Published online Oct 16, 2010. doi: 10.4253/wjge.v2.i10.349
Revised: July 13, 2010
Accepted: July 20, 2010
Published online: October 16, 2010
We report on a case of a 74 year old man who was diagnosed with a recurrence of non-invasive carcinoma of intraductal papillary mucinous neoplasm (non-invasive IPMN) by postoperative gastroscopy (GS). A pylorus preserving pancreatico duodenectomy for IPMN in the pancreatic head was performed. A histopathological study revealed non-invasive adenocarcinoma. At first, the local recurrence of the tumor around the superior mesenteric artery circumference was diagnosed and disappeared with gemcitabine. Later, the GS showed the elevated lesion with mucin hypersecretion in the remnant stomach. The lesion had a central dip and a fistula common to the pancreas was confirmed on fisterography. We diagnosed a recurrence of IPMN and administered chemotherapy again. However, he died of his original illness. There are no reports of postoperative recurrence of IPMN checked by GS. It should be remembered that the elevated lesion of the remnant stomach is considered as one of the recurrent patterns of IPMN.
- Citation: Uesato M, Nabeya Y, Miyazaki S, Aoki T, Akai T, Shuto K, Tanizawa T, Miyazaki M, Matsubara H. Postoperative recurrence of an IPMN of the pancreas with a fistula to the stomach. World J Gastrointest Endosc 2010; 2(10): 349-351
- URL: https://www.wjgnet.com/1948-5190/full/v2/i10/349.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i10.349
The prognosis of “non-invasive” intraductal papillary mucinous neoplasm (IPMN) is generally favorable[1-4] and postoperative recurrence is rare after a curative resection[1,2]. However, several reports have described peritoneal recurrence with frequencies ranging from 6% to 17% in patients with non-invasive IPMN[5].
This article reports on a rare case of postoperative recurrence from a non-invasive IPMN of the pancreas with a fistula to the remnant stomach. The potential significance of endoscopic surveillance for patients who undergo surgery for IPMN is also discussed.
A 74 year old Japanese man with epigastralgia was admitted to our hospital for treatment of a pancreas head tumor found at a clinic. By several imaging studies such as computed tomography (CT), ultrasonography and X-ray, the tumor was diagnosed as IPMN arising in the pancreas head with the dilated main pancreatic duct of the distal pancreas. In September 2001, the patient underwent a pylorus preserving pancreaticoduodenectomy for IPMN. The histopathological examination revealed that the tumor was a non-invasive type intraductal papillary adenocarcinoma with mucin hypersecretion (pT2, pN0, pM0, Stage IB according to TNM classificationIV) and a negative margin with no lymphovascular invasion. Pancreatoscopy showed no tumor in the remnant pancreas during the operation. He was carefully followed up without chemotherapy.
The patient developed a tumor around the superior mesenteric artery circumference 2 years and 7 mo after the operation. We diagnosed it as a local recurrence by the synchronous eruption of the tumor on CT and the tumor marker CEA level without a biopsy from the tumor.
He received chemotherapy with gemcitabine at a weekly dose of 1 000 mg/m2 three times every 4 wk as one cycle and continued on this regimen for sixteen cycles without adverse effects. This chemotherapy resulted in complete remission and the recurrence tumor was not detected.
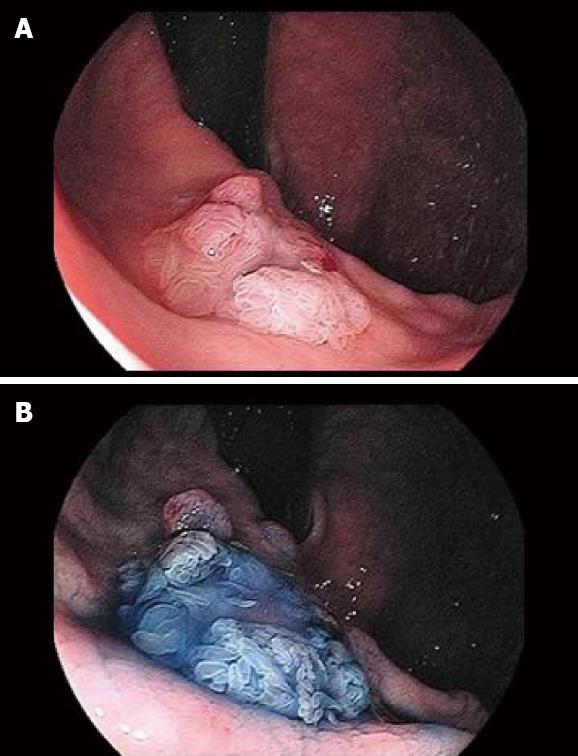
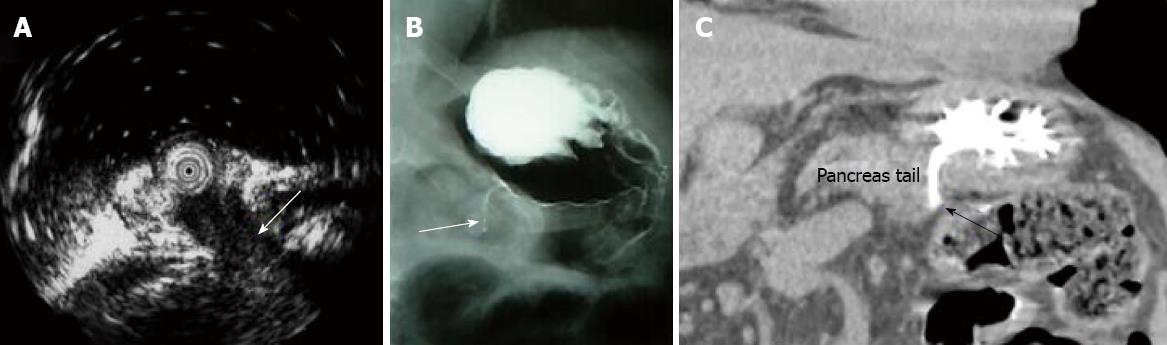
Surveillance gastroscopy (GS) revealed a 15 mm-sized villous elevated lesion with mucin hypersecretion in the remnant stomach 2 years and 3 mo after the first recurrence (Figure 1). The lesion had a central dip and was diagnosed as a fistula from the pancreas by the endoscopic ultrasonography (EUS; Figure 2A), fisterography (Figure 2B) and CT (Figure 2C). Immunohistochemical studies showed that the biopsy specimen from the elevated lesion was identical to the IPMN that had been removed earlier. Therefore, the lesion was diagnosed to be a recurrence of IPMN. However, he rejected the surgical treatment we offered because of his old age and thereafter received chemotherapy again. However, he died one year after the second recurrence.
This report presents a rare case of a postoperative recurrence from non-invasive IPMN with a fistula from the remnant pancreas through the gastric wall to the gastric mucosa. The lesion was initially found by surveillance GS which also suggested the diagnosis. In addition, we concluded that the gastric lesion was a recurrence of IPMN by the findings explained as follows: (1) GS in the remnant stomach showed the villous elevated lesion with mucin hypersecretion which was not like the configuration of conventional gastric cancer; (2) A fistula from the remnant pancreas through the gastric wall to the gastric mucosa was confirmed; and (3) The pathological findings were identical between the biopsy specimen of a new gastric lesion and the previously resected specimen of IPMN.
The prognosis of non-invasive IPMN is favorable with a five-year survival rate of 98.4%-100%[1,2,6]. However, cases of recurrent non-invasive IPMN are sometimes encountered in peritoneal recurrence[5]. In our case, the first recurrence pattern after a curative resection was similar. Nevertheless, no previous reports have described the recurrence pattern observed in the second recurrence featuring a fistula from the remnant pancreas to the stomach. The endoscopic images of the elevated tumor in the stomach initially appeared to be identical to the images of IPMN in the pancreatic duct[7]. Therefore, the definite diagnosis of recurrence could be attributed to the speedy and successful diagnostic procedures including EUS, fisterography and CT. Magnetic resonance cholangio pancreatography (MRCP) and CT are generally used in surveillance after the operation of IPMN. Nevertheless, if only MRCP and CT had been done, the recurrence of IPMN such as in our case may have been overlooked. The reasons being that the fistula was short and thin and the duct dilating lesion within the remnant pancreas was not revealed.
Penetration to another organ may be a characteristic of a primary IPMN but it may also be caused by either mucus pressure or carcinomatous invasion[8]. The carcinomatous invasion of IPMN may have led to penetration to the stomach in the current case because the CT showed that the recurrence in this case lacked a large mucus pool.
Nineteen percent of patients with IPMN demonstrate either a synchronous or metachronous malignant tumor in several organs[9]. In particular, gastric cancer is most frequent at 32.8% and the frequency of colorectal cancer is 21.8%[9,10]. Therefore, it is important to monitor the stomach preoperatively by a screening GS in patients who undergo surgery for IPMN in order to detect concomitant gastric cancer.
In conclusion, postoperative surveillance GS of the stomach in patients who undergo a resection of IPMN helps to find a metachronous gastric cancer at an early stage. On the other hand, if a gastric tumor featuring a fistula such as in our case was found in this group of patients, the lesion may be a recurrence of IPMN.
Peer reviewer: Mohammad Al-Haddad, MD, Indiana University School of Medicine, 550 N University Blvd, Indianapolis, IN 46202, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
| 1. | Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632-636; discussion 637. |
| 2. | Raut CP, Cleary KR, Staerkel GA, Abbruzzese JL, Wolff RA, Lee JH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582-594. |
| 3. | Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788-797; discussion 797-799. |
| 4. | Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, Barthet M, O’Toole D, Rufat P, Partensky C. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717-722. |
| 5. | Takahashi H, Nakamori S, Nakahira S, Tsujie M, Takahshi Y, Marubashi S, Miyamoto A, Takeda Y, Nagano H, Dono K. Surgical outcomes of noninvasive and minimally invasive intraductal papillary-mucinous neoplasms of the pancreas. Ann Surg Oncol. 2006;13:955-960. |
| 6. | Hatori T, Fukuda A, Onizawa S, Imaizumi T, Takasaki K. Follow-up strategy after surgical resection of intraductal papillary mucinous neoplasm of the pancreas (in Japanese with English abstract). Suizou. 2005;20:538-545. |
| 7. | Hara T, Yamaguchi T, Ishihara T, Tsuyuguchi T, Kondo F, Kato K, Asano T, Saisho H. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34-43. |
| 8. | Okada K, Furuuchi T, Tamada T, Sasaki T, Suwa T, Shatari T, Takenaka Y, Hori M, Sakuma M. Pancreatobiliary fistula associated with an intraductal papillary-mucinous pancreatic neoplasm manifesting as obstructive jaundice: report of a case. Surg Today. 2008;38:371-376. |
| 9. | Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, Sunamura M, Furukawa T, Yanagisawa A, Ariyama J. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241-246. |
| 10. | Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol. 2005;11:5688-5690. |