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 73. | Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 74. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 75. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [PubMed] |
| 76. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 77. | Kakushima N, Yahagi N, Fujishiro M, Kodashima S, Nakamura M, Omata M. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy. 2006;38:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Motohashi O, Nishimura K, Nakayama N, Takagi S, Yanagida N. Endoscopic submucosal dissection (two-point fixed ESD) for early esophageal cancer. Dig Endosc. 2009;21:176-179. [PubMed] [DOI] [Full Text] |
| 79. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 80. | Ishii N, Horiki N, Itoh T, Uemura M, Maruyama M, Suzuki S, Uchida S, Izuka Y, Fukuda K, Fujita Y. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife is a safe and effective treatment for superficial esophageal neoplasias. Surg Endosc. 2010;24:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 81. | Neuhaus H, Mayershofer R, Charton JP et al. Endoscopic submucosal dissection (ESD) early Barrett’s neoplasia (EBN) with a water-jet hybridknife (ESDH): first prospective clinical trial. Gastrointest Endosc. 2010;71:AB125. [DOI] [Full Text] |
| 82. | Yoshinaga S, Gotoda T, Kusano C, Oda I, Nakamura K, Takayanagi R. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 83. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 84. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 85. | Jung H, Choi K, Song H, Kim D, Lee G, Kim J. Endosocopic resection for superficial esophageal cancer: comparision between EMR and ESD method. Ann Oncol. 2008;19: vi45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Teoh AY, Cheung FK, Chiu PW, Ng EK. Endoscopic Submucosal Dissection Versus Endoscopic Mucosal Resection in Management of Superficial Squamous Esophageal Neoplasms. Gastrointest Endosc. 2008;67:AB188. [DOI] [Full Text] |
| 87. | Deprez PH, Piessevaux H, Aouattah T et al. ESD in Barrett’s esophagus high grade dysplasia and mucosal cancer: prospective comparison with CAP mucosectomy. Gastrointest Endosc. 2010;71:AB126. [DOI] [Full Text] |
| 88. | Vázquez-Sequeiros E, de Miquel DB, Olcina JR, Martín JA, García M, Lucas DJ, Garrido E, González C, Blanco AP, Arnau MR. Training model for teaching endoscopic submucosal dissection of gastric tumors. Rev Esp Enferm Dig. 2009;101:546-552. [PubMed] |
| 89. | Teoh AY, Chiu PW, Wong SK, Sung JJ, Lau JY, Ng EK. Difficulties and outcomes in starting endoscopic submucosal dissection. Surg Endosc. 2010;24:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Parra-Blanco A, Arnau MR, Nicolás-Pérez D, Gimeno-García AZ, González N, Díaz-Acosta JA, Jiménez A, Quintero E. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol. 2010;16:2895-2900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 506] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 92. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 93. | Bergman JJ. How to justify endoscopic submucosal dissection in the Western world. Endoscopy. 2009;41:988-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Ganz RA, Utley DS, Stern RA, Jackson J, Batts KP, Termin P. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc. 2004;60:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 95. | van Vilsteren FG, Bergman JJ. Endoscopic therapy using radiofrequency ablation for esophageal dysplasia and carcinoma in Barrett's esophagus. Gastrointest Endosc Clin N Am. 2010;20:55-74, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Frantz DJ, Dellon ES, Shaheen NJ. Radiofrequency ablation of Barrett’s esophagus. Tech Gastrointest Endosc. 2010;12:100-107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Roorda AK, Marcus SN, Triadafilopoulos G. Early experience with radiofrequency energy ablation therapy for Barrett's esophagus with and without dysplasia. Dis Esophagus. 2007;20:516-522. [PubMed] [DOI] [Full Text] |
| 98. | Sharma VK, Wang KK, Overholt BF, Lightdale CJ, Fennerty MB, Dean PJ, Pleskow DK, Chuttani R, Reymunde A, Santiago N. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 99. | Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Lightdale CJ, Santiago N, Pleskow DK. Endoscopic ablation of Barrett's esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 100. | Ganz RA, Overholt BF, Sharma VK, Fleischer DE, Shaheen NJ, Lightdale CJ, Freeman SR, Pruitt RE, Urayama SM, Gress F. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 101. | Pouw RE, Gondrie JJ, Sondermeijer CM, ten Kate FJ, van Gulik TM, Krishnadath KK, Fockens P, Weusten BL, Bergman JJ. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627-136; discussion 1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Gondrie JJ, Pouw RE, Sondermeijer CM, Peters FP, Curvers WL, Rosmolen WD, Krishnadath KK, Ten Kate F, Fockens P, Bergman JJ. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | Gondrie JJ, Pouw RE, Sondermeijer CM, Peters FP, Curvers WL, Rosmolen WD, Ten Kate F, Fockens P, Bergman JJ. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 104. | Sharma VK, Kim HJ, Das A, Dean P, DePetris G, Fleischer DE. A prospective pilot trial of ablation of Barrett's esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system). Endoscopy. 2008;40:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 105. | Hernandez JC, Reicher S, Chung D, Pham BV, Tsai F, Disibio G, French S, Eysselein VE. Pilot series of radiofrequency ablation of Barrett's esophagus with or without neoplasia. Endoscopy. 2008;40:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Sharma VK, Jae Kim H, Das A, Wells CD, Nguyen CC, Fleischer DE. Circumferential and focal ablation of Barrett's esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 107. | Velanovich V. Endoscopic endoluminal radiofrequency ablation of Barrett's esophagus: initial results and lessons learned. Surg Endosc. 2009;23:2175-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Vassiliou MC, von Renteln D, Wiener DC, Gordon SR, Rothstein RI. Treatment of ultralong-segment Barrett's using focal and balloon-based radiofrequency ablation. Surg Endosc. 2010;24:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Lyday WD, Corbett FS, Kuperman DA, Kalvaria I, Mavrelis PG, Shughoury AB, Pruitt RE. Radiofrequency ablation of Barrett's esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy. 2010;42:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 110. | Eldaif SM, Lin E, Singh KA, Force SD, Miller DL. Radiofrequency ablation of Barrett's esophagus: short-term results. Ann Thorac Surg. 2009;87:405-410; discussion 410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Muthasamy R, Lightdale CJ, Santiago N. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 112. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 971] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 113. | Semlitsch T, Jeitler K, Schoefl R, Horvath K, Pignitter N, Harnoncourt F, Siebenhofer A. A systematic review of the evidence for radiofrequency ablation for Barrett's esophagus. Surg Endosc. 2010;24:2935-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Beaumont H, Gondrie JJ, McMahon BP, Pouw RE, Gregersen H, Bergman JJ, Boeckxstaens GE. Stepwise radiofrequency ablation of Barrett's esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy. 2009;41:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, Devière J, Neuhaus H, Bergman JJ. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 116. | Pouw RE, Bisschops R, Pech O, Ragunath K, Weusten BL, Schumacher B, Rembacken B, Meining A, Messmann H, Schoon EJ. Seldenrijk CA, Mike Visser M, Antoon Lerut A, Jacques M. Deviere JM, Rosch T, Seewald S, Ten Kate FJ, Ell C, Neuhaus H, Bergman J. Safety Outcomes of Balloon-Based Circumferential Radiofrequency Ablation After Focal Endoscopic Resection of Early Barrett’s Neoplasia in 118 Patients: Results of an Ongoing European Multicenter Study. Gastointest Endosc. 2010;71:AB126. [DOI] [Full Text] |
| 117. | Pech O, Ell C. Editorial: Resecting or burning: what should we do with the remaining barrett's epithelium after successful ER of neoplasia? Am J Gastroenterol. 2009;104:2693-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | Wolfsen HC, Woodward TA, Raimondo M. Photodynamic therapy for dysplastic Barrett esophagus and early esophageal adenocarcinoma. Mayo Clin Proc. 2002;77:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Ackroyd R, Kelty CJ, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MW. Eradication of dysplastic Barrett's oesophagus using photodynamic therapy: long-term follow-up. Endoscopy. 2003;35:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett's esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc. 2003;58:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 121. | Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett's dysplasia or cancer. Aliment Pharmacol Ther. 2004;20:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 122. | Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, Bronner MP, Taylor SL, Grace MG, Depot M. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 123. | Pech O, Gossner L, May A, Rabenstein T, Vieth M, Stolte M, Berres M, Ell C. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett's cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc. 2005;62:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 124. | Prasad GA, Wang KK, Buttar NS, Wongkeesong LM, Lutzke LS, Borkenhagen LS. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett's esophagus. Gastrointest Endosc. 2007;65:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. | Ban S, Mino M, Nishioka NS, Puricelli W, Zukerberg LR, Shimizu M, Lauwers GY. Histopathologic aspects of photodynamic therapy for dysplasia and early adenocarcinoma arising in Barrett's esophagus. Am J Surg Pathol. 2004;28:1466-1473. [PubMed] |
| 126. | Mugurama N, Marcon NE. Technique and emerging role of cryotherapy. Tech Gastrointest Endosc. 2010;12:44-48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 127. | Johnston MH, Eastone JA, Horwhat JD, Cartledge J, Mathews JS, Foggy JR. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc. 2005;62:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 128. | Canto MI, Gorospe EC, Shin EJ, Dunbar KB, Montgomery EA, Okolo P. Carbon dioxide (CO2) cryotherapy is a safe and effective treatment of Barrett’s esophagus (BE) with HGD/intramucosal carcinoma. Gastrointest Endosc. 2009;69:AB341. [DOI] [Full Text] |
| 129. | Greenwald BD, Dumot JA, Horwhat JD, Lightdale CJ, Abrams JA. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus. 2010;23:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 130. | Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, Burdick JS, Abrams JA, Wang KK, Mallat D. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 131. | Dumot JA, Greenwald BD. Argon plasma coagulation, bipolar cautery, and cryotherapy: ABC's of ablative techniques. Endoscopy. 2008;40:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 132. | Van Laethem JL, Cremer M, Peny MO, Delhaye M, Devière J. Eradication of Barrett's mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut. 1998;43:747-751. [PubMed] |
| 133. | Bright T, Watson DI, Tam W, Game PA, Astill D, Ackroyd R, Wijnhoven BP, Devitt PG, Schoeman MN. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg. 2007;246:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 134. | Pinotti AC, Cecconello I, Filho FM, Sakai P, Gama-Rodrigues JJ, Pinotti HW. Endoscopic ablation of Barrett's esophagus using argon plasma coagulation: a prospective study after fundoplication. Dis Esophagus. 2004;17:243-246. [PubMed] [DOI] [Full Text] |
| 135. | Attwood SE, Lewis CJ, Caplin S, Hemming K, Armstrong G. Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol. 2003;1:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |