Published online Jun 16, 2011. doi: 10.4253/wjge.v3.i6.118
Revised: April 24, 2011
Accepted: May 4, 2011
Published online: June 16, 2011
AIM: To address the diagnostic value of the regular arrangement of collecting venules (RAC) among old age patients.
METHODS: A total of 390 consecutive patients whose Helicobacter pylori (H. pylori) status was known and who received upper gastrointestinal endoscopy, were retrospectively studied for the presence or absence of RAC as well as gastric mucosal atrophy. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC to detect normal gastric mucosa were assessed and were compared among two different age groups of patients.
RESULTS: The mean age ± standard deviation (SD) of included patients (n = 390), was 62.9 ± 13 years. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC to detect normal gastric mucosa were 91.7%, 66.1%, 18.8%, 99% and 68.1% respectively. Although the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC among patients < 60 years (n = 139) was 94.7%, 71.2%, 46.2%, 98.1%and 76.1%, respectively, it was 80%, 64.3%, 5.1%, 93% and 64.6%, respectively, among patients ≥ 60 years (n = 251). Younger Patients (< 60 years), have highly significant rates of RAC sensitivity, positive predictive value, and accuracy (P ≤ 0.001, ≤ 0.001 and ≤ 0.02, respectively). Older patients had highly significant rates of H. pylori infection and gastric mucosal atrophy (P≤ 0.01).
CONCLUSION: Although RAC is a valuable sign for real-time identification of normal gastric mucosa, its accuracy seems to be affected by the patient’s age.
- Citation: Alaboudy A, Elbahrawy A, Matsumoto S, Galal GM, Chiba T. Regular arrangement of collecting venules: Does patient age affect its accuracy? World J Gastrointest Endosc 2011; 3(6): 118-123
- URL: https://www.wjgnet.com/1948-5190/full/v3/i6/118.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i6.118
Helicobacter pylori (H. pylori) infection is a common, globally distributed phenomenon, and is considered the most important etiological risk factor for gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma (MALToma)[1-3]. In addition it is responsible for 50%-70% of atrophic gastritis cases among elderly[2,4,5].
The association between H. pylori infection and old age is well documented[6]. In Japan, around 50% of the general population is infected with H. pylori, with a notably increased infection rate of 70%-80% among people born before 1950[7,8].
Early detection and eradication of H. pylori infection is an important step in eliminating risk for gastric cancer. The real-time identification of an H. pylori infected stomach during endoscopy may not only reduce the sampling error and excessive workload for pathology departments, but also improve detection of early malignant lesions by indicating the need for more meticulous examination of the whole stomach[9].
Regular arrangement of collecting venules (RAC) is considered as a conventional endoscopic sign for identifying normal gastric mucosa[10]. Using standard endoscopy, RAC is visible as numerous minute starfish-like points covering the entire gastric corpus[11]. Previous studies[11-14] have reported reasonable accuracy of RAC in detecting normal gastric mucosa.
Decreased accuracy of RAC among older patients is a common clinical impression among Japanese endoscopists, although this has not yet been proven. In this study, we address the diagnostic value of RAC in this group of patients.
We retrospectively analyzed 402 consecutive patients who received upper gastrointestinal endoscopy from one author (SM) and whose H. pylori status was examined between November 1st, 2008 and October 31st, 2009 at Kyoto University Hospital. Patients whose medical records and endoscopic photos were available were eligible for inclusion. Twelve patients were excluded becasue of insufficient endoscopic images (n = 10) and associated portal hypertensive gastropathy (n = 2). Patients with a history of gastrointestinal bleeding, of taking non-steroidal anti-inflammatory drugs, or post anti- H. pylori eradication status were not excluded. Age, gender, H. pylori status and endoscopic data were registered and analyzed for included patients (n = 390).
The endoscopic records of included patients (n = 390) were examined for the presence or absence of the regular arrangement of collecting venules (RAC), gastric mucosal atrophy and endoscopic diagnosis.
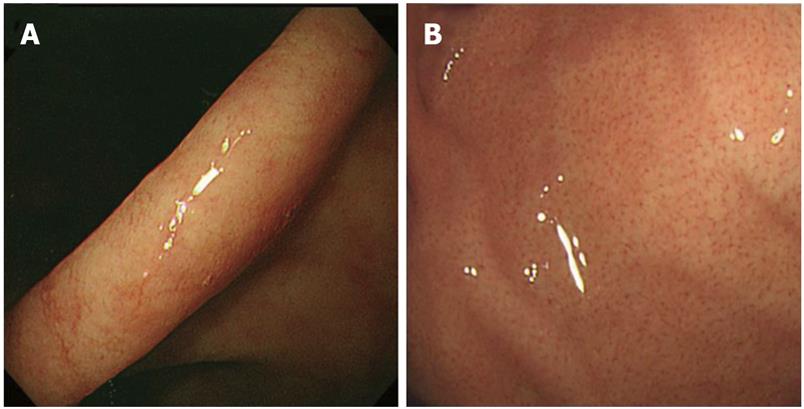
RAC was searched for at 2 sites; the lesser curvature of the lower body of the stomach (Figure 1A) and the greater curvature of the upper body (Figure 1B). RAC was recorded as positive if it was present and negative if it was absent.
After evaluation of RAC in both the lesser curvature of the lower gastric body and the greater curvature of the upper body and for descriptive purposes, we classified the patients into 3 groups: Group “A” comprised patients with positive RAC in both lower and upper body of the stomach, group “B” comprised patients with negative RAC in the lower body and those with positive RAC in the upper body and group “C” comprised patients with negative RAC in both of lower and upper gastric body.
The whole gastric mucosa was evaluated for gastric mucosal atrophy and was defined as either present or absent. The grade of atrophy was classified into no atrophy, closed type and open type[15].
All endoscopic data were collected by two investigators (AA and AE) who were trained in detection of RAC, without knowing H. pylori result. Disagreements was resolved by joint discussion with an experienced endoscopist (SM) to reach a consensus.
The gold standard of reference was H. pylori infection, which was detected either by immunohistochemistry (IHC) testing of gastric tissues (in 184 patients) or serological evaluation of H. pylori IgG antibodies (in 206 patients).
The results were expressed as the number and percentage or mean ± SD. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC [proportion of true results (both true positives and true negatives) in the population] were estimated, and correlation analysis was carried out using Pearson correlation (r). Comparison of categorical variables was done using the Chi-square test. Comparison of continuous variables was performed using Student’s t-test. A P value of less than 0.05 was required for significance. Statistical analysis was performed using SPSS statistical software (version 16, SPSS inc., Chicago, US).
Among 390 patients included in this study, 192 (49.2%) were women and 198 (50.8%) were men. Their ages ranged from 19 to 90 years (mean ± SD; 62.9 ± 13 years, median = 64.5 years).
The endoscopic diagnosis of the patients was as follows; atrophic gastritis in 136 cases (35%), gastric ulcer in 68 (18%), early gastric cancer in 61 (16%), duodenal ulcer in 21 (5%), advanced gastric cancer in 9 (2%), MALToma in 5 cases (1%), and other conditions in 90 cases (23%).
Group "A" included 24 out of 390 (6%) patients while group "B" had 85 (22%) patients. Group "C" was the largest and included 280 out of 390 (72%) patients. Positive RAC in the lower body but negative in the upper body was observed in one patient, who was negative for H. pylori.
Group “C” patients were significantly older than groups “A” and “B” (P≤ 0.001) (Table 1). There was no significant difference between groups “A”, “B”, and “C” regarding the gender (P > 0.05) (Table 1).
| Total | Group A | Group B | Group C | P value | |
| Number | 390 | 24 (6.2) | 85 (21.7) | 280 (71.8) | |
| Age (mean ± SD) years | 62.9 ± 13 | 48.5 ± 15.7 | 57 ± 13.2 | 66 ± 11.1 | ≤ 0.001 |
| < 60 years | 139 (35.6) | 19 (13.7) | 47 (33.8) | 73 (52.5) | |
| ≥ 60 years | 251 (64.4) | 5 (2) | 38 (15.2) | 207 (82.8) | |
| Gender | > 0.05 | ||||
| Male | 198 (50.8) | 8 (4.1) | 44 (22.3) | 145 (73.6) | |
| Female | 192 (49.2) | 16 (8.3) | 41 (21.4) | 135 (70.3) | |
| H. pylori status | ≤ 0.001, r = -0.29 | ||||
| Positive | 229 (58.7) | 2 (8.3) | 42 (49.4) | 185 (66.1) | |
| Negative | 161(41.3) | 22 (91.7) | 43 (50.6) | 95 (33.9) | |
| Grade of gastric atrophy | ≤ 0.001 | ||||
| No atrophy | 22 (5.6) | 10 (45.5) | 5 (22.7) | 7 (31.8) | |
| Closed-type atrophy | 198 (50.8) | 14 (7.1) | 63 (32) | 120 (60.6) | |
| Open-type atrophy | 170 (43.6) | 0 (0) | 17 (10) | 153 (90) |
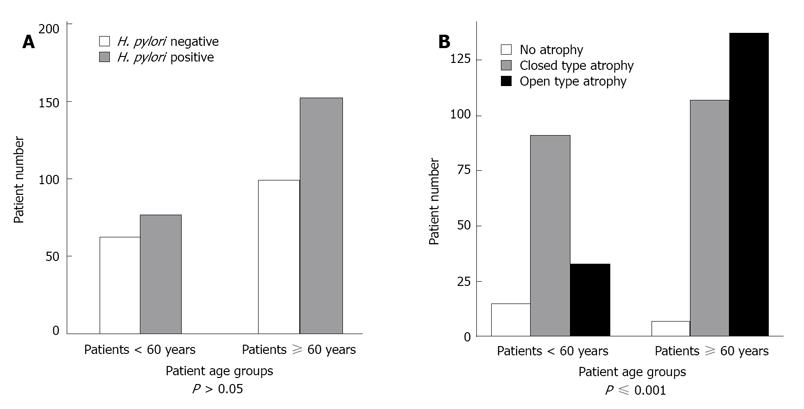
While 229(58.7%) patients were positive for H. pylori, 161 (41.3%) were negative (Table 1). H. pylori infection was detected by immunohistochemistry (IHC) in 184 (47.2%) patients where 89 (48.4%) patients were H. pylori positive and 95 (51.6%) were H. pylori negative. Serological testing was done in 206 (52.8%)and revealed that 140 (68%) patients were H. pylori positive and 66 (32%) H. pylori negative. Of group "A" patients, 22 (91.7%) patients were H. pylori negative and only 2 (8.3%) were H. pylori positive, while 185 (66.1%) patients of group “C” patients were H. pylori positive and 95 (33.9%) were H. pylori negative. We considered groups "A" and "C" as representative of the presence and absence of RAC respectively. A significant negative correlation was found between H. pylori infection and the presence or absence of RAC (r = -0.29, P≤ 0.001) (Table 1). The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC to identify normal gastric mucosa among included patients (n = 390) were found to be 91.7%, 66.1%, 18.8%, 99% and 68.1% respectively (Table 2). In order to detect any significant change in RAC accuracy among older patients (≥ 60 years), we further subdivided our patients into two groups according to their age (< 60 and ≥ 60 years). Patients < 60 years constituted 35.6% (139/390) while patients ≥ 60 years were 64.4% (251/390). RAC was negative in 52.5% (73/139) and 82.8% (207/251) of patients < 60 and ≥ 60 years respectively (Table 1). The Prevalence of H. pylori infection was 55.4% (77/139) and 60.6% (152/251) among patients in the younger and older groups, respectively (P > 0.05) (Figure 2A). In patients < 60 years, there were 19 (13.7%) patients who were RAC positive, 18 were H. pylori negative and only 1 patient was H. pylori positive, 73 (52.5%) patients were RAC negative, 21 of them were H. pylori negative and 52 patients were H. pylori positive (r = -0.43, P≤ 0.001) (Table 3) . On the other hand, among patients ≥ 60 years, there were 5 (2%) patients who were RAC positive, 4 of them were H. pylori negative and only 1 patient was H. pylori positive, 207 (82.8%) patients were RAC negative, 74 were H. pylori negative and 133 patients were H. pylori positive (r = -0.18, P≤ 0.003) (Table 3). The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC to detect normal gastric mucosa were all greatly reduced among older patients. These were 94.7 %, 71.2%, 46.2%, 98.1% and 76.1% among patients < 60 years, and 80%, 64.3%, 5.1%, 93% and 64.6% among patients ≥ 60 years, respectively. Comparing the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of RAC between the two age groups we found that the sensitivity, positive predictive value and accuracy were statistically significant (P≤ 0.001, ≤ 0.001 and 0.02 respectively) while the specificity and negative predictive value were not significant (P > 0.05) (Table 2). Open type gastric atrophy, closed type gastric atrophy and no gastric atrophy were detected in 43.6%, 50.8% and 5.6% of our patients, respectively. There was significant correlation between the gastric atrophy grade and patients with negative RAC (P≤ 0.001) (Table 1). Although there was significant correlation between gastric atrophy grade and greater age (≥ 60 years), (P≤ 0.001), (Figure 2B), a significant association was detected between gastric atrophy and H. pylori infection (P≤ 0.001).
| All Patients(n= 390 ) | < 60 years(n=139) | ≥ 60 years(n= 251) | P value | |
| Sensitivity | 91.70% | 94.70% | 80% | ≤ 0.001 |
| Specificity | 66.10% | 71.20% | 64.30% | > 0.05 |
| Positive predictive value | 18.80% | 46.20% | 5.10% | ≤ 0.001 |
| Negative predictive value | 99% | 98.10% | 93% | > 0.05 |
| Accuracy | 68.10% | 76.10% | 64.60% | 0.02 |
The main clinical significance of real-time detection of H. pylori infection during routine endoscopic examination relates to the identification of patients at high risk for gastric cancer and their need for meticulous gastric mucosal assessment. This is particularly relevant in elderly patients as they are vulnerable to higher rates of H. pylori infection and gastric cancer[16].
RAC is a conventional endoscopic marker for detecting normal gastric mucosa[10]. Previous studies[11,13,14] have reported good sensitivity, specificity and accuracy (around 95 %), for RAC in detecting normal gastric mucosa. This was in agreement with our results in terms of RAC sensitivity (91.7%). Our findings regarding high negative predictive values and low positive predictive values were in concordance with other reports[13], confirming that presence of RAC pattern virtually excludes H. pylori infection while its absence requires further evaluation. The only patient with positive RAC in the lower body but negative in the upper body showed negative to H. pylori IgG antibodies, although his Urea Breath Test was positive, suggesting mild H. pylori associated gastritis. There is no currently clear explanation of why does the signs of RAC remained in this patient.
Compared to previous studies[11,13,14] we detected lower rates for RAC specificity and accuracy (66.1%, 68.1% respectively). The main difference between our study and others is the age of studied population. Although other research investigated RAC in children and adolescents (median = 15 years)[14], as well as in middle aged patients (median age = 49 years old)[11], we investigated older patients (median age = 64.5 years). This enabled us to test the possible change of RAC sensitivity, specificity and accuracy among old patients. Our findings indicated a significant reduction in RAC sensitivity, specificity and accuracy among older patients (≥ 60 years), when compared with a group of younger patients (< 60 years).
Diagnosis of H. pylori infection in elderly patients is difficult due to multiple factors which may induce false negative results, including frequent hospitalization with multiple therapies, and the repeated use of antimicrobial therapies to treat pneumonia or urinary tract infections. Failure in the diagnosis of H. pylori infection is, therefore, frequent among this patient group especially in the presence of atrophic gastritis. In these cases the absence of bacteria in biopsy specimens, and negative H. pylori results from other diagnostic tests does not exclude the presence of ongoing infection[17,18]. Notably, the frequency of gastric atrophy was significantly higher among older patients in our study. Taken together these facts suggest that the lower RAC accuracy among older patients in our study may, at least in part, be a reflection of H. pylori detection failure, rather than an actual decrease in RAC accuracy. It should be noted that increasing age had no great impact on gastric atrophy among our patients, a fact which can be partially explained by the good correlation between H. pylori and gastric atrophy found in older patients.
In conclusion, our findings indicate that in areas with higher endemicity for H. pylori infection the presence of RAC is a sensitive endoscopic sign for identifying patients with normal gastric mucosa. RAC accuracy is decreased among older patients. Multiple factors should be considered before deciding on the H. pylori status of old patients. In addition to microscopic and serological examination, the absence of RAC as well as the presence of gastric atrophy during endoscopic assessment should be considered in making decisions.
The limitations of this study come from its retrospective nature, and from the inclusion of patients regardless of whether they had received eradication therapy for H. pylori or not. In addition, the investigators reviewed archived endoscopic images rather than conducting real-time endoscopy examinations.
