BODY
Pancreatic pseudocysts, abscesses, and walled-off pancreatic necrosis are types of pancreatic fluid collections that arise as a consequence of pancreatic injury. The basis of the pancreatic injury is disruption of the pancreatic duct or side branches that result in the formation of a collection of fluid with or without solid debris[3]. Pain, early satiety, biliary obstruction, and infection are all indications for drainage. Percutaneous-radiologic drainage, surgical drainage, and endoscopic drainage are the three accepted approaches to the drainage of pancreatic pseudocysts. The therapeutic approach to pancreatic pseudocysts has evolved over the past thirty years. What once was treated with a surgical or percutaneous approach is now being managed via endoscopy.
Radiologic guidance allows placement of a drainage pigtail catheter into the pancreatic pseudocyst allowing subsequent drainage. The catheter is connected to an external collection system and fluid is collected over several weeks. In order to monitor resolution of the pseudocyst, contrast is injected periodically into the cyst cavity and repeat imaging is performed. The percutaneous drainage approach is useful in high risk patients who would not tolerate the endoscopic or surgical approach because of confounding co-morbidities. However this technique makes the patient more prone to infection, produces significant patient discomfort, and might require multiple catheter exchanges because of catheter clogging. In those patients who have failed the percutaneous approach, a surgical option would be appropriate. In this setting, a fistula is surgically created between the pseudocyst and the stomach or the small bowel, allowing for complete drainage. Many studies have been performed that have shown a significantly higher mortality rate associated with surgical therapy compared with other approaches[4].
In the past thirty years, the endoscopic approach to pancreatic fluid collections has evolved greatly. The armamentarium of the endoscopist has allowed potentially successful drainage of pseudocysts and even walled-off pancreatic necrosis. The basis of the pancreatic injury is disruption of the pancreatic duct or side branches that result in the formation of a collection of fluid with or without solid debris. One of the first endosopic approaches to pancreatic pseudocysts was reported by Rogers where a woman with a history of alcohol abuse and pseudocysts due to recurrent pancreatitis presented with epigastric abdominal pain. An upper gastrointestinal series revealed a 10 cm pressure defect in the posterior aspect of the stomach which was confirmed endoscopically. An aspirating device constructed from the shaft of a 21 gauge needle and Teflon tubing was advanced through the biopsy channel and used to aspirate fluid from the aforementioned cyst. It is likely that this pseudocyst had communication with the pancreatic duct as repeat imaging three days later demonstrated that the cyst had refilled[5].
Kozarek et al in 1985 reported the first series of endoscopic drainage of pancreatic pseudocysts. They described endoscopic cystostomy in four high risk patients in whom surgery had been either unsuccessful or was felt to be contraindicated. An endoscopically visible bulge was identified in each case and the cystotomy was completed with a modified straight wire sphincterotome that was inserted through the stomach or the duodenum and into the pseudocyst[6]. Over the past twenty years the endoscopic approach to pseudocysts has evolved to include; transpapillary drainage, transmural drainage with or without endosonograpic guidance, or combined transmural and transpapillary drainage. Endoscopic pseudocyst drainage is safe when there are no associated pseudoaneurysms, gastric or duodenal varices demonstrated on noninvasive imaging modalities and the intended site of cyst wall puncture is within one cm of the bowel lumen. The approach that the endoscopist will employ for endoscopic drainage is based on the anatomical relationship of the collection to the stomach or duodenum, the presence of ductal communication, and the size of the collection[7].
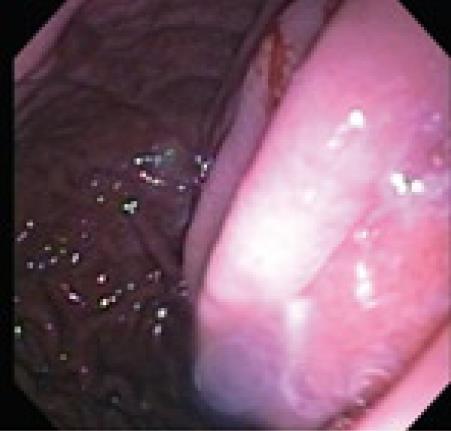
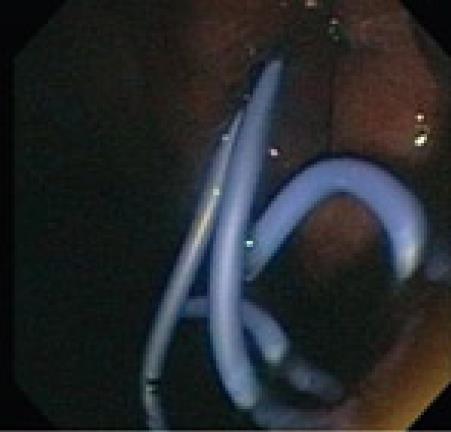
Since Kozarek demonstrated pseudocyst drainage endoscopically more than twenty years ago, the conventional endoscopic approach has been customized. Transluminal drainage may be accomplished via a transgastric, transduodenal, or even transesophageal route[8]. Studies performed to determine whether the transduodenal or transgastric approach had better long-term results, have not shown any superiority for either approach[9,10]. Conventional endoscopic drainage is feasible when a visible bulge is seen in the stomach or the duodenal wall (Figure 1). With a Seldinger technique, an aspirating needle is passed transluminally into the pseudocyst collection, and fluid is aspirated to confirm entrance into the pseudocyst. Once confirmation has been made that the collection is a pseudocyst, contrast medium is injected to confirm needle localization within the pseudocyst cavity. A 0.035 guide wire with a hydrophilic tip is inserted and coiled into the collection as the needle is removed. A cannula is now passed over the wire to perform an initial dilatation. If there is resistance due to a thick wall then needle-knife electrocautery can be performed in a forward pressure maneuver over the wire[11]. Once a tract has been made a biliary dilating balloon catheter can be used to further dilate the tract. Simultaneous endoscopic and fluoroscopic imaging confirms the position of the balloon and waist obliteration during balloon inflation. The balloon is removed and two double pig-tail stents are typically placed over the guide wire (Figure 2). Most authorities feel that the use of double pig tail stents will reduce stent migration and allow for expeditious drainage[12].
Figure 1 Visible bulge in a patient with a pancreatic pseudocyst.
Figure 2 Placement of two 7FR pigtail stents.
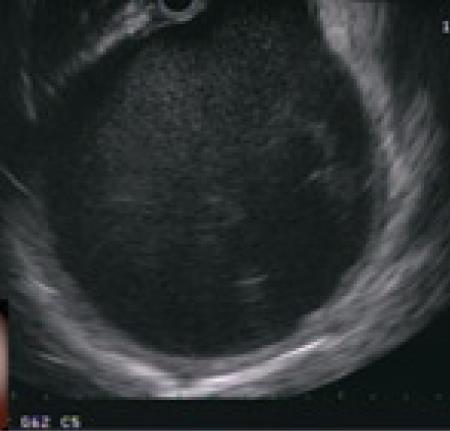
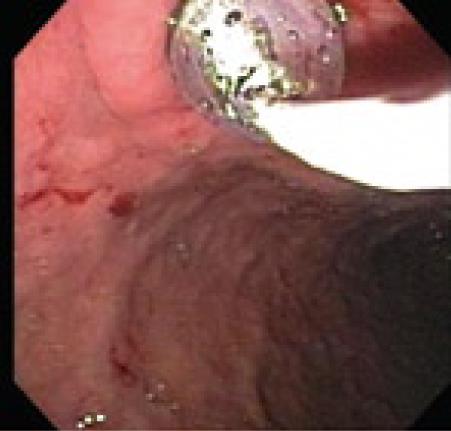
Endoscopic drainage performed without a visible endoluminal bulge carries significant risk of bleeding and perforation. In a series reported by Sahel et al[13] and Cremer et al[10] perforation occurred in 2 of 20 patients without visible endoluminal bulge and bleeding occurred in 2 of 33 patients who had no endoluminal bulge. Grimm et al[14] reported a case of pseudocyst puncture under direct endosonographic guidance using an electronic oblique scanning echoendoscope in a patient without endoscopic evidence of extramural pseudocyst bulging. Endoscopicultrasound (EUS) can be used to confirm that the pseudocyst is adjacent to the stomach or duodenum with the distance measuring less than one cm and to exclude associated pseudoaneurysms (Figure 3). Until recently the echoendoscope was replaced, after the enterostomy site was marked, with a duodenoscope and the enterostomy was performed. Transmural drainage of pancreatic fluid collections performed entirely under EUS guidance using Doppler-equipped therapeutic channel echoendoscopes was first described by Giovannini et al[15]. Recently a “one-step” technique for drainage of pancreatic fluid collections using the Needle-Wire Oasis System has been described. The first step of this procedure is to puncture the pseudocyst using the needle-wire by applying electrocautery. When the needle wire is inside the pseudocyst, the internal rigid part of the needle-wire is removed and the guide wire is coiled into the pseudocyst. The second step is to dilate the tract using a dilator catheter (Figure 4) and finally deliver the stent using a pusher, similar to the technique adopted for biliary stenting[16].
Figure 3 Endosonographic view of pancreatic pseudocyst.
Figure 4 Balloon dilatation after cautery.
Studies have been performed to evaluate which is the best modality to drain pancreatic pseudocysts; the conventional endoscopic approach or endoscopic ultrasound guided. Kaheleh et al prospectively compared their experience in EUS-guided cystenterostomy with that in contemporaneous group of patients with pseudocysts drained using conventional transmural drainage. A total of 99 patients underwent endoscopic management of pancreatic pseudocysts. Patients with bulging lesions without obvious portal hypertension underwent conventional endoscopic drainage; all others underwent endoscopic ultrasound drainage. Patients were followed with cross sectional imaging during clinical visits and results were compared at 1 and 6 mo post procedure. Forty-six underwent endoscopic ultrasound drainage and the remaining 53 had the conventional drainage performed. There were no significant differences between the two groups regarding either short-term or long-term success. Complications occurred in 19% of the endoscopic ultrasound group vs 18% of the conventional drainage group, and consisted of bleeding in three cases, infected collection in eight, stent migration in three, and pneumoperitoneum in five[17]. Varadarajulu et al performed a randomized study and compared the rate of technical success between EUS and Esophagogastroduodenoscopy (EGD) for transmural drainage of pancreatic pseudocysts. Those included in the study were patients with a history of pancreatitis and symptomatic pancreatic pseudocysts that were greater than 4 cm in size. Technical success was defined as the ability to access and drain a pseudocyst by placement of a transmural stent. Complications were assessed at 24 h and at day 30. Treatment success was defined as the complete resolution or decrease in size of the pseudocysts to less than 2 cm on CT, in association with clinical resolution of symptoms at 6 wk follow-up. Thirty patients were randomized to undergo pseudocyst drainage; fifteen were randomized to EUS and the remaining fifteen to the EGD approach. Of the fifteen under the EUS approach, 14 underwent successful drainage while the procedure was technically successful in only five of fifteen randomized to EGD. Reasons for technical failure in these ten patients were: the absence of luminal compression in nine and active bleeding after attempted puncture of the pseudocyst in one patient. All ten patients who failed drainage by EGD underwent successful drainage of pseudocyst on crossover to EUS[18]. More studies are needed in this comparison. However, it seems that EUS, given its excellent safety profile, should be considered first-line treatment modality for endoscopic drainage of pancreatic pseudocysts.
The transpapillary approach is preferable when communication is demonstrated between the pancreatic ductal system and the pseudocyst. A pancreatogram is obtained during ERCP and if the pseudocyst fills with contrast medium, then communication with the main pancreatic ductal system is confirmed. Visualization of the main pancreatic duct beyond the site of communication between the duct and the pseudocyst is not always seen, either because of duct disconnection or the preferential flow of contrast medium into the pseudocyst cavity. Pancreatic sphincterotomy is performed as it will facilitate the introduction of stents and/or dilating devices and may promote transpapillary flow around the stent. Using a hydrophilic guidewire, the leak should be traversed so that patency of the main pancreatic duct is achieved. Stent sizes are dependent on the pancreatic duct diameter but are usually 7Fr, and continuity of the main pancreatic duct should be accomplished when placing the stent across the ductal leak[19,20]. The use of both transpapillary and transmural drainage should be considered in very large pseudocysts (> 6 cm) or cases in which there is a pancreatic ductal abnormality.