Published online Apr 16, 2010. doi: 10.4253/wjge.v2.i4.112
Revised: March 27, 2010
Accepted: April 3, 2010
Published online: April 16, 2010
Colorectal cancer is a major cause of death in the western world and is currently the second commonest cause of death from malignant disease in the UK. Recently a “driving test” for colonoscopists wishing to take part in the National Health Service Bowel Cancer Screening Program has been introduced, with the aim of improving quality in colonoscopy. We describe the accreditation process and have reviewed the published evidence for its use. We compared this method of assessment to what occurs in other developed countries. To the authors’ knowledge no other countries have similar methods of assessment of practicing colonoscopists, and instead use critical evaluation of key quality criteria. The UK appears to have one of the most rigorous accreditation processes, although this still has flaws. The published evidence suggests that the written part of the accreditation is not a good discriminating test and it needs to be improved or abandoned. Further work is needed on the best methods of assessing polypectomy skills. Rigorous systems need to be in place for the colonoscopist who fails the assessment.
- Citation: Kelly NM, Moorehead J, Tham T. Is the ‘driving test’ a robust quality indicator of colonoscopy performance? World J Gastrointest Endosc 2010; 2(4): 112-120
- URL: https://www.wjgnet.com/1948-5190/full/v2/i4/112.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i4.112
Colorectal cancer is a major cause of death in the western world and is currently the second commonest cause of death from malignant disease in the UK. High quality video colonoscopy is a central tenet in the investigation of symptomatic patients with bowel disorders, and is part of the UK National Health Service Bowel Cancer Screening Programme (NHS BCSP).
Colonoscopy is not a perfect test. Several studies have highlighted important limitations in its accuracy. Ineffective bowel preparation, inability to consistently intubate the caecum and rapid withdrawal times are all important contributors to missed lesions at colonoscopy[1-3]. Moreover, a systematic review of tandem colonoscopy has shown a miss rate of 22%[4] for polyps < 10 mm. Recent large computed tomography colonography studies have confirmed a significant miss rate[5-7]. In these studies, segmental unblinding was used to ascertain the miss rate for optical colonoscopy. It was revealed that 2%-12% of polyps > 10 mm were missed. Assuming that the colonoscopies in a trial setting would all have been performed by experienced colonoscopists, the miss rate in clinical practice, where experience varies, might actually be higher. Perhaps the most important driver for change in the UK was a large prospective study of colonoscopy demonstrating poor practice. Prior to commencement of the NHS BCSP in June 2006 this study showed an adjusted caecal intubation rate of only 56.9%[8], well below the recommended target of 90%.
Undoubtedly there are inherent limitations to colonoscopy and sensitivity will never reach 100%. It is also unclear what proportion of missed lesions is due to correctable aspects of colonoscopy technique and performance. With these factors in mind, as well as the risks inherent in the procedure, it was decided that bowel cancer screening with colonoscopy would only begin in centres with high achievement on the Global Rating Scale, and by colonoscopists who had had a formalised assessment of their skills. The Joint Advisory Group on Gastrointestinal Endoscopy (JAG), a national body tasked with a role in the quality assurance of endoscopy training and services across the UK, has subsequently instigated a rigorous accreditation process for colonoscopists whose practices include colonoscopy as part of the NHS BSCP.
We sought to examine the accreditation process, the evidence for its use, and the wider implications for endoscopists and endoscopic practice.
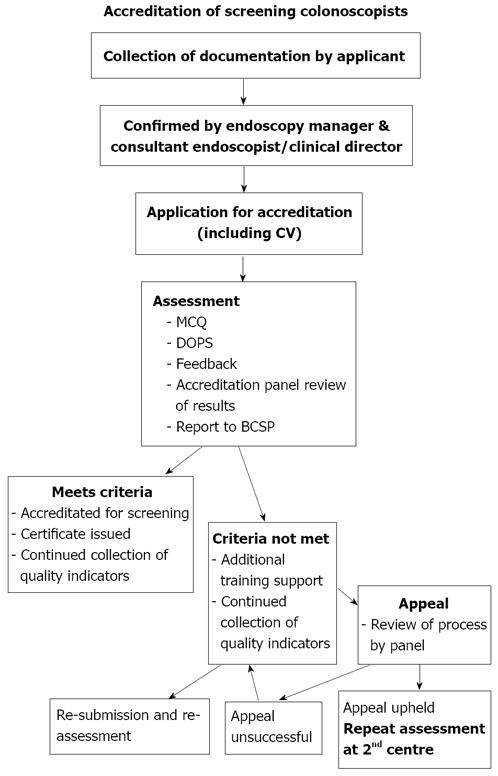
The NHS BCSP has set out in detail the criteria required for accreditation in screening colonoscopy in their document ‘Accreditation of screening colonoscopists: BCSP Implementation Guide No.3 (Version 8: 12th February 2009)’[9]. The process is summarised in Figure 1 and outlined below.
In order to be assessed for accreditation in screening colonoscopy, individuals must first fulfil the following criteria: (1) Be attached to a screening centre approved for the BCSP; (2) Minimum lifetime colonoscopy experience > 1000 procedures; (3) > 150 procedures in year preceding assessment; (4) Unadjusted completion rate on an intention to treat basis of > 90% over preceding year; (5) Documentation of preceding year audit data including number, median sedation levels (under 70 years/over 70 years), completion rate, details of failures, polyp detection rate (expected to be > 20%) and complications; and (6) Audit should have been verified by Endoscopy Unit Sister and consultant colleague/clinical director/medical director.
If the above criteria are met, on submission of an application to the NHS BCSP, individuals will be invited to attend one of several accredited assessment centres, where they will be assessed.
The first part of the assessment process is a written test comprising a one-hour multiple-choice questionnaire (MCQ) consisting of 30 questions. This is based on a list of topics for study and suggested sources for reading given in the pre-assessment documentation. The MCQ paper is positively marked, and candidates are encouraged to answer all questions. The main part of the accreditation process consists of direct observation of practical colonoscopy on two consecutive cases, where skills are scored against standardised criteria by two assessors. This is know as a directly observed procedural skill (DOPS) assessment, and is used widely in the UK to assess practical skills of trainee doctors. This DOPS assessment of endoscopy is also normal practice for trainee registrars in gastroenterology who are required to complete many such procedures during their time in training, before being deemed competent to practice independently. However, those undergoing the accreditation process for bowel cancer screening will generally have already been deemed competent to practise independently.
Candidates are assessed on consent, pre-procedure preparation, sedation practice, and colonoscopic technique, including therapeutic ability and the discussion of results and management with the patient. Assessors allocate a grade score for each criterion assessed and use these grades to inform their final decision as to whether or not a candidate has met the criteria required for accreditation. Interestingly, the assessors are advised that some aspects of a domain may be irrelevant to cases in an assessment, but should still be marked. For example, a patient may have no polyps and therefore require no therapy. Assessors are advised that a grade 3 or 4 (pass grades) can still be awarded in these domains. In this way, a colonoscopist undergoing assessment could be deemed ‘highly skilled’ at polypectomy without ever having been observed removing a polyp. We know that approximately 30% of patients undergoing screening will have adenomas requiring removal for histopathological analysis[10,11]. As it is impossible to select patients with polyps for the accreditation colonoscopy, this represents a significant weakness in the process.
Following the assessment, candidates are given written and verbal feedback on their performance. Written feedback covers areas of good practice as well as areas for further training or development. Results and feedback take place in private and do not last longer than 10 min.
There are two possible outcomes of the assessment process: (1) The candidate meets the criteria for formal accreditation; and (2) The candidate does not yet meet the criteria/needs further development.
After initial accreditation, candidates must submit to further accreditation procedures on an annual basis, after which accreditation is renewed, provided that candidates can meet the following criteria: (1) An intention to undertake > 150 screening colonoscopies per annum; (2) Agree to the submission of quality monitoring data on an annual basis, while continuing to meet the application criteria; and (3) Maintenance of an acceptable level of complications over a prolonged period, specifically, below the national average as defined in recent published series[8].
Prior to the institution of this formalised assessment for screening colonoscopists, there was considerable anxiety amongst the endoscopic community. Concerns were raised about the assessment process itself, and the appropriateness of such a test. There were also concerns about validity and reliability.
Barton attempted to allay some of these concerns with two studies published in abstract form in 2008[12,13]. The first study examined the performance and outcomes of candidates in the assessment and their perceptions of the process[12]. 76 assessments undertaken by 67 candidates were reviewed. These initial colonoscopists were experienced practitioners with a mean number of colonoscopies of 2490 (range 500-7500) and a mean polyp detection rate of 29% (range 18%-53%). It is interesting that the procedural experience of some of these candidates fell outside the current guidelines laid out in the NHS BCSP accreditation document (minimum lifetime number procedures > 1000, polyp detection rate > 20%). The mean score in the MCQ paper was 80% (range 59%-98%). No pass mark was given, although it appears that no candidates failed their accreditation as a result of their MCQ paper score. From this we can infer that the pass mark was < 59%.
8 assessments had to be repeated - 3 for breach of protocol with the DOPS assessment and 5 as a true re-sit assessment. Of 73 secure assessments, 54 (74%) candidates met the criteria required for accreditation, giving an overall failure rate of 26%. The perceptions of the process by candidates highlighted particular flaws with the MCQ paper, notably a degree of ambiguity in some questions, poor clarity of some images, and concerns about the content and relevance of some questions. The comments regarding the DOPS assessment were of particular interest. Some commented that the process was hugely stressful, and that a degree of luck was involved in the allocation of cases. Others commented that some of the difficulties lay with assessment taking place in an unfamiliar unit. Despite these comments, candidates felt welcomed, and that the assessment was fair. Only 5 appeals against the results were referred back to the Accreditation Panel,with one upheld as a breach of protocol.
The validity and reliability of the MCQ and DOPS assessment have also been assessed[13]. In this study self reported, verified performance and demographic data, as well as assessment data from both the MCQ and DOPS over two cases assessed by two assessors, were collected prospectively from 76 candidates as well as 17 assessors. Semi-structured questionnaires were completed by both the candidates and the assessors. In 2284 paired judgements of 76 candidates, during 151 cases, there was 96% congruence across the pass/fail divide, 98% for major domains. The expert global opinion agreed with the grading system in 74/76 (97%) of cases. Gradings correlated weakly with self-reported caecal intubation rates and MCQ scores (r = 0.24 and 0.27, P < 0.01). Overall, 27/30 candidates felt the DOPS assessment was fair/very fair, while 27/32 felt the MCQ was fair/very fair. Of the assessors, 12/16 felt the DOPS was valid/very valid, while 17/17 felt the overall process was fair/very fair.
These data suggest that the DOPS assessment is reliable and valid. The MCQ test appears to be a poor discriminator, as there have been no failures. In addition, no data for evaluating the validity of the test for important outcomes such as polyp detection rate, miss rates, patient comfort and complications exist.
Significant questions remain as to what happens the candidate who fails the test and how this should impinge on their daily practice.
However, the accreditation process sets out clear guidelines to cope with this eventuality. In the first instance, if a candidate feels the process was flawed, they have a right to appeal, although candidates cannot dispute the judgement of the assessors or the Accreditation Panel. If the appeal against the process is successful, then the candidate would have to undergo a repeat assessment at a second centre (Figure 1).
If a candidate does not meet the criteria for accreditation at their first sitting, they are eligible for one more attempt within a 12 mo period with two different assessors. If they do not meet the criteria at the second attempt, then they cannot re-apply until 12 mo have passed from the date of their first assessment.
If there are serious concerns about the competency of an individual colonoscopist, procedures are in place which allow assessors and the Accreditation Panel to take appropriate action, including informing the medical director of the trust where the candidate is employed, if necessary.
One can infer from the guidelines that it is entirely conceivable for candidates to fail the accreditation process repeatedly and continue their routine NHS service colonoscopy practice outwith the NHS BCSP. However, if a failed candidate continued his/her NHS practice, but was unfortunate enough to encounter a complication, followed by a legal challenge, would his/her practice be defensible?
With the NHS BCSP accreditation process in place, there are currently 2 forms of colonoscopy accreditation for practicing endoscopists in England - the aforementioned BSCP accreditation and that of the standard JAG. Whilst these two forms of accreditation are similar, there are subtle differences between them, with the BCSP accreditation having slightly more stringent criteria (Table 1).
| Criteria | JAG accreditation | NHS BCSP accreditation |
| Lifetime number | > 200 | > 1000 |
| Lifetime perforations | < 0.5% | Not documented |
| Number last 12 mo | > 100 | > 150 |
| Sedation | ||
| Under 70 years | Midaz < 5 mg/Pethidine < 50 mg | Midaz < 5 mg/Pethidine < 50 mg |
| 70 years + | Midaz < 2.5 mg/Pethidine < 25 mg | Midaz < 2.5 mg/Pethidine < 25 mg |
| Caecal intubation | > 90% | > 90% |
| Polyp detection & Removal | > 10% | > 20% |
| Data certified | Endoscopic supervisor | Endoscopy sister & consultant colleague/clinical director/medical director |
The NHS BCSP criteria place greater emphasis on experience, with large volumes of endoscopy practice required, which precludes newly qualified trainees from becoming screening colonoscopists. Of the key quality criteria caecal intubation rate is the same (> 90%) in both processes whilst polyp detection and removal is higher in the NHS BCSP accreditation (> 20% vs > 10%). Despite the NHS BCSP accreditation process having been developed to minimise the risk of complications, the current eligibility criteria do not require candidates to include their lifetime perforation rate.
Unlike the NHS BCSP accreditation, there is no formal written assessment for JAG accreditation in colonoscopy. However, new Specialty Trainee registrars have to complete a knowledge- based written assessment [Specialty Certificate Examination (SCE)] in order to qualify for their Certificate of Completion of Training prior to obtaining a substantive post. Whilst this is not directed at colonoscopy per se we suspect that many of the topics covered in the 30 question MCQ for NHS BCSP will also be covered in the SCE. As outlined above, the evidence published has shown that the MCQ is a poor discriminator and no candidates have failed accreditation on this part.
The formative DOPS assessments in the two groups are virtually identical, the only difference being that NHS BCSP candidates must achieve < 4 grade 2 s across the minor domains (with JAG accreditation candidates being allowed < 6).
In England, the current accreditation will undoubtedly lead to a to a ‘two-tier’ colonoscopy service. Over time, 3 distinct groups of colonoscopists will be in practice - those who have JAG accreditation but who do not, because of need or eligibility, have NHS BCSP accreditation; those who have NHS BCSP accreditation; and lastly those who have attempted NHS BCSP accreditation and failed. Clearly the latter gives the greatest cause for concern, as some of these practitioners may have failed on grounds that would also have caused them to fail to achieve JAG accreditation. As discussed previously, missed cancers are sometimes unavoidable, but the question is whether it is morally, ethically or legally justifiable for individuals who have failed a formalised accreditation process, to continue to be permitted to practice on symptomatic patients?
Within the UK there is considerable disparity in accreditation practice for screening colonoscopists. England, Scotland, Northern Ireland and Wales all have different criteria for accreditation that vary in the methods of assessment, number of procedures carried out, and key quality criteria. In England the accreditation process is as previously described.
In Scotland the Bowel Cancer Screening Programme has not followed the route of accreditation of screening colonoscopists. There are however, some criteria set for screening colonoscopists: (1) There should be a caecal intubation rate of at least 90% (the endoscopist should be able to clearly demonstrate that they had reached the caecum, an indication that they were able to examine the entire colon); (2) A minimum of 150 colonoscopies a year should be being performed; and (3) A JAG- accredited colonoscopy course should have been attended.
In Wales the process is different again. They have developed a 3-phase assessment process that JAG has approved for operation.
Phase 1 - Pre-assessment: Attendance at a preparatory course covering therapeutic techniques, mock DOPS assessments, and review of performance data.
Phase 2 - Progress review visit: A member of the assessment faculty visits the potential screening colonoscopists in order to check their progress in achieving their training goals.
Phase 3 - Assessment visit: The formal summative assessment process involves at least two members drawn from the Welsh Assessment Team and in many cases an external assessor from England is also included. The assessors aim to perform all necessary assessments on the day of the assessment visit to the potential screener’s Trust. The candidates must complete an MCQ (JAG approved) as in the English programme.
Perhaps the Northern Ireland proposals (Northern Ireland Cancer Network Draft Document) most closely mirror English accreditation process. Figure 2 below summarizes the route by means of which potential screening colonoscopists must progress prior to approval in Northern Ireland.
In the USA, training in gastrointestinal endoscopy is outlined in the Gastroenterology Core Curriculum[14]. This curriculum suggests a minimum number of colonoscopy procedures of 140 including a minimum of 30 snare polypectomy and haemostasis before competency can be assessed. The curriculum also suggests objective performance criteria for the evaluation of colonoscopy, namely intubation of the splenic flexure, intubation of the terminal ileum (desirable skill) and retroflexion. Similar to that of the UK, the core curriculum in the US also has a formalised diagnostic colonoscopy procedural competency form. This competency form highlights the key quality criteria (caecal intubation, ileal intubation, retroflexion in the rectum and polypectomy ability), although, unlike JAG, it does not set minimum standards for competence.
Hospitals or institutions where endoscopy takes place grant accreditation in the US. The American Society of Gastrointestinal Endoscopy[15] has issued guidelines for this process. Determination of competence based on these guidelines is as follows: (1) The applicant should have completed a residency program that incorporates structured experience in gastrointestinal endoscopy. Competence should be documented by the instructor(s); (2) The applicant should be able to demonstrate proficiency in endoscopic procedure(s) and clinical judgement equivalent to that obtained in a residency program; and (3) The applicant’s endoscopic director should confirm in writing the training, experience (including the number of cases for each procedure for which privileges are requested) and actual observed level of competency. Such experience includes indications, complications and their management, and alternative approaches. The training director’s opinion and recommendation should be considered prima facie evidence for the trainee’s acceptance as an individual qualified in gastrointestinal endoscopy.
In Canada responsibility for accreditation for colonoscopy is the responsibility of the endoscopists local institution or facility. However, The Canadian Association of Gastroenterology has suggested specific recommendations as a guide for institutions[16]. These Canadian guidelines list key quality criteria similar to those issued by other national societies: (1) Technical competence can be assessed after 150 procedures, however, completion of a specified number of colonoscopies does not imply competence; (2) Competence should be based on the completion of > 100 unassisted procedures; (3) Caecal intubation should have taken place in at least 85%-90% of all cases and in > 95% of screening cases in healthy adults; (4) Mean completion time of approximately 30 min with an emphasis on methodical, careful withdrawal; (5) Withdrawal times generally in excess of 7 min; (6) Photodocumentation of caecal intubation is encouraged for quality assurance purposes; (7) When colonoscopy is performed for cancer screening, adenomata should be detected in > 25% of men and > 15% of women > 50 years of age; (8) As a threshold for competency, > 30 supervised unassisted snare polypectomies should have been completed; (9) Perforation rate of 0.2% for all patients and < 0.1% for patients undergoing screening; and (10) Post-polypectomy bleeding rate of < 1%.
As part of an Endoscopy Quality Initiative the Canadian Gastroenterology Association are currently implementing the UK Global Rating Scale in an effort to improve and maintain high standards of endoscopy service.
In Australia, training is assessed by the Conjoint Committee for the Recognition of Training in Gastrointestinal Endoscopy. This is a national body comprising representatives from the Gastroenterological Society of Australia, the Royal Australasian College of Physicians (RACP), and the Royal Australasian College of Surgeons (RACS).
Training is assessed by the Conjoint Committee, usually in the context of the Specialist Advanced Training Program of either the RACP or RACS. Full recognition is therefore dependent on appropriate training, experience and supervision pursuant to those Training Programs. Assessment for certification of training in colonoscopy primarily involves assessment of the caecal intubation rate in intact colons at the completion of training. The reference criteria are as follows[17]. Candidates must: (1) Perform a minimum of 100 unassisted, supervised, complete colonoscopies to the caecum, and preferably to the ileum in patients with intact colons (i.e. with no prior colonic resection); (2) Perform successful snare polypectomies on a minimum of 30 patients; and (3) Achieve at least a 90% caecal intubation rate by the completion of training.
We are unaware of further assessment or accreditation of colonoscopic performance in Australia at this time.
New Zealand has a similar accreditation process, although they allow a caecal intubation rate of only 85%[18]. At present neither Australia nor New Zealand have population screening programs in place for screening for colorectal cancer.
The authors are unaware of any formalised accreditation process similar to that in England that occurs under the auspices of JAG and NHS BCSP in other countries. Although the accreditation process in other countries does not incorporate a ‘driving test’ or DOPS, we are not aware of any evidence that the quality of colonoscopy performed in these countries is substandard.
In general, higher medical training in the UK has become more structured and formalised with the introduction of Modernising Medical Careers. Trainees now have to submit written evidence of competencies, including practical procedural competencies, using Directly Observed Procedural Skills (DOPS) forms. Many specialties (cardiology, respiratory, gastroenterology) have guidelines for the minimum number of executions of any one procedure before an individual trainee can undergo a competency assessment. Competency assessments are written and recorded in the trainee’s logbook and therefore represent a quality assurance initiative. Higher surgical training in the UK follows similar procedures. Trainee surgeons have to undergo workplace-based assessments and record details of all procedures carried out, including the degree of independence involved, complications, etc. This logbook, along with the workplace- based assessments, form the basis for the annual review of competence of each trainee, and assess the trainee’s suitability to progress to the next stage of, or complete, the training program.
In the UK the JAG offers the quality framework and standards by which endoscopic practice is measured. JAG offers accreditation in the following endoscopic procedures: (1) Diagnostic upper GI endoscopy; (2) Therapeutic upper GI endoscopy; (3) Flexible sigmoidoscopy; (4) Colonoscopy; and (5) Endoscopic retrograde pancreatography.
JAG suggests that practicing endoscopists might use the frameworks provided to demonstrate competence and for their own endoscopic professional development. This will not only benefit patients by improving practice, but will also protect endoscopists in the event that a known complication of endoscopy occurs.
Although there is no legal requirement to complete JAG accreditation in endoscopic procedures at present, this may change as Department of Health and GMC initiatives for revalidation and recertification begin. Recertification is a new idea that was set out in the Government’s 2007 White Paper - ‘Trust, Assurance and Safety - the Regulation of Health Professionals’[19]. The recertification component of this White Paper is aimed at doctors who are on the GMC’s specialist register or GP register. In future, these doctors will need to demonstrate, through recertification, that they continue to meet the particular standard(s) that apply to their specialty or area of practice. It is entirely conceivable that, for practicing gastrointestinal endoscopists, this could mean JAG accreditation/re-accreditation. We can only speculate as to what the future holds across other medical or surgical disciplines. For example, will competent, practicing surgeons have to have their surgical skills assessed by their peers as in the UK colonoscopy ‘driving test’ in order to be able to continue their practice?
The main alternative to a formalised external accreditation process would be for colonoscopists to submit an annual record of procedures carried out, with key quality measures (caecal intubation rate, ilieal intubation rate, withdrawal time, polyp detection and removal rates) and complication rates noted. These data could be scrutinised by a fellow gastroenterologist working in the same trust, co-signed and presented to a national body for accreditation. The addition of a ‘driving test’ may not add to the validity of this process.
Large randomised controlled trials have shown that screening for colorectal cancer using faecal occult blood testing and subsequent colonoscopy for those with positive tests reduces mortality[20-22]. Where the screening program differs from other screening programs in the UK (e.g. breast cancer, cervical cancer), is the invasive nature of the diagnostic test (colonoscopy), and the moderate risk of serious and potentially fatal complications in an asymptomatic population. For these reasons it seems right and proper that those carrying out screening colonoscopy are skilled practitioners with the highest completion rates and the lowest complication rates. How best to ensure this remains a contentious issue.
At present the UK appears to have one of the most rigorous accreditation processes, although even this still has some major flaws. The evidence published to date suggests that the written part of the accreditation is not a good discriminating test, and it needs to be improved or abandoned. The fact that candidates are able to pass their accreditation without having completed a colonoscopy or removed a polyp during the assessment is also not ideal, although how one would rectify this is not clear. One possibility would be to increase the number of procedures required for accreditation. This would provide the dual benefits of a longer observation time, and also increase the chance of the need for polypectomy. The drawbacks would be the time and expense of the longer assessment. Given that peer assessment is only required once, this may be an option worth pursuing.
The other major flaw in the process as it stands is the separation between JAG accreditation and NHS BCSP accreditation. It would seem more logical to have a single accreditation process through which all colonoscopists should pass. This would include senior registrars coming to the end of their training as well as existing consultants, who would benefit from the accreditation process both for the screening program and for the GMC recertification process when it comes online in the future. Marrying the two accreditation processes does not seem an unreasonable proposition, as the actual DOPS assessments are nearly identical. As outlined above, the main differences are in the entry criteria, specifically the lifetime number of procedures and the polyp detection/removal rate. We know that colonoscopic skills improve over time, but we also know that experience does not necessarily equate to expertise. Perhaps the total number of colonoscopies required to undergo either accreditation should be in the region of 400-500, in order to allow adequate exposure to the relevant procedures for developing the technical skills required, but also to ensure that newly appointed skilled colonoscopists would be in a position to undergo NHS BCSP accreditation if required. This would also help NHS trusts with future workforce planning issues. Polyp detection and removal rate is a more rigorous quality measure. Perhaps standard JAG accreditation should reflect this, and introduce the higher figure of 20% in order to match the BCSP accreditation.