Regular arrangement of collecting (RAC) venules is considered a valuable endoscopic sign for identifying the Helicobacter pylori (H. pylori) negative stomach, but whether it is affected by the patient age or not, has not yet been investigated. Therefore, the authors decided to perform this study to detect the accuracy of Regular arrangement of collecting venules (RAC) particularly in older patients.
The role of RAC for diagnosing the H. pylori negative stomach is well documented. The current issue is whether or not its accuracy is affected by the patient’s age.
Previous studies reported good accuracy of this marker. However, these studies were done on young and middle age patients. This current study is very useful for clarifying that the accuracy of RAC is decreased in older patients. Therefore, the absence of RAC during endoscopic assessment should be considered in the diagnosis of H. pylori infection in elderly patients.
Although the presence of RAC could exclude H. pylori infection, its absence requires further evaluation particularly in older patients.
The authors conducted a retrospective study regarding the accuracy of RAC as a marker in different age groups. Although it was designed in a retrospective manner, the results of this study provided essential information about clinical usage of RAC as a marker.
Peer reviewer: Sheng-Lei Yan, MD, Division of Gastroenterology, Department of Internal Medicine, Chang Bing Show Chwan Memorial Hospital, No.6, Lugong Rd., Lugang Township, Changhua County 505, Taiwan, China; Sanjiv Mahadeva, MBBS, MRCP, CCST, MD, Associate Professor, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia
S- Editor Wang JL L-Editor Hughes D E-Editor Zhang L
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 3. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 4. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 496] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 5. | Kuipers EJ, Klinkenberg-Knol EC, Vandenbroucke-Grauls CM, Appelmelk BJ, Schenk BE, Meuwissen SG. Role of Helicobacter pylori in the pathogenesis of atrophic gastritis. Scand J Gastroenterol Suppl. 1997;223:28-34. [PubMed] |
| 6. | Salles N, Mégraud F. Current management of Helicobacter pylori infections in the elderly. Expert Rev Anti Infect Ther. 2007;5:845-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham DY. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760-766. [PubMed] |
| 8. | Chiba T, Seno H, Marusawa H, Wakatsuki Y, Okazaki K. Host factors are important in determining clinical outcomes of Helicobacter pylori infection. J Gastroenterol. 2006;41:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Yagi K, Honda H, Yang JM, Nakagawa S. Magnifying endoscopy in gastritis of the corpus. Endoscopy. 2005;37:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. [PubMed] [DOI] [Full Text] |
| 12. | Gonen C, Simsek I, Sarioglu S, Akpinar H. Comparison of high resolution magnifying endoscopy and standard videoendoscopy for the diagnosis of Helicobacter pylori gastritis in routine clinical practice: a prospective study. Helicobacter. 2009;14:12-21. [PubMed] [DOI] [Full Text] |
| 13. | Machado RS, Viriato A, Kawakami E, Patrício FR. The regular arrangement of collecting venules pattern evaluated by standard endoscope and the absence of antrum nodularity are highly indicative of Helicobacter pylori uninfected gastric mucosa. Dig Liver Dis. 2008;40:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Nakayama Y, Horiuchi A, Kumagai T, Kubota S, Kobayashi M, Sano K, Ota H. Discrimination of normal gastric mucosa from Helicobacter pylori gastritis using standard endoscopes and a single observation site: studies in children and young adults. Helicobacter. 2004;9:95-99. [PubMed] [DOI] [Full Text] |
| 15. | Kim DH, Kim GH, Kim JY, Cho HS, Park CW, Lee SM, Kim TO, Kang DH, Song GA. Endoscopic grading of atrophic gastritis is inversely associated with gastroesophageal reflux and gastropharyngeal reflux. Korean J Intern Med. 2007;22:231-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Franceschi M, Di Mario F, Leandro G, Maggi S, Pilotto A. Acid-related disorders in the elderly. Best Pract Res Clin Gastroenterol. 2009;23:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Sipponen P. Update on the pathologic approach to the diagnosis of gastritis, gastric atrophy, and Helicobacter pylori and its sequelae. J Clin Gastroenterol. 2001;32:196-202. [PubMed] |
| 18. | Pilotto A, Salles N. Helicobacter pylori infection in geriatrics. Helicobacter. 2002;7 Suppl 1:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |