INTRODUCTION
Primary intestinal lymphangiectasia (PIL) was first described by Waldmann et al[1] in 1961. In PIL, impaired lymphatic drainage causes lymph leakage into the bowel lumen, which results in protein-losing enteropathy. Hypoalbuminemia, lymphocytopenia, hypogamma-globulinemia and fat-soluble vitamin deficiency anemia are common laboratory findings in PIL[2]. Patients complain of persistent diarrhea, abdominal pain, malabsorption, peripheral edema and chylous effusion. Obstructive ileus of the small intestine may develop, requiring partial jejunectomy[3]. In this condition, a sudden blockade of lymphatic drainage occurs in the affected area, followed by the massive dilation of submucosal channels and possible obstructive ileus. Congenital lymphedema is also associated with a selective deficit of naive CD4+ T lymphocytes. PIL with very low CD4+ counts and immunoglobulin G levels is related to recurrent and opportunistic infections and associated with increased morbidity and mortality[4].
The diagnosis of PIL is based on histological analysis of surgical specimens or endoscopic biopsies that reveal lacteal juice and dilated mucosal and submucosal lymphatic vessels. Typical mucosal findings upon endoscopy include diffuse swelling and enlarged whitish villi. Esophagogastroduodenoscopy and colonoscopy can be used to visualize parts of the small bowel, duodenum and terminal ileum, but capsule endoscopy is more useful to explore the entire small bowel mucosa[5]. Double-balloon enteroscopy can localize lesions and small bowel tissue can be obtained for pathological confirmation[6].
Only a few reports describe the diagnosis of PIL using capsule endoscopy and double-balloon enteroscopy[7]. No prior cases were similar to the case described in the present study, in which a deletion was found on chromosome 4. We present clinical, radiological, endoscopic and histological findings for a patient with PIL who was diagnosed using these techniques. Genetic analysis was also performed because the patient’s protein-losing enteropathy occurred at a very young age.
CASE REPORT
An 18-year-old male was transferred from the pediatrics department for endoscopic evaluation. He had been diagnosed with protein-losing enteropathy 15 d after birth based on laboratory findings and symptoms, but no cause was identified. Recurrent abdominal pain, diarrhea, hypoalbuminemia and malnutrition led to frequent hospitalization throughout his childhood. At the age of 11 years, the patient underwent a Denver shunt operation to control chylous ascites. He adhered to a low-fat diet supplemented with medium chain triglycerides (MCT) to avoid lacteal engorgement and to prevent lymphatic rupture with ensuing protein loss.
At the age of 18 years, he presented with a 3 wk history of abdominal pain, diarrhea over 20 times a day, general weakness and poor oral intake. He had short stature due to malnutrition. The physical examination showed edema in his ankles and legs. Total protein and albumin were low (3.2 and 1.8 g/dL). Sodium and potassium were within normal ranges (141 and 4.3 mmol/L), whereas ionized calcium was lower than normal (3.90 mg/dL). Urinalysis did not show proteinuria. The indicator levels of thyroid function, T3 and free T4, were low (76.31 and 0.66 ng/dL). His pituitary function and mental capacity were normal.
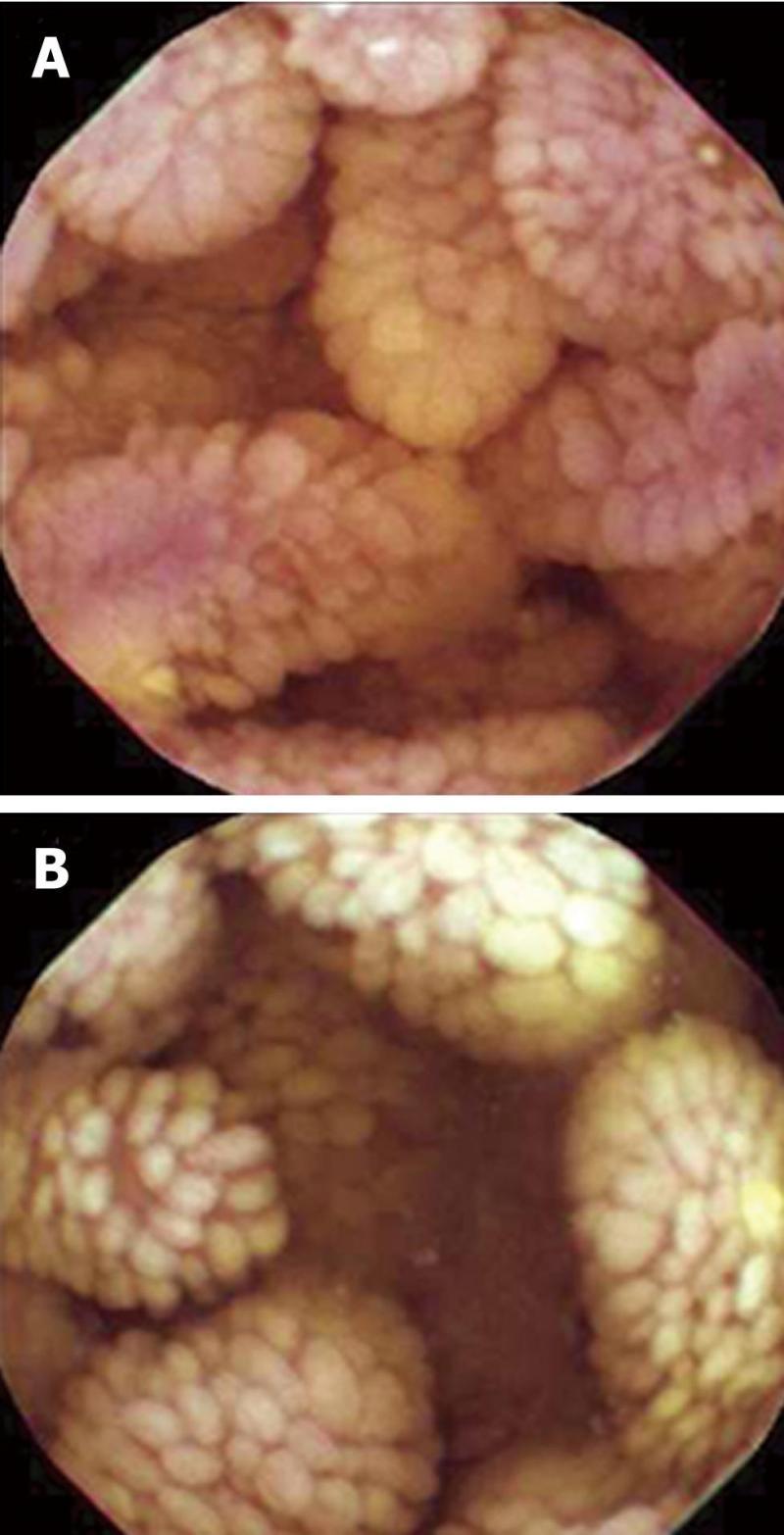
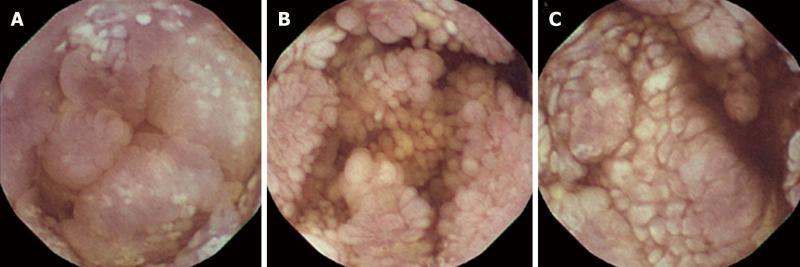
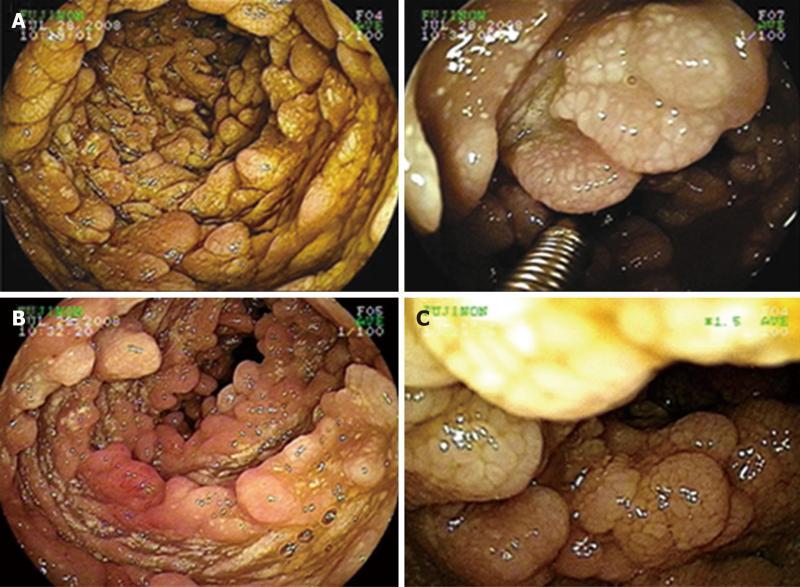
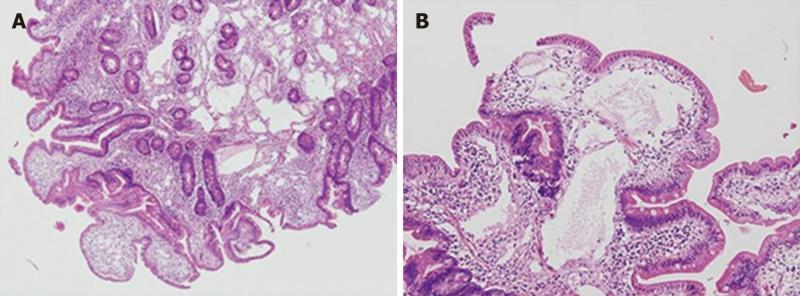
Double-contrast small bowel series with barium revealed diffuse mucosal fold thickening and increased granularity in the duodenum, jejunum and ileum; however, barium transit time was within the normal range. Abdominal ultrasonography showed diffuse small bowel wall thickening with a small amount of ascites. Abdomino-pelvic computerized tomography (CT) showed markedly thickened and enhanced mucosal layers of the jejunum and ileum. Capsule endoscopy (MiroCam®, Intromedic Co., Korea) revealed diffusely elongated, circumferential and polypoid mucosae covered with enlarged whitish villi involving the entire small bowel. These findings suggested intestinal lymphangiectasia (Figure 1), which was confirmed by double-balloon enteroscopy (Fujinon Inc., Japan). PIL involved the duodenum (A), jejunum (B) and ileum (C) (Figure 2). Multiple polypoid lesions throughout the small bowel were observed by enteroscopy and were consistent with the capsule endoscopic findings. The intestinal mucosa was slightly atrophic and covered with enlarged whitish villi and numerous lymphangitic follicles (Figure 3). Endoscopic biopsies using forceps were performed on the proximal jejunum. Histological examination demonstrated dilated lymphatic vessels with positive immune reaction of the lymphatic endothelium, compatible with lymphangiectasia (Figure 4).
Figure 1 Capsule endoscopy shows diffuse edematous mucosae covered with enlarged and swollen villi in the jejunum (A), and diffuse finger-like elongated mucosa covered with enlarged whitish villi in the ileum (B).
Figure 2 Capsule endoscopic findings in the duodenum (A), jejunum (B) and ileum (C).
Figure 3 Double balloon enteroscopy was performed with biopsy forceps to obtain a small bowel specimen of polypoid mucosa covered with enlarged whitish villi (A); and additional double balloon enteroscopic findings (B, C).
Figure 4 Histology of mucosal tissue in the jejunum shows multiple dilated lymphatics.
A: HE, × 40; B: HE, × 100.
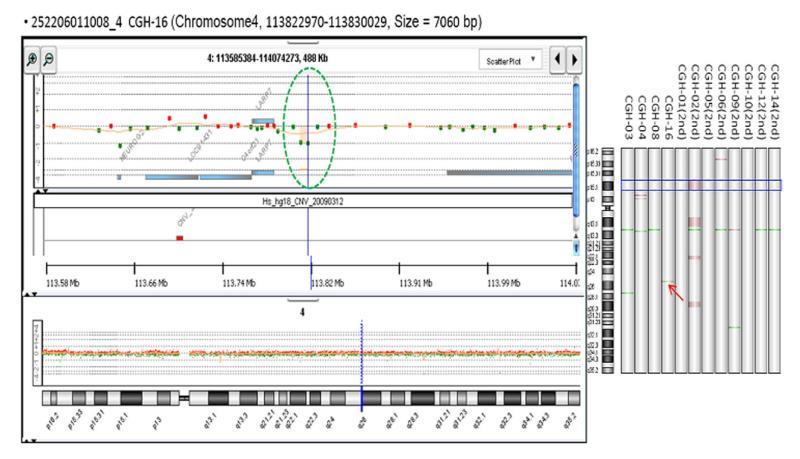
To evaluate the possibility of a genetic cause, a chromosomal study was performed which revealed a normal male karyotype (46, XY) with a deletion of chromosome 4q25 (Figure 5). A chromosomal study of the patient’s father revealed a normal male karyotype (46, XY).
Figure 5 The deletion on chromosome 4q25.
During hospitalization, the patient underwent conservative treatment that included a high protein, high calcium diet with intravenous electrolytes and albumin replacement. He has had regular check-ups and received conservative care such as albumin replacement since being discharged.
DISCUSSION
PIL is a rare congenital disorder caused by abnormal lymphatic function. Intestinal lymphangiectasis can also occur as a secondary effect of tuberculosis, sarcoidosis, Crohn’s disease, Budd-Chiari syndrome, lymphoma, congestive heart failure, constrictive pericarditis, systemic lupus erythematosus and retroperitoneal fibrosis[8]. A rare, previous case of intestinal lymphangiectasia caused by multiple myeloma involved the mesenteric lymph nodes[9]. PIL was suggested because there was no evidence of pancreatic disease, systemic lupus erythematosus, Menetrier’s disease, other intestinal disorders, other disorders of intestinal lymphatics or intestinal lymphoma[10]. Ruptured lymphatic vessels resulting from inflammatory diseases of the small intestine or malrotation were reported to be mechanisms of secondary intestinal lymphangiectasis. Lymph leakage into the bowel lumen, which leads to hypoalbuminemia and lymphopenia, is a basic mechanism of intestinal lymphangiectasis[11]. In PIL, the etiology of lymphatic dysfunction is unknown. Some studies point to genetic factors in hereditary lymphatic disorders, while other data suggest that regulatory signals involved in lymphangiogenesis, such as vascular endothelial growth factor receptor 3 and LYVE-1, are associated with intestinal lymphagiectasia[12]. Vascular endothelial growth factors-C and -D may also stimulate lymphangiogenesis[13]. Mutations in the FOXC2 (MFH-1) gene or deletion of the pik3r1 gene may result in lymphangiectasia, arrested lymphatic sprouting and maturation defects[14,15]. Defects in chromosome 4 may be related to Rieger syndrome, head and neck squamous cell carcinoma and postoperative atrial fibrillation[16-18]. However, the present case is unique because a deletion was found on chromosome 4. We hypothesized that the deletion of 4q25 and PIL were related because a recent study identified a chromosome 4q25 variant that was associated with diseases such as atrial fibrillation[18]. However, the relationship between deletions on chromosome 4q25 and lymphatic disorders such as PIL remains to be investigated. Mutations related to PIL have never previously been reported.
Diagnoses of PIL are typically confirmed by the presence of intestinal lymphangiectasia based on endoscopic findings and the corresponding histology in intestinal biopsy specimens[11]. Intestinal tissue is acquired through double balloon enteroscopy if a suspicious lesion is observed in the small intestine. Abdominal ultrasonography and CT show diffuse wall thickening and mesenteric edema[19]. Typical mucosal lesions can be identified using endoscopy. Donzelli et al[20] described two types of lymphangiectatic plaques on the surface of the duodenal mucosa that were detected via duodenoscopy. One type had a diameter of less than 1 mm, while the other exceeded 3 mm in diameter. Asakura et al[21] reported three cardinal endoscopic findings in protein-losing enteropathy: scattered white spots, white villi and chyle-like substances covering the mucosa in the jejunum. In cases of sustained protein-losing enteropathy, capsule endoscopy may assist diagnosis. Chamouard et al[22] described a case of PIL that was detected using Given M2A video capsule endoscopy. Fang et al[10] reported the case of a female patient who was diagnosed with PIL by M2A capsule endoscopy and was confirmed by pathological examination. In our case, we were able to examine the patient’s small bowel completely and detect these typical findings via capsule endoscopy following accurate pathological diagnosis according to double balloon enteroscopy. However, the roles of capsule endoscopy and double balloon enteroscopy as prognosticators have not been reported.
Outcomes of PIL may be predicted according to whether complications, such as infection, malignancy (lymphoma) and serous effusion (pleural or pericardic), occur. There is no gold standard treatment for PIL. A low-fat diet with supplemental MCT forms the cornerstone of management in PIL[23]. MCT are directly absorbed into the portal venous system, which prevents lacteal engorgement[24]. Dietary intervention is more effective in children than in adults, therefore early diagnosis of intestinal lymphangiectasia and consistent attention to diet are very important for early nutrition and growth[25]. Small bowel resection may be helpful to treat localized disease[26]. Antiplasmin, octreotide and corticosteroids are treatment options, but their efficacies are variable and insufficient[11]. In our patient, the course of disease apparently stabilized after treatment; however, he has reported episodes of protein-losing enteropathy during occasional periods of dietary therapy cessation. Antiplasmin, octreotide and corticosteroids may be future options if his symptoms progress despite dietary therapy.
We report the case of a patient with PIL involving the entire small bowel, which we confirmed using capsule endoscopy and double-balloon enteroscopy-guided tissue pathology. Capsule endoscopy showed diffusely swollen mucosae and enlarged whitish villi, and genetic analysis revealed a deletion on chromosome 4. However, we did not identify the specific gene associated with chromosome 4q25 that may be related to PIL. The LARP7 gene, which is located near chromosome 4q25, may be involved. The possible relationship between the deletion at chromosome 4q25 and PIL warrants further study.
Peer reviewers: Sheng-Lei Yan, MD, Division of Gastroenterology, Department of Internal Medicine, Chang Bing Show Chwan Memorial Hospital, No.6, Lugong Rd., Lugang Township, Changhua County 505, Taiwan, China; Marmo Riccardo, Postgraduate Specialist, Head, Gastroenterology Unit, Azienda Sanitaria Locale Salerno, Presidio Ospedaliero Polla (SA), Via Luigi Curto Polla, 84037, Italy; Rami Eliakim, Professor, Chair of Medicine, Head, Department of Gastroenterology, Rambam Health Care Campus, Bat Galim, PO Box 9602, Haifa 31096, Israel
S- Editor Zhang SJ L- Editor Roemmele A E- Editor Zheng XM