Published online Jul 16, 2010. doi: 10.4253/wjge.v2.i7.263
Revised: June 22, 2010
Accepted: June 29, 2010
Published online: July 16, 2010
AIM: To assess the additive effect of lubiprostone on the quality of colon preparation in diabetics given single-dosed polyethylene glycol electrolyte (PEG) for colonoscopy.
METHODS: This was an investigator-initiated, single-center, single-blinded prospective trial comparing the efficacy of L + PEG to PEG alone on colon preparation quality in diabetics undergoing screening colonoscopy. The study was approved by our institution’s IRB. The PEG was given as a single-dose to address patient-compliance concerns voiced by our IRB with split-dosing. All patients received only clear liquids the day prior to colonoscopy. Experimental group (Grp L) received PEG + 1 dose L 2 h prior to and 2 h after PEG completion. Control group (Grp C) received only PEG the evening prior to the colonoscopy. Patients were randomly assigned to one of the 2 groups. The endoscopist was blinded to which colon prep was given and all colonoscopies were complete. Upon colonoscopy completion, the endoscopist rated the colon prep-quality by a validated 5-point Likert scale (1-excellent to 5-inadequate).
RESULTS: Sixty patients were enrolled in the study; 30 Grp L and 30 Grp C. Overall, patients were excluded due to study non-completion in 12 (41%) Grp L and 5 (17%) Grp C, P = 0.04. Average colon preparation score Grp L = 2.47 and Grp C = 3.00, P = 0.09. Although this was not statistically significant, there was a trend towards improved colon prep in Grp L. Statistical significance may have been achieved if completion rates had been similar between both study groups.
CONCLUSION: Use of 2-L capsules with PEG resulted in a trend towards improved colon prep over PEG alone in diabetic patients when given as a single-dose regimen.
- Citation: Grigg E, Schubert MC, Hall J, Rahhal F, Raina D, Sridhar S, Chamberlain SM. Lubiprostone used with polyethylene glycol in diabetic patients enhances colonoscopy preparation quality. World J Gastrointest Endosc 2010; 2(7): 263-267
- URL: https://www.wjgnet.com/1948-5190/full/v2/i7/263.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i7.263
Colonoscopy allows visualization of the entire colon and is indicated to identify etiologies of anemia, bleeding or inflammation in the gastrointestinal (GI) tract. Currently, colonoscopy is also the procedure of choice for colon cancer/polyp screening and surveillance[1]. Colon preparation cleansing quality determines the difficulty, speed and completeness of colonoscopy, especially in terms of lesion detection[2]. The impact of adequate colon preparation also has important cost implications as poor bowel cleansing results in shortened interval between colonoscopies, longer procedure times, decreased patient satisfaction and increased lesion miss rates[3]. The current types of colon preparations available are either larger volume polyethylene glycol electrolyte (PEG)-based or smaller volume sodium phosphate-based preparations. Despite the same efficacy between PEG vs sodium phosphate-based preparations according to a meta-analysis of randomized-controlled trials[4], sodium phosphate-based preps have been associated with fluid overload, electrolyte abnormalities (transient increase in serum sodium and phosphorus and decrease in calcium levels) and acute phosphate nephropathy in diabetic patients, even with normal renal function[5-8]. Thus, PEG-based colon cleansing solutions are the most commonly used colonoscopy preparations for diabetic patients. A large population-based study has shown diabetes to be an independent risk for colon cancer compared to the general population[9]. However, recent data has shown that diabetic’s bowel cleansing with PEG-based prep is not as efficient as non-diabetic’s[10]. PEG (Nulytely) is an osmotically-balanced bowel cleansing regimen that may be safely administered to patients with electrolyte imbalances, advanced liver disease and those with poorly compensated congestive heart failure and renal failure[11]. A 4 liter volume of PEG is taken orally in its entirety the evening before colonoscopy or as a split-dose (each 2 liters the night before and 5 h prior to colonoscopy). Lubiprostone (Amitiza, Sucampo Pharmaceuticals, Inc., Bethesda, MD; Takeda Pharmaceuticals America, Inc., Deerfield, IL) is a locally acting type-2 chloride channel activator which causes intestinal fluid secretion resulting in softened stool and increased intestinal transit without the loss of either net intravascular fluid or electrolytes[12]. Lubiprostone is currently approved by the US Food and Drug Administration (FDA) at a 24 mcg dose taken twice daily orally for chronic idiopathic constipation in adults and an 8mcg dose taken twice daily orally for irritable bowel syndrome with constipation in women ≥ 18 years old[13]. Long term use of lubiprostone causes no clinically significant changes in serum electrolyte levels[13]. Lubiprostone has been safely used in diabetic patients and is only contraindicated in patients with known or suspected mechanical GI obstruction. In addition, lubiprostone should be avoided in pregnant patients and is a category C medication[13]. A prior trial with non-diabetics using a 24 mcg lubiprostone capsule (L) given in a single dose with split-dose PEG showed improvement in prep quality[14]. The purpose of our study was to assess whether the addition of lubiprostone to a single-dose of 4 liters of PEG the evening before colonoscopy would affect the quality of colon preparation in diabetics.
This was an investigator-initiated, single-center, single-blinded prospective trial comparing the efficacy of L + PEG to PEG alone on colon preparation quality in adult-onset diabetic mellitus (AODM) undergoing screening colonoscopy. This study was approved by our institution’s IRB prior to implementation.
We prospectively offered enrollment to all adult-onset diabetic outpatients who were referred to the Gastroenterology clinic at the Medical College of Georgia in Augusta, Georgia for a screening colonoscopy from July, 2008 to March, 2010. Patients were at least 50 years of age with known AODM. The study participants were enrolled in the trial by one of two Gastroenterology attending physicians or a Gastroenterology fellow. Women must have been post-menopausal or surgically sterile. Patients had to be able to read and write in English and give a valid, informed consent. Patients with the following characteristics were excluded from the study: suspected acute or chronic pseudo-obstruction, active GI hemorrhage, known inflammatory bowel disease, chronic diarrhea, prior colonic resection, acute diverticulitis, known colonic mass, clinical evidence of decompensated liver disease, renal disease or patients on dialysis, current or previous use of lubiprostone and allergy to lubiprostone.
Subjects were assigned to the Control group (Grp C) or Experimental group (Grp L) on an odd/even basis. After research informed consent had been obtained, subjects were given a study ID numbered 1 through 60. Subjects with an odd number were assigned to Grp C while subjects with an even number were assigned to Grp L. Subjects were then given a randomization package by the hospital research pharmacist consisting of the preparation orders, supplies, instructions and the date of their procedure by the investigator obtaining informed consent. The endoscopists were blinded to which preparation was given.
All patients received 4 liters of PEG preparation (Nulytely, Braintree Laboratories, Inc., Braintree, MA; 420 g polyethylene glycol 3350, 5.72 g sodium bicarbonate, 11.2 g sodium chloride, 1.48 g potassium chloride and one optional 2.0 g flavor pack) given as a single-dose to address patient compliance concerns voiced by our IRB with split-dosing. In addition, all patients received only a clear liquid diet the day prior to colonoscopy. Grp L received PEG plus 2 lubiprostone capsules, 1 capsule the 2 h prior to PEG and 1 capsule 2 h after PEG completion. Grp C received only PEG the evening prior to colonoscopy. The study’s sponsor prohibited a placebo-pill to be given in Grp C (a single-blinded trial). All patients were instructed to start drinking the PEG solution around 6pm the evening before their colonoscopy and ingest about 8 oz every 10 min until completion of 4 liters.
All the colonoscopies were carried out in the endoscopy center at the Medical College of Georgia. The colonoscopies were performed by two experienced endoscopists using the Olympus colonoscopes (Olympus Optical Co., Tokyo, Japan). A complete colonoscopy was defined as reaching the cecum which was determined by visualization and documentation of the ileocecal valve and appendiceal orifice. Patients either received moderate conscious sedation by administering a combination of fentanyl and midazolam intravenously or monitored anesthesia care with diprivan.
The primary measured endpoint of this study was the quality of colon cleansing preparation as rated by a blinded endoscopist using a validated 5-point Likert scale[15].
One of two gastroenterology attending physicians graded all the bowel preparations upon completion of the colonoscopy and was blinded to what bowel cleansing prep the patient had taken. The colon prep quality was rated based on global colon assessment using a modified Ottawa bowel preparation scale with 1 being excellent and 5 considered inadequate (Table 1).
| Score | Definition |
| 1-excellent | Small volume of clear liquid or great than 95% of the colonic mucosal surface seen |
| 2-good | Large volume of clear liquid covering 5%-25% of the surface, but greater than 90% of surface seen |
| 3-fair | Some semisolid stool that could be suctioned or washed away, but great than 90% of surface seen |
| 4-poor | Semisolid stool that could not be suctioned or washed away but great than 90% of the surface seen |
| 5-inadequate | Solid stool obscuring mucosal detail and contour despite aggressive washing and suctioning; repeat preparation and colonoscopy needed |
The study was designed to determine whether L + PEG improved colon prep quality in AODM patients undergoing screening colonoscopy vs PEG alone. It was expected that at least 100 patients would complete the trial. A sample size of 100 patients would detect a 30% difference in the percentage of patient with excellent (1) or good (2) prep quality with 89% power and a two tailed P value of 0.05. However, due to loss of funding from the pharmaceutical company, the study was terminated early. The quality of colonoscopy preparations was compared using chi-square statistics. Exact methods were used if there were small or zero cell counts.
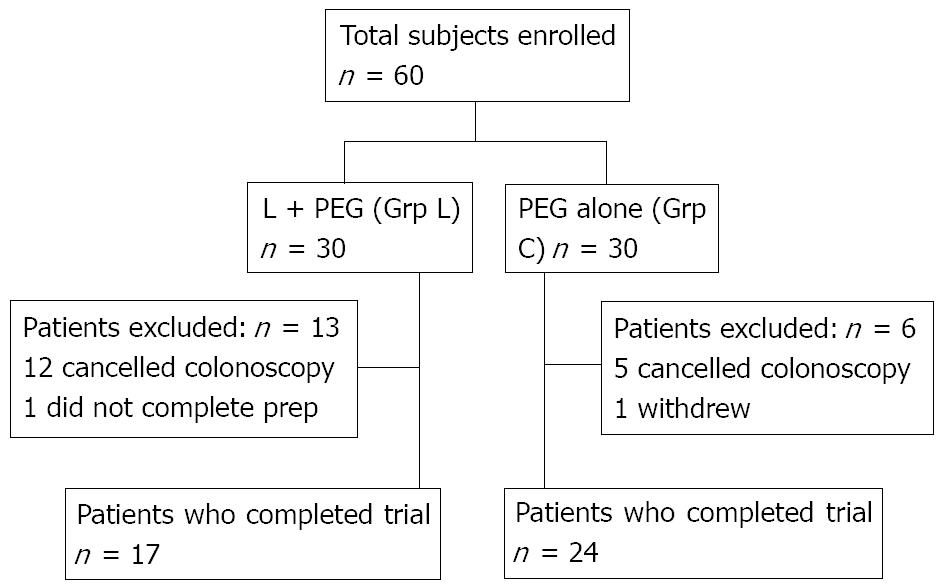
A total of 60 patients were enrolled and randomized in the clinical trial; 30 in Grp L and 30 in Grp C (Figure 1). Overall, 13 patients were excluded in Grp L and 6 patients in Grp C. In Grp L, 12 patients (41%) cancelled their procedure and 1 did not complete the prep. In Grp C, 5 patients (17%) cancelled their procedure and 1 withdrew from the trial. The no-show rate between Grp L and Grp C was statistically significant (P = 0.04).
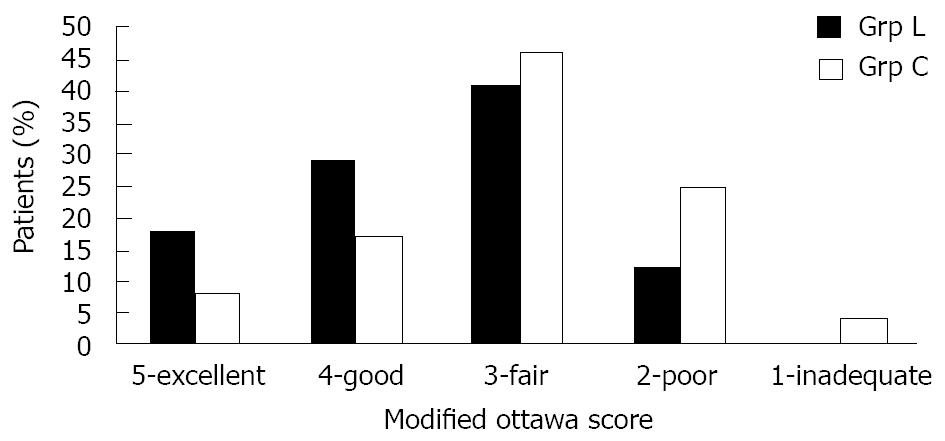
The quality of the bowel preparation as evaluated by the endoscopist for each study group is shown in Figure 2. Overall, 8 out of 17 patients (47%) in Grp L had an excellent or good colon prep quality versus 6 out of 24 patients (25%) in Grp C (P = 0.14). The average colon preparation score in Grp L was 2.47 and 3.00 in Grp C (P = 0.09). Unfortunately, in order to achieve a statistical significance of ≤ 0.05 an anticipated effect (f2) of 0.37 would have had to have been achieved (f2 = 0.18 in this study). Although this trial did not show statistical significance, there was a trend towards improved colon prep quality in Grp L.
There were no serious adverse events associated with this study. However, 1 patient in Grp L had a known history of paroxysmal atrial fibrillation and was in normal sinus rhythm during the time of study enrollment but was in atrial fibrillation with controlled rate the morning of colonoscopy. All of the colonoscopies were complete, except 1 in Grp C due to inadequate prep quality.
Colonoscopy remains the preferred method for colon cancer and polyp screening and a successful procedure requires adequate bowel preparation[16]. The ideal colon preparation should allow consistent and reliable visualization of the colonic mucosal surface with a safety profile that is acceptable for all types of patients. Unfortunately, no current bowel cleansing preparation meets these criteria[14].
Our study is the first to evaluate the use of lubiprostone in combination with a more practical single-day PEG regimen on the effect of bowel prep quality in diabetics undergoing screening colonoscopy. Use of 2 lubiprostone 24 mcg capsules with a PEG single-dose regimen resulted in a trend towards improved colon preparation versus PEG alone in patients with AODM. Statistical significance may have been achieved if completion rates had been similar between both study groups. It is also possible that even higher doses of lubiprostone will be required to achieve better colon prep-quality in diabetics when single-dose PEG is used.
This study does have some limitations. Firstly, it was performed at a single-center and was only single-blinded. The study’s sponsor prohibited a placebo-pill to be given in Grp C. Secondly, previous studies have shown improved bowel cleansing with split-dose PEG[16] but, due to concerns voiced by our IRB regarding patient compliance with a split-dose PEG, we had to use single-dose PEG in our trial. Lastly, the original trial was powered for a sample size of 100 patients in order to achieve statistical significance; however this trial was terminated early due to loss of funding from the pharmaceutical company. Thus, it is possible that a type II error could have occurred because the study group was insufficiently powered. Statistical significance may have been achieved if the trial had been fully completed.
In conclusion, this study showed that there is a trend towards improved colon prep quality in diabetic patients undergoing screening colonoscopy who received a combination of L + PEG versus PEG alone. Given that 2 doses of lubiprostone has an average retail cost under $9.00 US, combining lubiprostone to standard PEG may be a reasonable and cost-effective option to achieve better bowel cleansing in difficult to prep adult-onset diabetic patients[17]. In addition, with almost no adverse events reported, adding lubiprostone may be a viable option to achieve optimal bowel prep in diabetics especially if split-dose PEG is used. A larger double-blinded trial will be required to further evaluate these findings. The medical community must continue to develop safe, effective and well tolerated methods for bowel cleansing in order to maximize the effect of colon cancer/polyp screening.
Diabetic patients may have difficulty in obtaining acceptable colonoscopy preparation quality.
Achievement of superior colonoscopy preparation quality is of utmost importance to allow for colonic lesion detection in colonoscopy. Research is ongoing to provide patients colonoscopy preparation medication regimens that are easily taken while achieving preparation quality for patient compliance and wide-spread clinical application.
Addition of Lubiprostone to polyethylene glycol–based colonoscopy preparations may enhance colonoscopy preparation quality in a diabetic population that is difficult to prep.
Future larger studies utilizing lubiprostone with other patient populations and colonoscopy preparation regimens will need to be performed to confirm that lubiprostone is a cost-effective adjunctive medication in colonoscopy preparation.
Lubiprostone (Amitiza, Sucampo Pharmaceuticals, Inc., Bethesda, MD; Takeda Pharmaceuticals America, Inc., Deerfield, IL) is a locally acting type-2 chloride channel activator which causes intestinal fluid secretion resulting in softened stool and increased intestinal transit without the loss of either net intravascular fluid or electrolytes.
The article should be accepted as initial, innovative research utilizing lubiprostone to improve colonoscopy preparation in the difficult to prep diabetic population. However, the reviewers noted that conclusions drawn from the study’s result would be limited by its small sample size.
Peer reviewers: Varut Lohsiriwat, MD, MSc, Assistant Professor, Department of Surgery, Faculty of Medicine Siriraj Hospital, Bangkok 10700, Thailand; Alberto Arezzo, MD, PhD, Endoscopic Surgery Unit International Evangelic Hospital, Genova 16122, Italy; Nevin Oruc, MD, Associate Professor, Department of Gastroenterology, Ege University Faculty of Medicine, Izmir 35100, Turkey
| 1. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. |
| 2. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of GI Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. |
| 3. | Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696-1700. |
| 4. | Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276-282. |
| 5. | Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85:422-427. |
| 6. | Kolts BE, Lyles WE, Achem SR, Burton L, Geller AJ, MacMath T. A comparison of the effectiveness and patient tolerance of oral sodium phosphate, castor oil, and standard electrolyte lavage for colonoscopy or sigmoidoscopy preparation. Am J Gastroenterol. 1993;88:1218-1223. |
| 7. | Cohen SM, Wexner SD, Binderow SR, Nogueras JJ, Daniel N, Ehrenpreis ED, Jensen J, Bonner GF, Ruderman WB. Prospective randomized, endoscopic blinded trial comparing precolonoscopy bowel cleansing methods. Dis Colon Rectum. 1994;37:689-696. |
| 8. | Beyea A, Block C, Schned A. Acute phosphate nephropathy following oral sodium phosphate solution to cleanse the bowel for colonoscopy. Am J Kidney Dis. 2007;50:151-154. |
| 9. | Limburg PJ, Anderson KE, Johnson TW, Jacobs DR Jr, Lazovich D, Hong CP, Nicodemus KK, Folsom AR. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2005;14:133-137. |
| 10. | Taylor C, Schubert ML. Decreased efficacy of polyethylene glycol lavage solution (golytely) in the preparation of diabetic patients for outpatient colonoscopy: a prospective and blinded study. Am J Gastroenterol. 2001;96:710-714. |
| 11. | Lichtenstein GR, Cohen LB, Uribarri J. Review article: Bowel preparation for colonoscopy--the importance of adequate hydration. Aliment Pharmacol Ther. 2007;26:633-641. |
| 12. | Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther. 2007;25:1351-1361. |
| 13. | Amitiza (lubiprostone) package insert. Bethesda, MD: Sucampo Pharmaceuticals, Inc.. 2009;. |
| 14. | Stengel JZ, Jones DP. Single-dose lubiprostone along with split-dose PEG solution without dietary restrictions for bowel cleansing prior to colonoscopy: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2008;103:2224-2230. |
| 15. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. |
| 16. | Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z, Tarchichi M, Sharara AI. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005;62:213-218. |
| 17. | Aoun E; Drugstore. com, Inc: Drugstore. com: Amitiza 2010; Available from: http://www.drugstore.com. |