In addition, there should be a more rigorous system in place for the individual who fails the assessment. An accreditation process needs to be rigorous - if no one fails the test, this would suggest that the test is too easy. Candidates who fail should have the opportunity to retake the test at the earliest possible opportunity, and there should be systems in place to allow rapid and high quality retraining if necessary.
Furthermore, the process itself should be continuously measured and evaluated in order to ensure it is equitable and fair. Such audit procedures will also help to identify any deficiencies in the training of gastroenterology registrars or even regional variations in practice.
In conclusion, the ‘driving test’ has become an accepted process for establishing that a trainee has achieved the required competency to practice independently as a colonoscopist. However, it is not clear if, for a previously trained colonoscopist, the ‘driving test’ in addition to performance data, is necessary for the selection of screening colonoscopists or as a tool for revalidation.
To go back to the analogy of a driving test, once a driver passes, does he/she need to be retested after many years of driving? Is there evidence that doing such a thing will reduce accidents? Or is the presence of an unblemished driving record proof enough that he/she is a safe driver?
Peer reviewers: Satoru Kakizaki, MD, PhD, Assistant Professor, Department of Medicine and Molecular Science, Gunma University, Graduate School of Medicine, 3-39-15 Showa-machi, Maebashi, Gunma 371-8511, Japan; Marmo Riccardo, Postgraduate Specialist, Head, Gastroenterology Unit, Azienda Sanitaria Locale Salerno, Presidio Ospedaliero Polla (SA), Via Luigi Curto Polla 84037, Italy
| 1. | Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297-2303. |
| 2. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. |
| 3. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. |
| 4. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. |
| 5. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. |
| 6. | Cotton PB, Durkalski VL, Pineau BC, Palesch YY, Mauldin PD, Hoffman B, Vining DJ, Small WC, Affronti J, Rex D. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713-1719. |
| 7. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. |
| 8. | Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277-283. |
| 9. | Accreditation of screening colonoscopists: National Health Service Bowel Cancer Screening Program Implementation Guide. 2009;8:3. |
| 10. | Steele RJ, McClements PL, Libby G, Black R, Morton C, Birrell J, Mowat NA, Wilson JA, Kenicer M, Carey FA. Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut. 2009;58:530-535. |
| 11. | Denis B, Ruetsch M, Strentz P, Vogel JY, Guth F, Boyaval JM, Pagnon X, Ebelin JF, Gendre I, Perrin P. Short term outcomes of the first round of a pilot colorectal cancer screening programme with guaiac based faecal occult blood test. Gut. 2007;56:1579-1584. |
| 12. | Barton R. Outcomes and perceptions of a high-stakes colonoscopy skills assessment: The Joint Advisory Group/Bowel Cancer Screening Programme Screening Colonoscopy Assessment. Gut. 2008;57 Suppl 1:A1-A172 (no.139). |
| 13. | Barton R. Validity and reliability of an accreditation assessment for colonoscopy. Gut. 2008;57 Suppl 1:A1-A172 (no.004). |
| 14. | The Gastroenterology Core Curriculum, Third Edition. Gastroenterology. 2007;132:2012-2018. |
| 15. | Wexner SD, Litwin D, Cohen J, Earle D, Ferzli G, Flaherty J, Graham S, Horgan S, Katz BL, Kavic M. Principles of privileging and credentialing for endoscopy and colonoscopy. Gastrointest Endosc. 2002;55:145-148. |
| 16. | Romagnuolo J, Enns R, Ponich T, Springer J, Armstrong D, Barkun AN. Canadian credentialing guidelines for colonoscopy. Can J Gastroenterol. 2008;22:17-22. |
| 17. | Conjoint Committee for the Recognition of Training in Gas¬trointestinal Endoscopy. Information for registrants. http://www.conjoint.org.au. |
| 18. | New Zealand Society of Gastroenterology - Endoscopy Training. http://www.nzsg.org.nz/training/endoscopy. |
| 19. | Trust, Assurance and Safety: The Regulation of Health Professionals in the 21st Century. London: Department of Health. 2007;. |
| 20. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. |