Published online Mar 16, 2025. doi: 10.4253/wjge.v17.i3.104966
Revised: February 6, 2025
Accepted: February 25, 2025
Published online: March 16, 2025
Processing time: 63 Days and 5.3 Hours
Inguinal hernias are common after surgery. Tension-free repair is widely accepted as the main method for managing inguinal hernias. Adequate exposure, coverage, and repair of the myopectineal orifice (MPO) are necessary. However, due to differences in race and sex, people’s body shapes vary. According to European guidelines, the patch should measure 10 cm × 15 cm. If any part of the MPO is dissected, injury to the nerves, vascular network, or organs may occur during surgery, thereby leading to inguinal discomfort, pain, and seroma formation after surgery. Therefore, accurate localization and measurement of the boundary of the MPO are crucial for selecting the optimal patch for inguinal hernia repair.
To compare the size of the MPO measured on three-dimensional multislice spiral computed tomography (CT) with that measured via laparoscopy and explore the relevant factors influencing the size of the MPO.
Clinical data from 74 patients who underwent laparoscopic tension-free inguinal hernia repair at the General Surgery Department of the First Affiliated Hospital of Anhui University of Science and Technology between September 2022 and July 2024 were collected and analyzed retrospectively. Transabdominal preperitoneal was performed. Sixty-four males and 10 females, with an average age of 58.30 ± 12.32 years, were included. The clinical data of the patients were collected. The boundary of the MPO was measured on three-dimensional CT images before surgery and then again during transabdominal preperitoneal. All the preoperative and intraoperative data were analyzed via paired t-tests. A t-test was used for comparisons of age, body mass index, and sex between the groups. In the comparative analysis, a P value less than 0.05 indicated a significant difference.
The boundaries of the MPO on 3-dimensional CT images measured 7.05 ± 0.47 cm and 6.27 ± 0.61 cm, and the area of the MPO was 19.54 ± 3.33 cm2. The boundaries of the MPO during surgery were 7.18 ± 0.51 cm and 6.17 ± 0.40 cm. The errors were not statistically significant. However, the intraoperative BD (the width of the MPO, P = 0.024, P < 0.05) and preoperative AC (the length of the MPO, P = 0.045, P < 0.05) significantly differed according to sex. The AC and BD measurements before and during surgery were not significantly different according to age, body mass index, hernia side or hernia type (P > 0.05).
The application of this technology can aid in determining the most appropriate dissection range and patch size.
Core Tip: Computed tomography 3-dimensional (CT 3D) reconstruction techniques are combined with laparoscopic measurements to obtain myospubic foramen (MPO) size data. To study the effects of different gender, age, body mass index and other factors on the size of the musopubic foramen. Through the analysis of CT 3D imaging and laparoscopic measurement results, the value of CT 3D reconstruction technology in guiding surgery was clarified. Through a retrospective analysis of 74 patients undergoing laparoscopic tension-free inguinal hernia repair, it was found that the combination of CT 3D reconstruction technology and laparoscopic measurement could effectively obtain relevant data of myospubic foramen to reduce postoperative recurrence and adverse reactions.
- Citation: Zhang L, Chen J, Zhang YY, Liu L, Wang HD, Zhang YF, Sheng J, Hu QS, Liu ML, Yuan YL. Three-dimensional reconstruction under computed tomography and myopectineal orifice measurement under laparoscopy for quality control of inguinal hernia treatment. World J Gastrointest Endosc 2025; 17(3): 104966
- URL: https://www.wjgnet.com/1948-5190/full/v17/i3/104966.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i3.104966
Inguinal hernias are common and typically treated surgically. Researchers have reported that the annual incidence rate is 100-300 people per 100000 people[1]. Researchers have also estimated that more than 20 million hernia repair surgeries are performed worldwide every year[2,3]. Tension-free repair surgery is widely accepted as the standard repair method; however, the recurrence rate is approximately 4%, and the incidence of postoperative chronic pain is 12%. Insufficient dissection of the myopectineal orifice (MPO) and folding and dislocation of the mesh due to hematoma formation are also considered risk factors for recurrence[4]. Postoperative chronic pain may be related to an overly extensive surgical range.
The Rives-Stoppa technique involves placement of the mesh in the preperitoneal space so that it covers the MPO while also separating it from the nerve plane. As there are possibly three hernia openings, this placement technique is ideal for preventing hernia recurrence and chronic pain, particularly from hydrostatic and anatomical perspectives[5]. Some studies revealed that a large mesh covering the iliac vessels and the bladder may lead to urogenital or vascular diseases in the future. However, only covering the MPO (such as in the Rives operation) can effectively repair the defect and avoid these drawbacks[6,7]. According to Fruchaud’s concept, the MPO is composed of the rectus abdominis as the medial pillar, the iliopsoas as the lateral pillar, the penile roof representing the lower edge, and the upper edge being the arcuate margin formed by the internal oblique muscle and the transverse oblique muscle. The MPO is a weak area in the inguinal wall through which all inguinal and pubic hernias protrude. Among them, the transversalis fascia is the only (low) plane resistant to intra-abdominal pressure[8]. Therefore, the mesh used for laparoscopic inguinal hernia repair should completely cover the MPO and overlap the adjacent muscle and bone by 2-3 cm. Thus, surgeons must have a comprehensive understanding of the anatomical structure of the MPO so that they can appropriately limit the dissection range and ensure that the patch fully covers the MPO, as doing so will reduce the risks of recurrence and postoperative adverse reactions.
Since Bassini performed the first hernia repair surgery more than 100 years ago, modern meshes have been increasingly used to repair all types of hernias as they can be applied with minimal tension and do not distort the normal anatomical structure. This technique is simple, fast, relatively painless and effective, thereby enabling a quick return to unrestricted physical activities[9]. However, a wide variety of meshes are used in clinical practice, and their application is associated with a series of complications, such as recurrence, inguinal discomfort, pain, and seroma formation. Research revealed that the recurrence rate after hernia repair is 10%-24%[10], and the postoperative mesh infection rate is 0.5%-9.0%[11]. Therefore, the patient’s postoperative condition depends on the size and shape of the mesh. Therefore, the method that provides the most accurate measurement of the MPO must be identified because the size and shape of the patch are selected on the basis of the size of the MPO. Therefore, in this study, the size of the MPO measured via 3-dimensional (3D) multislice spiral computed tomography (CT) before hernia repair was compared with that measured during surgery to verify the measurement accuracy of 3D multislice spiral CT. In the future, the size of the patch could possibly be predicted on the basis of preoperative 3D CT measurements, thereby reducing the dissection range, recurrence rate after hernia repair and risk of adverse reactions.
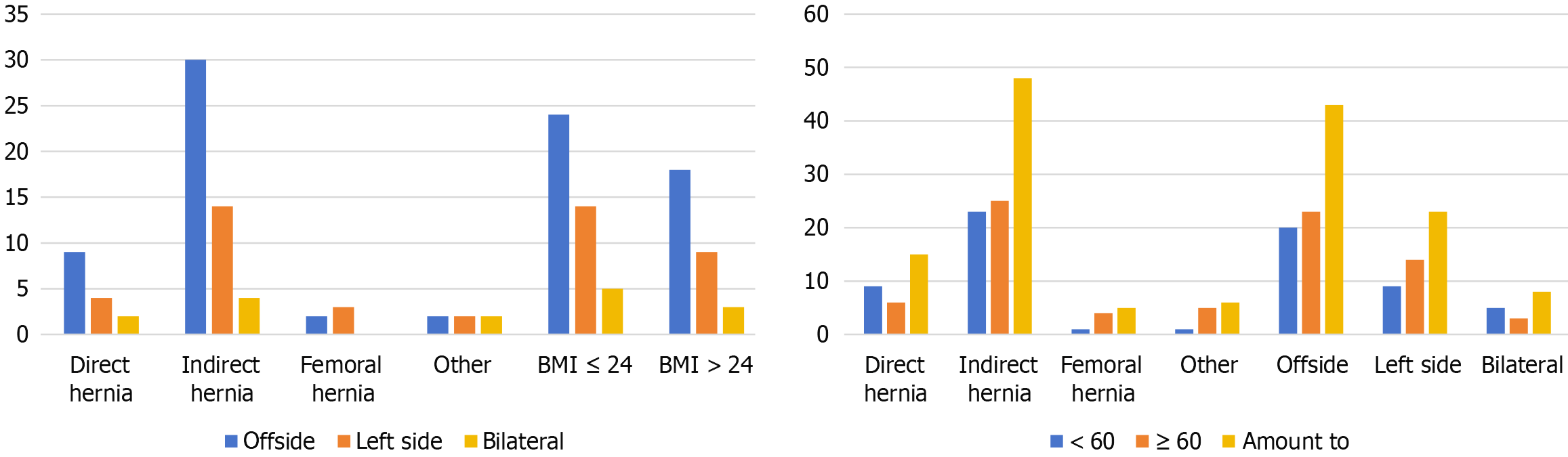
The study was approved by the ethics committee of our hospital, and then the clinical data of 74 hernia patients who underwent transabdominal preperitoneal (TAPP) at the First Affiliated Hospital of Anhui University of Science and Technology between September 2022 and July 2024 were selected. Among the patients, 64 were male and 10 were female, with an average age of 58.30 ± 12.32 years. Eight patients had bilateral hernias, 43 had right-side hernias, and 23 had left-side hernias. Fifteen were direct hernias, 48 were indirect hernias, 5 were femoral hernias, and 6 were other types (different types of bilateral hernias and other types of hernias) (Table 1). All patients underwent thin-slice CT examinations of the lower abdominal wall or pelvis before the operation. The MPO was measured preoperatively via three-dimensional CT and then intraoperatively in all patients. Patients with missing information were excluded. There were 50 patients with preoperative and intraoperative measurements and 74 patients with only preoperative measurements.
| Grouping | Quantity | Percentage | Cumulative percentage |
| Hernia side | |||
| Bilateral | 8 | 10.81 | 10.81 |
| Right | 43 | 58.11 | 68.92 |
| Left | 23 | 31.08 | 100.00 |
| Hernia type | |||
| Oblique hernia | 48 | 64.86 | 72.97 |
| Direct hernia | 15 | 20.27 | 93.24 |
| Femoral hernia | 5 | 6.76 | 100.00 |
| Other | 6 | 8.11 | 8.11 |
| Age classification | |||
| < 60 years | 34 | 45.95 | 45.95 |
| ≥ 60 years | 40 | 54.05 | 100.00 |
| BMI classification (n = 73) | |||
| ≤ 24 | 43 | 58.90 | 58.90 |
| > 24 | 30 | 41.10 | 100.00 |
| Sex | |||
| Male | 64 | 86.49 | 86.49 |
| Female | 10 | 13.51 | 100.00 |
| Occupational classification (n = 72) | 74 | 100.0 | 100.0 |
The inclusion criteria were as follows: (1) Male and female patients aged ≥ 18 years; (2) Who were clinically diagnosed with an inguinal hernia and scheduled to undergo TAPP; and (3) Who underwent thin-slice CT examinations of the lower abdominal wall or pelvis before the operation and agreed to voluntarily participate in the study. The exclusion criteria were as follows: (1) Patients who had poor quality 3D CT images; (2) A history of lower abdominal surgeries that affect the anatomical structure of the MPO; and (3) Were considered unsuitable for inclusion in the study after evaluation by the researchers. The research protocol was approved by the hospital’s ethics committee (approval number: 2022-KY-416-001). The patients’ sex, age, weight (body mass index, BMI), and type of inguinal hernia were recorded. A 3D model was created using CT measurements of the MPO. Moreover, CT and laparoscopic measurements were used together to determine the most appropriately sized patch to avoid excessive dissection and secondary injuries.
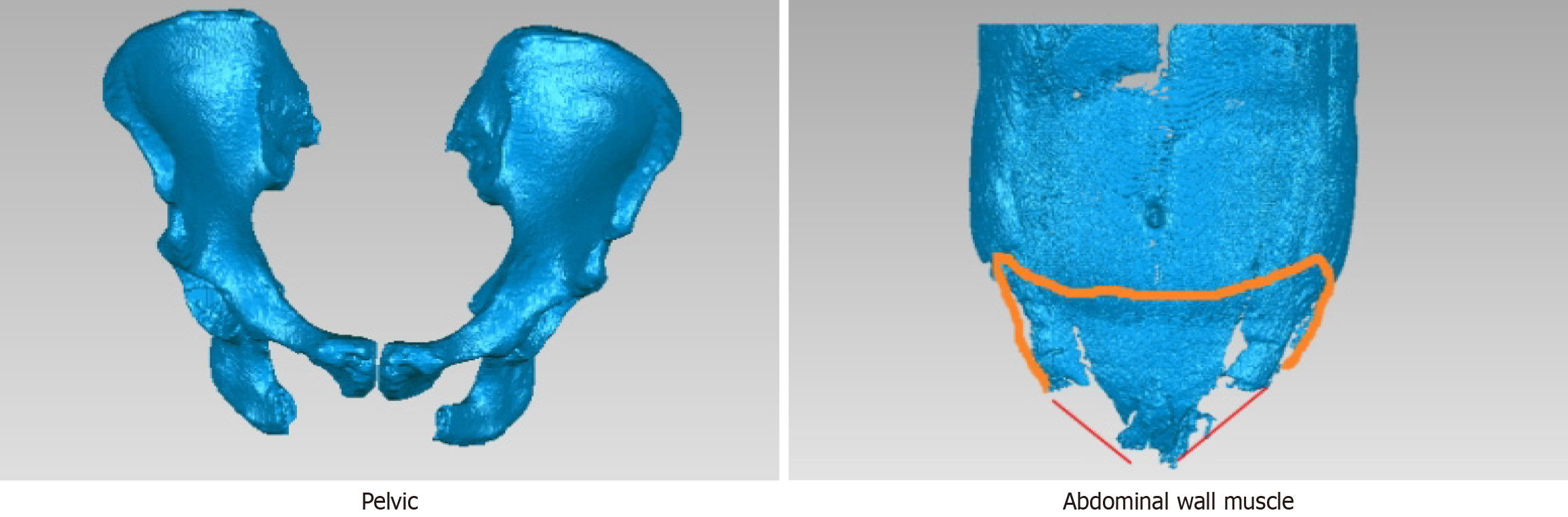
The GE-Revolution™ CT scanner was used to scan the lower abdomen. For the plain scan, the scanning range was from below the umbilical level to the pelvic floor (including the entire hernia sac). The images were segmented, and then a 3D model of the MPO and surrounding tissues was created (Figure 1). The procedure for constructing the 3D model was as follows: First, the CT images that were saved in DICOM format were imported into Mimics 21.0 software and segmented into three views. The positions and demarcation points of the abdominal wall muscles were calibrated in the cross-sectional view, and the boundaries of the muscle tissues were outlined. The position of the ilium was calibrated in the coronal view, and the boundary was outlined to determine the modeling area. Then, the adaptive threshold algorithm and the manual adjustment method were adopted. The threshold range for the ilium was set to 50-150 HU, and the threshold range for the abdominal wall muscles was set to -50-50 HU. The rectus abdominis, latissimus dorsi, iliopsoas, vertebrae, ilium, and pubis were segmented according to different thresholds. The Gaussian filtering algorithm was used to denoise the segmented images. Then, on the basis of the voxel reconstruction algorithm, a three-dimensional grid was created, the pixel values of the denoised images were mapped, and the linear interpolation method was used to estimate the pixel values of the unfilled voxels to fill the three-dimensional grid and generate a model. Finally, based on the finite element analysis used to optimize the parameters, the three-dimensional model of the MPO was saved in STL format and then printed. This method improves post-processing for radiologists and assists clinicians in diagnosis and precise surgical planning.
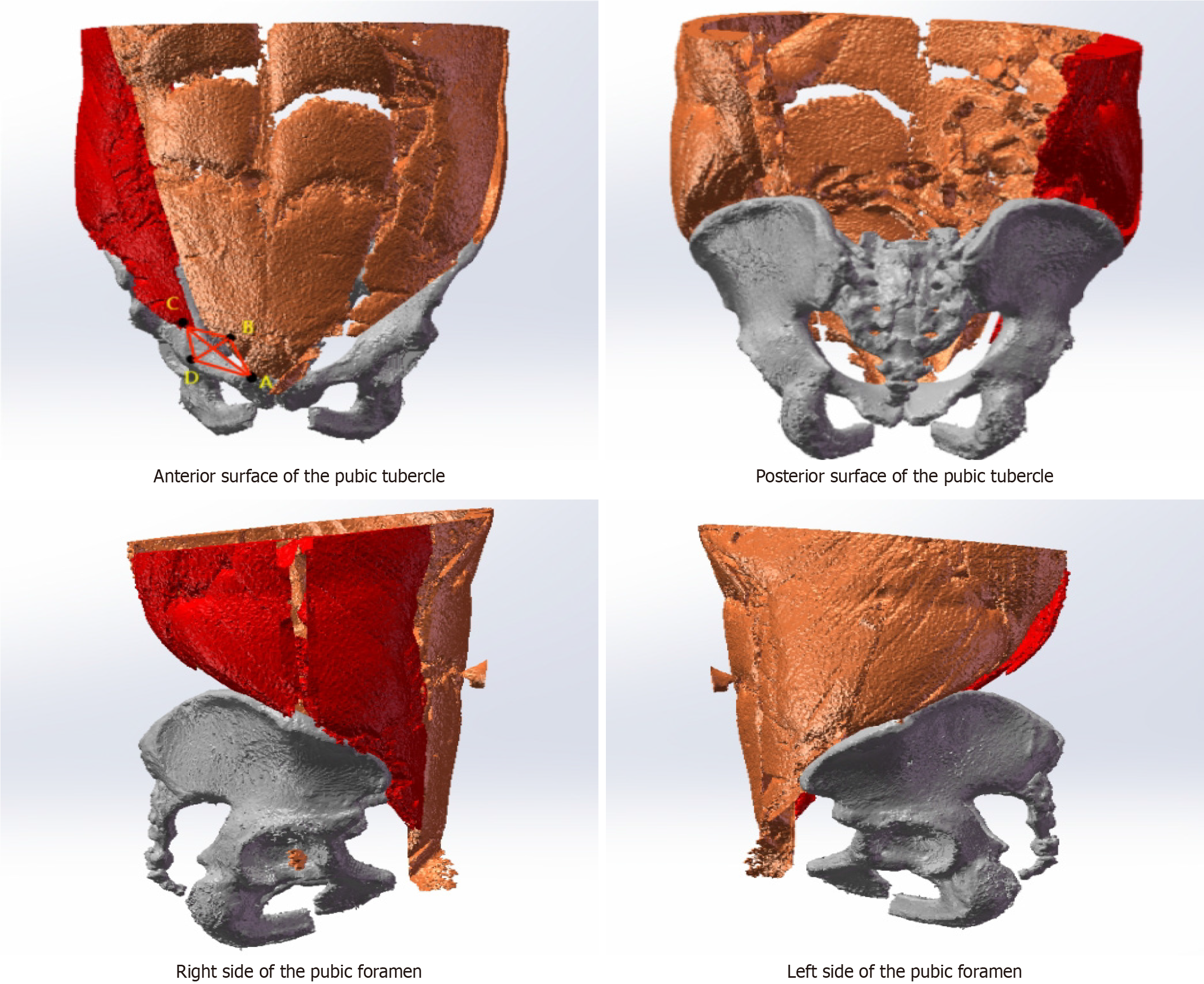
By comparing the key views and imaging features of the MPO, we identified four key landmarks to determine the position of the MPO: Point A, the innermost edge of the iliopsoas muscle; point B, the lowest point of the transverse abdominis muscle; point C, the intersection point of the horizontal line passing through Point A and the iliopsoas muscle; and point D, the intersection point of the vertical line passing through point B and the pubis (Figure 2). Along the edges of the rectus abdominis muscle, conjoint tendon, iliopsoas muscle and pubis, these four points are connected end to end to form an approximately elliptical shape, which is called the MPO. The pelvic parameters were obtained directly from the measurements determined by the software.
In this study, the abovementioned data were divided into male and female groups, young and middle-aged groups
The TAPP surgical procedure is as followed: After successful induction of general anesthesia, the affected side was elevated, and the patient was placed in the Trendelenburg position. A pneumoperitoneum needle was inserted at the upper edge of the umbilicus to establish pneumoperitoneum, and a 10-mm trocar was used to maintain pneumoperitoneum. Then, 5-mm trocars were inserted at the outer edges of the left and right rectus abdominis muscles. The opening of the hernia ring on the affected side was observed, and the opposite side was examined to ascertain whether a hidden hernia was present. The contents of the hernia (omentum, intestine, and bladder) and the relative relationship between the inferior epigastric artery and the opening of the hernia ring were observed to determine whether it was an indirect hernia, a direct hernia, a femoral hernia, etc. The peritoneum was incised approximately 1 cm above the opening of the inner ring. The medial incision did not extend beyond the medial umbilical ligament, and the lateral incision did not extend beyond the anterior superior iliac spine. The Retzius space and the Borgros space were fully dissected. The preperitoneal dissection range was roughly as follows: Medial to the pubic symphysis and beyond the midline, lateral to the iliopsoas muscle and the anterior superior iliac spine, superior to 2-3 cm above the conjoint tendon, inferomedial to approximately 2 cm below the pectineal ligament, and inferolateral to the fascia of the spermatic cord. This dissection range ensured that a sufficiently large patch could be inserted. Moreover, the Retzius space should be dissected between the surface of the bladder and the pubis, and the vascular plexus on the surface of the pubis should not be damaged. When dissecting the lateral Borgros space, dissection should be performed superficial to the transversalis fascia to protect the lateral iliohypogastric nerve. The hernia sac was mobilized, the adhesions between it and the transversalis fascia were severed, and the medial vas deferens and the lateral spermatic cord vessels were dissected to completely free the hernia sac.
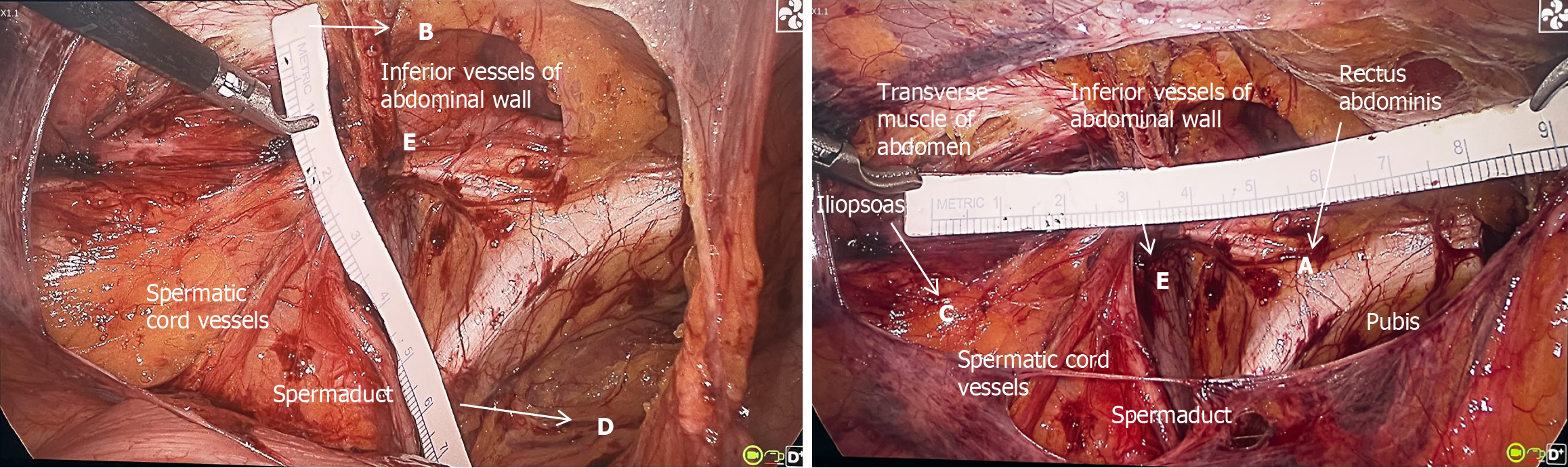
During the operation, the convergence points (Point E) of the spermatic cord vessels, the vas deferens (round ligament of the uterus) and the inferior epigastric vessels were located. The intersection point (point A) of the horizontal line passing through point E and the medial edge of the rectus abdominis muscle was determined. The intersection point (point B) of the vertical line passing through Point E and the lower edge of the transverse abdominis muscle was identified. The intersection point (point C) of the horizontal line passing through point E and the medial edge of the iliopsoas muscle was identified. The intersection point (point D) of the vertical line passing through point E and the pubis was located. AC represents the length of the MPO, and BD represents the width of the MPO. A sterile paper ruler (specification model: 0.8 cm × 15 cm, Chenjie Paper-Plastic Nursing) was used to measure the distance between the AC and the BD during the operation. The method used to measure A, B, C, D, and E during surgery is shown in Figure 3.
The diameter of the opening of the inner hernia ring, the distances between the edge of the inner ring opening and the lateral edge of the iliopsoas muscle, the lower edge of the transverse abdominis muscle, the pubic ramus, and the lateral edge of the medial rectus abdominis muscle were measured. The patch was trimmed according to the measured distances, and then the patch was applied to cover the dissected surface. The principle is to replace the transversalis fascia and cover the entire MPO, ensuring sufficient overlap with the surrounding muscular and bony tissues. The medial part of the patch should extend beyond the midline by 1-2 cm (for bilateral hernias, both sides must be included), the lateral part should extend to the anterior superior iliac spine, the upper part should reach approximately 2-3 cm above the conjoint tendon (arcuate upper edge), the inferomedial part should reach approximately 2 cm below the pectineal ligament, and the inferolateral part should extend to approximately 6 cm from the fascia of the spermatic cord (at the level of the middle of the psoas major muscle). The area covered by the patch is the same as that included in the dissection range of the abovementioned preperitoneal space. When it is a bilateral hernia, the two patches should cross and overlap at the midline. The medial part should extend beyond the midline to avoid the recurrence of direct hernias. The inferomedial part should be placed in the pubovesical space, and the inferolateral part should be more than 0.5 cm from the fascia of the peritoneum[12]. After the patch (TransEasy Medical Tech. Co., Ltd. EasyProsthes®, Light Weight 3D Mesh, 12 cm × 16 cm, TransEasy Medical Tech. Co., Ltd. EasyProsthes®, Flat Mesh, Size: 10 cm × 15 cm, BIOSIS HEALING, MP4-10.8 × 16-R/L, common3D, MP1-10.8 × 16-R/L, Light Weight 3D Mesh) is placed, it should be continuously sutured to the peritoneal flap with absorbable sutures, the pneumoperitoneum should be resolved, and the trocar openings should be sutured closed, marking the completion of the operation.
SPSS AU statistical software was used for paired-sample t test analysis of the distance between the AC (the distance between point A and point C) and BD (the distance between point B and point D) measured by 3D multislice spiral CT three-dimensional reconstruction data based on multi-slice spiral CT and that measured during the operation. Normally distributed data are presented as means ± SDs. A P value greater than 0.05 indicated no statistically significant difference between the two groups. To analyze the impacts of sex, age, BMI, hernia side, and hernia type on AC and BD, t-tests were used. A P value less than 0.05 indicated a statistically significant difference between the two groups.
Seventy-four patients underwent preoperative 3D CT of the bones, muscles, inguinal canals and other tissues. All surgeries were completed smoothly. Among the 74 patients, 64 were male and 10 were female. The average age of the patients was 58.30 ± 12.32 years, and there was no significant difference in the average age between males and females (P > 0.05). The average projected area of the MPO was 19.54 ± 3.33 cm2 (Table 2). The distances between AC and BD were 7.18 ± 0.51 cm and 6.17 ± 0.40 cm, respectively. There was no statistically significant difference between the AC and BD values determined under laparoscopy and those determined under 3D CT (P > 0.05) (Table 3). These findings indicate that the size of the MPO measured via 3D multislice spiral CT is similar to that measured during laparoscopy. The AC and BD measurements were correlated with sex (P < 0.05).
| Name | Sample size | Minimum value | Maximum value | The average value | SD | Median |
| Intraoperative AC | 50 | 6.000 | 8.500 | 7.052 | 0.474 | 7.000 |
| Intraoperative BD | 50 | 5.000 | 7.500 | 6.266 | 0.609 | 6.400 |
| Preoperative AC | 74 | 6.220 | 8.410 | 7.204 | 0.474 | 7.275 |
| Preoperative BD | 74 | 5.220 | 7.410 | 6.113 | 0.402 | 6.115 |
| MPO area, cm2 | 74 | 12.670 | 26.840 | 19.535 | 3.325 | 19.640 |
| Name | Pairing 1 | Pairing 2 | Difference (pairing 1-pairing 2) | t | P |
| Intraoperative AC paired with preoperative AC | 7.05 ± 0.47 | 7.18 ± 0.51 | -0.12 | -1.890 | 0.065 |
| Intraoperative BD paired with preoperative BD | 6.27 ± 0.61 | 6.17 ± 0.40 | 0.09 | 1.010 | 0.317 |
There was no significant correlation between the AC or BD measurement and the MPO area and age or BMI (Table 4). There was no significant correlation between the AC or BD measurement and the MPO area or the affected side (P > 0.05). There was no significant correlation between the AC or BD measurement and the MPO area or indirect/direct hernia (P > 0.05) (Table 5). In this study, an indirect hernia was the most common type of hernia, as was right-side hernias. Patients aged 60 years or older composed more of the study population than patients younger than 60 years did (Figure 4). In several studies, the authors reported that the MPO was oval or trapezoidal. In our study, the MPO was a three-dimensional structure composed of two triangles. Therefore, the area of the MPO is calculated by summing the areas of the two triangles (Figure 2).
| Intraoperative AC | Intraoperative BD | Preoperative AC | Preoperative BD | MPO area | |
| Gender | |||||
| Male (n = 64) | 7.09 ± 0.47 | 6.35 ± 0.61 | 7.25 ± 0.46 | 6.13 ± 0.41 | 19.59 ± 3.12 |
| Female (n = 10) | 6.85 ± 0.49 | 5.83 ± 0.35 | 6.92 ± 0.46 | 6.03 ± 0.37 | 19.17 ± 4.64 |
| t | 1.324 | 2.333 | 2.042 | 0.667 | 0.278 |
| P | 0.192 | 0.024a | 0.045a | 0.507 | 0.786 |
| Age | |||||
| < 60 (n = 34) | 7.05 ± 0.34 | 6.36 ± 0.60 | 7.18 ± 0.46 | 6.14 ± 0.37 | 20.07 ± 3.31 |
| ≥ 60 (n = 40) | 7.06 ± 0.59 | 6.17 ± 0.61 | 7.22 ± 0.49 | 6.09 ± 0.43 | 19.08 ± 3.31 |
| t | -0.088 | 1.110 | -0.407 | 0.554 | 1.274 |
| P | 0.930 | 0.272 | 0.685 | 0.582 | 0.207 |
| BMI | |||||
| ≤ 24 (n = 43) | 6.97 ± 0.40 | 6.20 ± 0.60 | 7.17 ± 0.43 | 6.10 ± 0.36 | 19.40 ± 3.34 |
| > 24 (n = 30) | 7.17 ± 0.55 | 6.36 ± 0.62 | 7.24 ± 0.54 | 6.13 ± 0.47 | 19.75 ± 3.40 |
| t | -1.536 | -0.898 | -0.542 | -0.286 | -0.432 |
| P | 0.131 | 0.374 | 0.590 | 0.775 | 0.667 |
| Intraoperative AC | Intraoperative BD | Preoperative AC | Preoperative BD | MPO area | |
| Hernia side | |||||
| Right (n = 43) | 7.07 ± 0.48 | 6.29 ± 0.58 | 7.26 ± 0.49 | 6.11 ± 0.40 | 19.56 ± 3.30 |
| Left (n = 23) | 6.96 ± 0.55 | 6.15 ± 0.67 | 7.10 ± 0.47 | 6.04 ± 0.42 | 19.10 ± 3.42 |
| t | 0.620 | 0.683 | 1.270 | 0.689 | 0.536 |
| P | 0.539 | 0.499 | 0.209 | 0.493 | 0.594 |
| Hernia type | |||||
| Oblique hernia (n = 48) | 7.02 ± 0.49 | 6.33 ± 0.60 | 7.21 ± 0.48 | 6.12 ± 0.39 | 19.59 ± 3.25 |
| Direct hernia (n = 15) | 7.12 ± 0.46 | 6.22 ± 0.66 | 7.26 ± 0.48 | 6.15 ± 0.44 | 19.98 ± 3.12 |
| t | -0.548 | 0.496 | -0.347 | -0.250 | -0.414 |
| P | 0.586 | 0.622 | 0.729 | 0.804 | 0.680 |
An inguinal hernia is an external abdominal hernia that occurs in the inguinal region. That is, the organs or tissues inside the abdominal cavity protrude through the defect in the abdominal wall in the inguinal region. Defects in the abdominal wall can be congenital or acquired[13]. The factors that contribute to the development of an inguinal hernia include BMI, high intra-abdominal pressure, a history of appendectomy, contralateral hernia, thoracic or abdominal aortic aneurysm, a history of prostatectomy, peritoneal dialysis, chronic obstructive pulmonary disease, smoking, and collagen vascular diseases[2,14].
The aim of inguinal hernia surgery is to address the MPO. The MPO was first discussed by the French doctor Fruchaud. The MPO extends from the transverse abdominis muscle above, to Cooper’s ligament below, to the lateral side of the rectus abdominis muscle medially, and to the iliopsoas muscle laterally. Sufficient dissection of the MPO is necessary for successful repair of the inguinal hernia. The preperitoneal space is the optimal location for patch placement as the patch can adhere to the abdominal wall more closely because of intra-abdominal pressure[6]. Moreover, injury to adjacent structures, mesh folding, and hematoma formation are common factors that contribute to inguinal hernia recurrence after surgery[4]. Researchers have also reported that the mesh shrinks by approximately 20%, which further contributes to patch displacement and inguinal hernia recurrence[15]. To date, no studies have revealed the specific area of the MPO even though well-known measurement methods have been used. Cadaver studies of hernias revealed that the width and height of the MPO were 7.8 ± 3.0 cm and 6.5 ± 1.9 cm, respectively[16].
In a cadaver study, Wolloscheck et al[17]reported that the size of the MPO was 7.8 cm × 6.5 cm × 4.5 cm and that the mesh should be no smaller than 10 cm × 8 cm for successful hernia repair. Takahiro performed total extraperitoneal laparoscopy and reported that, in males, hernias with an opening less than 3 cm in length had a horizontal distance of 7.64 ± 1.04 cm and a vertical distance was 3.62 ± 0.76 cm. However, hernias with an opening greater than or equal to 3 cm had a horizontal distance of 7.97 ± 1.01 cm and a vertical distance of 5.04 ± 1.01 cm[18]. By establishing a three-dimensional model using computed tomography angiography data and the MPO area, the authors concluded that the projected area of the MPO had a significant positive correlation with age and had no correlation with pelvic parameters such as the width or height of the MPO[3].
There was not much difference in the comparison of the two measurement methods in this study. To further explore the area of the MPO in the East Asian population, the length and width and the projected area of the MPO were measured via 3D CT before the operation to predict the actual area of the MPO, determine the most appropriate patch size, accurately measure the patch area suitable for Chinese people, and reduce the preperitoneal dissection range with the aim of reducing the incidence of secondary injuries. In the medium and long term, the probability of patch shrinkage, hernia recurrence, infection and pain can be reduced.
TAPP involves entering the abdominal cavity by creating a hole at the level of the umbilicus. The operating holes are established on the lateral side of the rectus abdominis muscle at the level of the umbilicus. The peritoneum is incised and dissected at the opening of the inner ring, and the Retzius and Borgros spaces are then dissected. The dissection area is smaller in TAPP than in total extraperitoneal. Therefore, TAPP is suitable for ascertaining whether there is a hidden hernia on the opposite side of the inguinal region and whether there are adhesions in the abdominal cavity. Additionally, for larger scrotal hernias or incarcerated hernias, measures such as reducing the hernia sac, transecting the hernia sac or suturing the opening of the inner ring can be implemented.
The boundaries of the MPO are as follows: The internal oblique muscle, the rectus abdominis muscle on the medial side and the iliopsoas muscle on the lateral side extending above the pubis. As the MPO is characterized as relatively weak with no muscle present, it is involved in the development of all inguinal hernias. Moreover, MPO measurements are widely available for European and American populations and relatively scarce for East Asian populations. Therefore, patch data differs after measurement using different methods[3]. For patients with inguinal hernias, after preperitoneal insufflation and dissection, the anatomy of the inguinal region is different from its preoperative state. CT may be insufficient for evaluating the anatomy of the lower abdominal wall to determine which type of mesh should be used. However, it can help the patch adhere to the preperitoneal space[19]. The success of treatment depends on adequate preperitoneal dissection, proper patch placement and sufficient coverage of the MPO to prevent hernia recurrence.
The use of both 3D CT and laparoscopy for measuring the MPO marks the transition from empirical treatment to personalized management. The MPO is a weak area where inguinal hernias occur that is surrounded by the transverse abdominal muscle, rectus abdominis muscle, iliopsoas muscle, and Cooper’s ligament. Its shape is significantly affected by the patient’s age, sex, weight, disease duration, etc. Traditional inguinal hernia repair involves palpation and two-dimensional imaging to determine the defect range. However, it is easy to underestimate the invasiveness of complex hernias (such as recurrent hernias, saddle hernias, and incarcerated hernias) on 3D CT images. Thin-slice 3D CT (slice thickness < 1 mm), through multiplanar reformation and volume rendering (volume rendering algorithm), can stereoscopically quantify the transverse diameter, longitudinal diameter, and area of the MPO. A comparison of the MPO values revealed that measurements obtained via 3D CT were comparable to those obtained via TAPP. Thus, it is possible to preliminarily determine the projection area and length-width dimensions of the patient’s MPO before surgery, determine the appropriate size and area of the patch preoperatively, avoid excessive dissection of the MPO, and reduce the risk of injury to the nerves, blood vessels, vas deferens, and bladder, all of which are within the range of dissection. Therefore, selection of the most appropriate patch size and area will enable the patch to be precisely anchored to the bony structures and ligamentous and muscular structures of the pelvic floor. It can also reduce the risks of patch displacement and seroma formation due to an overly large dissection area during the operation, as well as patch folding and distortion due to an overly small dissection range. Ultimately, precise preoperative CT measurements can guide appropriate dissection during surgery and facilitate the accurate selection and placement of suitable patches, all of which are crucial for high-quality laparoscopic inguinal hernia repair. The findings of this study provide a deeper understanding of the anatomy of structures in the inguinal region and the principles of repair for optimal functional outcomes, thereby providing a new dimension for the precise treatment of inguinal hernias.
Before inguinal hernia repair, the area of the MPO as well as the size of structures located within the MPO should be accurately measured via 3D multislice spiral CT. The application of this technology can aid in determining the most appropriate dissection range and patch size. The patch must be appropriately sized to ensure full coverage of the MPO and thereby successful repair of the inguinal hernia.
| 1. | Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 626] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 2. | HerniaSurge Group. International guidelines for groin hernia management. Hernia. 2018;22:1-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1263] [Article Influence: 180.4] [Reference Citation Analysis (1)] |
| 3. | Song Z, Yang D, Wang Y, Bu X, Yang J, Wu J, Nie X, Song H, Gu Y. Three-dimensional visualization and measurement of myopectineal orifice in non-inguinal hernia patients. Surg Radiol Anat. 2020;42:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Daes J, Felix E. Critical View of the Myopectineal Orifice. Ann Surg. 2017;266:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, de Lange D, Fortelny R, Heikkinen T, Kingsnorth A, Kukleta J, Morales-Conde S, Nordin P, Schumpelick V, Smedberg S, Smietanski M, Weber G, Miserez M. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2009;13:343-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 949] [Cited by in RCA: 900] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 6. | Pélissier EP, Blum D, Marre P, Damas JM. Inguinal hernia: a patch covering only the myopectineal orifice is effective. Hernia. 2001;5:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Pélissier EP. Inguinal hernia: the size of the mesh. Hernia. 2001;5:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Stoppa R, Warlaumont C, Marrasse E, Verhaeghe P. [Pathogenesis of inguinal hernias]. Minerva Chir. 1989;44:737-744. [PubMed] |
| 9. | Lichtenstein IL, Shulman AG, Amid PK, Montllor MM. The tension-free hernioplasty. Am J Surg. 1989;157:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 740] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 10. | Dumanian GA, Tulaimat A, Dumanian ZP. Experimental study of the characteristics of a novel mesh suture. Br J Surg. 2015;102:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Tanabe K, Mori S, Kita Y, Wada M, Kenji B, Itaru O, Takaaki A, Satoshi I, Kosei M, Natsugoe S. A rare case report of bilateral recurrent inguinal hernia due to persistent Müllerian duct syndrome treated by transabdominal preperitoneal repair. Medicine (Baltimore). 2020;99:e19079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Cao Y, Ding Z, Qiang H. Analysis on Influencing Factors of Recurrence after Indirect Inguinal Hernia Laparoscopic Surgery. J Healthc Eng. 2022;2022:2978745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Chen S, Tang J. [China Guideline for Diagnosis and Treatment of Adult Groin Hernia (2018 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:721-724. [PubMed] |
| 14. | Lau H, Fang C, Yuen WK, Patil NG. Risk factors for inguinal hernia in adult males: a case-control study. Surgery. 2007;141:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Seker D, Oztuna D, Kulacoglu H, Genc Y, Akcil M. Mesh size in Lichtenstein repair: a systematic review and meta-analysis to determine the importance of mesh size. Hernia. 2013;17:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Wolloscheck T, Konerding MA. Dimensions of the myopectineal orifice: a human cadaver study. Hernia. 2009;13:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wolloscheck T, Gaumann A, Terzic A, Heintz A, Junginger T, Konerding MA. Inguinal hernia: measurement of the biomechanics of the lower abdominal wall and the inguinal canal. Hernia. 2004;8:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Hiratsuka T, Shigemitsu Y, Etoh T, Kono Y, Suzuki K, Zeze K, Inomata M. Appropriate mesh size in the totally extraperitoneal repair of groin hernias based on the intraoperative measurement of the myopectineal orifice. Surg Endosc. 2021;35:2126-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Chrzan R, Karbowski K, Pasternak A. Do we really need three-dimensional convex inguinal hernia meshes? Hernia. 2020;24:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |