Published online Feb 16, 2025. doi: 10.4253/wjge.v17.i2.101284
Revised: October 14, 2024
Accepted: November 5, 2024
Published online: February 16, 2025
Processing time: 156 Days and 4.7 Hours
Discordance between endoscopic and histologic assessments in Crohn’s disease (CD) have been observed, however the prevalence and cause are unclear.
To assess if a protocolized approach to biopsy collection facilitates understanding of this discordance in patients with ileal CD.
Patients with known ileal CD underwent colonoscopy with endoscopic disease activity assessment. Three protocolized biopsies were taken respectively from an ulcer edge, 7-mm, and 14-mm away from the ulcer edge in patients with discrete ileal ulcer(s). In patients with no ulcers as controls, the same 3-site biopsy protocol was applied in a randomly selected area of endoscopically-unremarkable terminal ileal mucosa. A blinded pathologist assessed mucosal inflammation in each biopsy using 3 validated histologic indices.
Twenty-four participants had visible ulcer(s) on endoscopy and 12 served as no-ulcer controls. Of biopsies taken from an ulcer edge, only 67% showed histologic evidence of active (neutrophilic) inflammation, and 33% showed histologic features of ulcer or erosion; all were from either large (n = 4) or very large (n = 4) ulcers. In the no-ulcer controls, no biopsies showed histologic features of ulcer or erosion, but 8% showed active inflammation.
A striking discordance exists between endoscopic and histologic assessments for mucosal inflammation in patients with active ileal CD, even in biopsies targeted at an ulcer edge, while a higher concordance is seen in patients with no endoscopic disease activity. It remains unclear how to incorporate histologic disease activity into the treatment paradigm. Further research is needed to optimize biopsy protocols and histologic assessments for CD.
Core Tip: Discordance exists between endoscopic and histologic assessments for mucosal inflammation in patients with active ileal Crohn’s disease (CD), even in biopsies targeted at an ulcer edge, while a higher concordance is seen in patients with no endoscopic disease activity. Microscopic patchiness of mucosal inflammation in CD likely contributes to the discrepancy. Given the discordance, ulcer and erosion should not be included in the histologic evaluation scheme for CD.
- Citation: Lee SD, Mau B, Avalos CJ, Clark-Snustad KD, Kamp KJ, Gui X. Discordance between endoscopic and histopathologic assessment in ileal Crohn’s disease. World J Gastrointest Endosc 2025; 17(2): 101284
- URL: https://www.wjgnet.com/1948-5190/full/v17/i2/101284.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i2.101284
Therapeutic targets in inflammatory bowel disease (IBD) currently include clinical and endoscopic remission[1,2]. Consideration has been given to adding transmural healing in Crohn’s disease (CD) and histologic healing in ulcerative colitis (UC) to measure deeper remission based on observations that histologic inflammation remains in a significant number of patients who have no endoscopic inflammation[2]. However, it is a known phenomenon in both CD and UC that discrepancies exist between endoscopic and histologic assessments regarding the presence and degree of mucosal inflammation. Biopsies taken from areas of mucosal ulceration may show no evidence of ulcer or even no significant active inflammation on histology. Alternatively, histopathologic active inflammation may be identified in biopsies taken from endoscopically noninflamed and even normal-appearing mucosa. Such discordance has been attributed to sampling variation or the focality/patchiness of Crohn’s inflammation. An alternative explanation is that a true discrepancy exists between the histologic and endoscopic features for unknown reasons[3].
In current clinical practice, patients with CD undergo colonoscopy and multiple biopsies are collected from inflamed and normal appearing mucosa from each bowel segment (ileum, right colon, transverse colon, left colon, and rectum). Then all biopsies from each bowel segment (including both inflamed and normal appearing tissue) are placed in one container and sent to pathology. Thus, the proximity of individual biopsies in relation to an endoscopic feature (e.g. ulcer, inflamed mucosa, noninflamed mucosa, ulcer margin, mucosa adjacent to ulcer) is not specified, complicating our understanding of the correlation between endoscopic and histologic assessments of the same area of mucosa.
Given the non-uniform involvement of CD, we opted to limit our study population to patients with a history of known ileal disease within reach of colonoscopy. In this patient population, we obtained biopsies from a single region of bowel, the terminal ileum, in a protocolized manner that permitted identification and assessment of biopsies taken at an ulcer edge and at prespecified distances from endoscopic ulceration. We aimed to evaluate if a protocolized biopsy approach targeting the ulcer edge would improve the concordance between endoscopic and histologic assessments of inflammatory changes in ileal Crohn’s.
Institutional review board approval was received for this prospective study. Eligible patients were adults ≥ 18 years old with a history of ileal/ileocolonic CD who underwent ileocolonoscopy with biopsy collection per standard of care between 2022-2023 at an academic hospital in Washington State. We excluded patients with J-pouch, ileostomy, or colostomy, those with ileal strictures, and those taking non-steroidal anti-inflammatory drugs within 8 weeks of the colonoscopy. Patients on the endoscopy schedule were invited to participate, and eligible, consented patients proceeded with the study. Biopsies were collected in a protocolized manner during colonoscopy and sent for histopathologic evaluation. Participant demographics and clinical characteristics were obtained through the medical record and patient questionnaires.
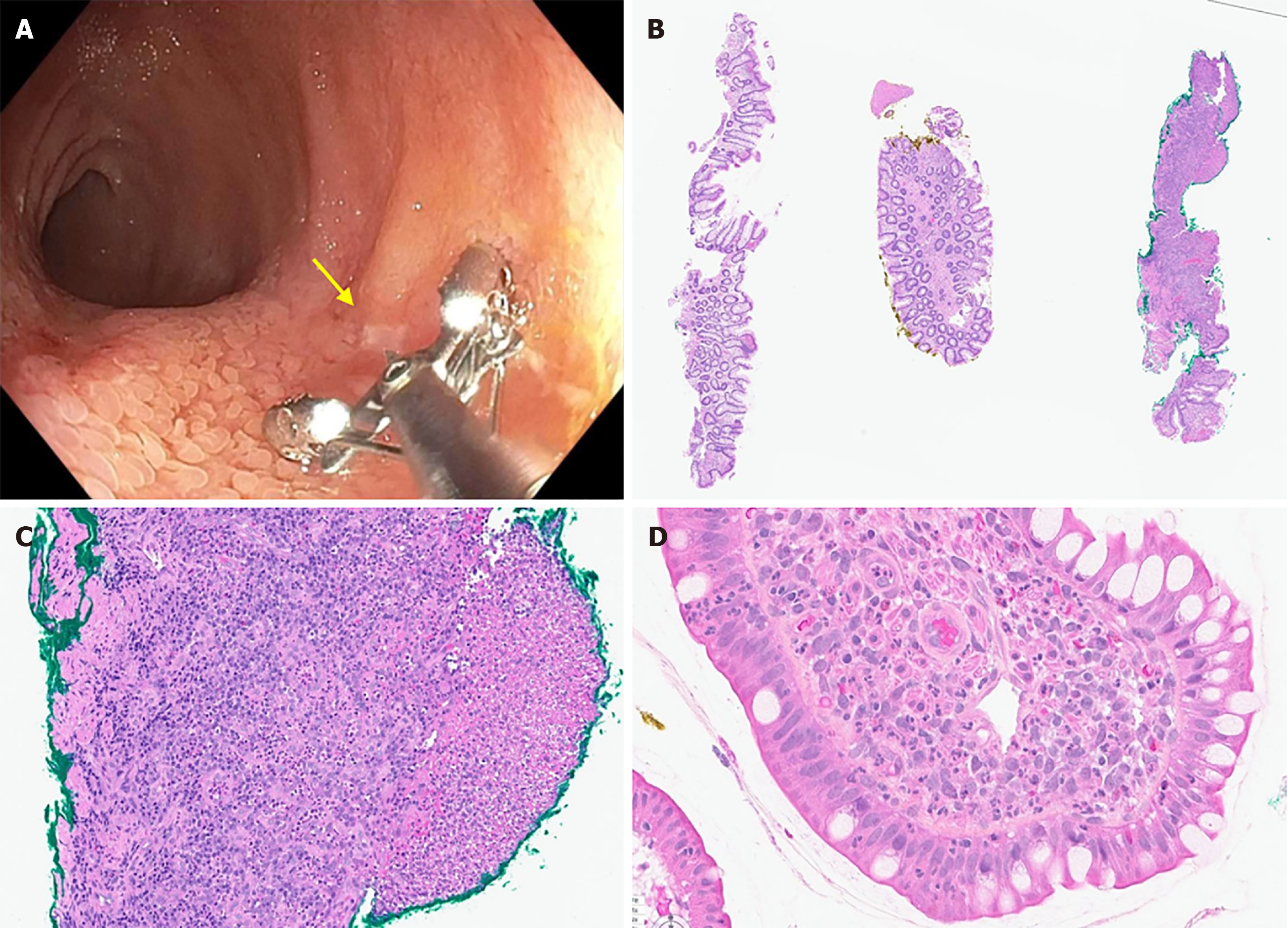
During colonoscopy, the ileum was meticulously washed prior to biopsy. Endoscopic disease activity was evaluated with the Simple Endoscopic Score for Crohn's Disease (SESCD) and the size of any ileal ulcer was measured by a single endoscopist (Scott D Lee). In patients with endoscopically visible ulcer(s), 3 biopsies were taken around the ulcer. The biopsies were taken at the ulcer edge, 7-mm away from the ulcer edge, and 14-mm away from the ulcer edge. 7-mm is the width of the open biopsy forceps and provided a straightforward measurement method. In patients without an endoscopically visible ulcer, the same 3-biopsy protocol was performed with biopsies collected from a randomly chosen area of endoscopically non-inflamed terminal ileal mucosa. To identify the specific biopsy location for microscopic examination and to permit blinding of the pathologist, one biopsy was marked with yellow dye, one with green dye (Surgipath® Tissue Marking Dye, Leica Biosystems), and one remained undyed. The 3 biopsies were placed in the same container with fixative (10% neutral buffered formalin) and otherwise handled as routine. The color (or lack of dye) used for each biopsy was randomized, and the pathologist was blinded to the color scheme. A gastrointestinal pathologist (Xianyong Gui) evaluated the histology of each biopsy and further analyzed the mucosal inflammation using three validated histologic indices for IBD, including Global Histology Activity Score (GHAS)[4], Robarts Histopathology Index (RHI)[5], and Picasso Histological Remission Index[6]. The presence or absence of ulcer/erosion, active (neutrophilic) inflammation, and chronic (lymphoplasmacytic) inflammation is included in each of the 3 indices and was the focus of the histologic assessment. Statistical analyses were performed using R Studio (version 2023.06.1). We considered data from the no-ulcer group as independent samples as they each represented tissue from a non-ulcerated mucosa. Continuous data are presented as mean and standard deviation and categorical data are presented as absolute number and percentage.
81 patients were prescreened and contacted, 45 consented to participate, and 36 were included in the analysis. Patients were excluded from analysis due to technical reasons (n = 5 due to stricture or extensive disease as we were unable to obtain biopsies adjacent to the ulcer that were free of ulcer, n = 1 due to dye application failure) or due to failure to collect biopsies related to procedural timing (n = 3). Participants were on average 44.6 years old (SD: 15.3, range: 20-72) with 21 males and 15 females. Average disease duration was 18.4 years (SD: 9.8, range: 2-45). 24 participants had endoscopically visible ulcer(s) (mean ileal SESCD score: 6.2) and 12 served as no-ulcer controls with no endoscopically visible inflammation or ulcerations (mean ileal SESCD score: 0). For those with ulcers, 5 had aphthous ulcers (0.1-0.5cm, mean ileal SESCD: 3.2), 11 had large ulcers (0.5-2 cm, mean ileal SESCD: 6.2), and 8 had very large ulcers (> 2 cm, mean ileal SESCD: 8.1). See Figure 1 for sample images of histologic and endoscopic assessments.
Of all biopsies taken from an ulcer edge, 67% (16/24) showed histologic evidence of active (neutrophilic) inflammation (RHI > 3 and PHI > 0) and 46% (11/24) showed chronic (lymphoplasmacytic) inflammation. Only 33% (8/24) showed histologic features of ulcer or erosion, and they were seen only in patients with large (n = 4) or very large (n = 4) ulcers. Of biopsies from 7-mm distant to ulcer, 29% (7/24) showed evidence of active and 21% (5/24) showed chronic inflammation. Of the biopsies 14-mm away from ulcer, 17% (4/24) showed active and 8% (2/24) showed chronic inflammation. Of all biopsies, the mean RHI score was highest at the ulcer edge (11.1, SD: 10.6) and lower at 7-mm (2.0, SD: 2.9) and 14-mm (1.5, SD: 3.1) away from the ulcer edge (Table 1).
| Histology | Ulcer group | No ulcer controls | ||
| Ulcer edge | 7 mm from ulcer | 14 mm from ulcer | ||
| Total sample | ||||
| Ulcer subscore1 | 8 (33) | 0 | 0 | 0 |
| Active (neutrophilic) histologic inflammation2 | 16 (67) | 7 (29) | 4 (17) | 3 (8) |
| Chronic (lymphoplasmacytic) histologic inflammation3 | 11 (46) | 5 (21) | 2 (8) | 1 (3) |
| RHI | ||||
| Total sample | 11.1 ± 10.6 | 2.0 ± 2.9 | 1.5 ± 3.1 | 0.9 ± 1.7 |
| Subset with active histologic inflammation2 | 16.6 ± 8.8 | 6.1 ± 1.2 | 7.5 ± 3.9 | 6 ± 0 |
| GHAS | ||||
| Total sample | 6.5 ± 4.8 | 2.3 ± 3.2 | 1.9 ± 2.7 | 1.1 ± 1.9 |
| Subset with active histologic inflammation2 | 8.8 ± 3.7 | 5.3 ± 2.8 | 6.3 ± 2.8 | 5.7 ± 1.2 |
| PHRI | ||||
| Total sample | 1.9 ± 1.6 | 0.4 ± 0.7 | 0.4 ± 0.8 | 0.1 ± 0.5 |
| Subset with active histologic inflammation2 | 2.9 ± 1.1 | 1.1 ± 0.9 | 1.8 ± 0.5 | 1.3 ± 1.2 |
| Large (0.5-2 cm) or very large (> 2 cm) ulcers4 | ||||
| Ulcer subscore1 | 8 (42) | 0 | 0 | |
| Active (neutrophilic) inflammation2 | 16 (84) | 6 (32) | 4 (21) | |
| Chronic (lymphoplasmacytic) inflammation3 | 11 (58) | 4 (21) | 2 (11) | |
| Aphthous ulcers (0.1-0.5 cm)5 | ||||
| Ulcer subscore1 | 0 | 0 | 0 | |
| Active (neutrophilic) inflammation2 | 0 | 1 (20) | 0 | |
| Chronic (lymphoplasmacytic) inflammation3 | 0 | 1 (20) | 0 | |
Of all biopsies taken from the 5 patients with aphthous ulcers (characterized as SESCD subscore of 1 for ulcer size), none showed histologic evidence of ulcer or active inflammation at the ulcer edge. Only one (20%) showed active as well as chronic inflammation on histology at 7-mm from ulcer edge. None showed active or chronic inflammation at 14-mm. Biopsies taken from large or very large ulcers had better correlation with histologic assessment, with 42% (8/19) having a positive ulcer subscore, 84% (16/19) showing active inflammation, and 58% (11/19) showing chronic inflammation. Biopsies taken 7-mm and 14-mm away from large and very large ulcers showed no histologic evidence of ulcer and had lower rates of active disease. In the no-ulcer controls, none of the biopsies showed features of ulcer or erosion, as expected; however, the endoscopically non-inflamed mucosa showed active inflammation in 8% of biopsies and chronic inflammation in 3% on histology.
When considering the ileal SESCD score, only those participants with an ileal SESCD score greater than or equal to 4 (which is considered active ileal disease) showed histologic evidence of an ulcer or of active inflammation (Table 2). Of those with active endoscopic inflammation, 80% had histologic ulcers and 55% had active inflammation.
Significant discordance between endoscopic and histologic assessments for mucosal ulceration and inflammation in CD has been described, however our understanding of the prevalence of the discordance, as well as the cause, is confounded by the fact that biopsies of inflamed and non-inflamed tissue are often placed in the same container for histopathology. Thus, the location of a particular biopsy in related to an endoscopic ulcer (or normal appearing tissue) is not generally specified to the pathologist in clinical practice.
In our study, we obtained biopsies in a protocolized fashion that permitted histopathologic assessment of biopsies collected at pre-specified distances from an endoscopic ulcer. Our results continue to show a striking discordance between endoscopic and histologic assessments of the same area of mucosa. We report that the majority of biopsies taken at an ulcer edge fail to show histologic features of ulcer (defined by loss of mucosa with associated granulation tissue with or without fibrinopurulent exudate) or erosion (defined by presence of fibrinopurulent exudate with or without destruction of surface epithelium but no granulation tissue). Our results suggest that endoscopic ulceration more closely correlates with the histologic finding of neutrophils in the epithelium (i.e., active inflammation), however even so, less than a third of biopsies taken in mucosa around an ulcer showed active histologic inflammation, and fewer had chronic inflammation. Prior studies have also described that one may observe significant histologic inflammation in endoscopically non-inflamed mucosa, thus histologic remission has been considered a deeper remission[2]. Our study demonstrated this finding as well. However, we report better concordance in patients with no visible ulceration, with only 8% of biopsies from endoscopically normal-appearing mucosa showing findings of active inflammation on histology.
One explanation for these findings is the patchiness of inflammation in CD, a well-known characteristic feature. Based on this study, we believe that the microscopic patchiness of inflammation in CD is variable and has poor concordance with endoscopic patchiness. Therefore, endoscopists should anticipate that the majority of biopsy samples taken at or around an ulcer may not demonstrate features of ulcer on histologic evaluation, and that many samples may not even demonstrate features of histologic inflammation. Particularly, small aphthous ulcers are unlikely to show histopathologic evidence of ulcer or even active inflammation.
Our study findings clearly demonstrate the poor endoscopic-histologic correlation in assessing mucosal inflammation and disease activity in CD, and raise concern about the appropriateness and reliability of current biopsy protocols and histologic scoring for assessing histologic remission and drug effects in clinical trials in patients with CD[3]. Admittedly, this is a single institution study and a single biopsy was collected at each location. Sampling variation may occur, but we believe this was minimized by our biopsy protocol.
It is important to consider that the majority of current histological scores for IBD were developed for UC, which has a continuous and diffuse inflammatory presentation, as opposed to the discontinuous and patchy/focal nature of inflammation in CD. Additionally, all of the histologic indices were originally designed for colonic disease and not validated in small intestinal disease, however they have been used for both colonic and small bowel diseases. These differences raise concerns about the accuracy of using any UC-originated histologic index for CD. In recognition of this, our study included the Global Histologic Disease Activity Score, which is currently the only histologic index designed for CD and widely used in clinical trials of CD. However, the only difference between GHAS and other histologic indices is that the GHAS includes a section estimating the percentage of biopsies affected by disease in order to reflect the focality and extent of inflammation in each sampled region of bowel.
Another factor highlighted by our study is that even when a biopsy is taken at an ulcer edge, the histopathologic assessment often does not show features of an ulcer. This raises concern about the utility of including the assessment of ulcer/erosion on histologic indices for CD, as this may not accurately reflect the disease activity. Future work is needed to determine a more representative and reproducible biopsy protocol and histologic index for CD, especially in assessing histologic remission. This should take into consideration a more robust characterization of the focality of CD[3] and possible involvement in endoscopically unremarkable intervening mucosa. A CD-centered histologic index should also be validated in both small bowel disease and Crohn’s colitis.
While histologic assessment is an important component of CD evaluation, our results suggest that current histologic scores of biopsies taken at an ulcer edge to evaluate Crohn’s inflammation do not appear to add additional information that would alter clinical treatment, beyond the known endoscopic disease activity. However, biopsy collection remains important to evaluate for alternative etiologies of the endoscopic findings (e.g. infection, malignancy). Currently, endoscopic disease activity/severity has been validated as a treatment target, and this should be the primary finding used to alter clinical therapy.
In conclusion, if an endoscopist observes an ulcer, approximately two-thirds of the time biopsy findings may be discordant, with no histologic features of mucosal ulceration. Approximately one-third of the time the biopsy may not even show active (neutrophilic) inflammation. The relationship between endoscopic and histologic assessments is better for larger ulcers than for aphthous ulcers, and the histologic score for active inflammation is on average highest at the ulcer edge and lower further from the ulcer. Furthermore, if an endoscopist does not observe an ulcer, it is unlikely that an ulcer will be observed on histology. Sometimes, although rare, active (8%) or chronic (3%) inflammation may be identified on histology of biopsies taken from endoscopically normal appearing areas. Overall, some degree of discordance between endoscopic and histologic findings at and around an ulcer can be expected, and we do not recommend therapy change based on histologic findings alone. Ultimately, it is still unclear how to incorporate histologic disease activity into the treatment paradigm. Further research is needed to optimize biopsy protocols and histologic assessments for CD.
| 1. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1404] [Article Influence: 140.4] [Reference Citation Analysis (115)] |
| 2. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1614] [Article Influence: 403.5] [Reference Citation Analysis (1)] |
| 3. | Almradi A, Ma C, D'Haens GR, Sandborn WJ, Parker CE, Guizzetti L, Borralho Nunes P, De Hertogh G, Feakins RM, Khanna R, Lauwers GY, Mookhoek A, Pai RK, Peyrin-Biroulet L, Riddell R, Rosty C, Schaeffer DF, Valasek MA, Singh S, Crowley E, Feagan BG, Jairath V, Pai RK. An expert consensus to standardise the assessment of histological disease activity in Crohn's disease clinical trials. Aliment Pharmacol Ther. 2021;53:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 4. | D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 604] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 5. | Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, Diebold MD, Danese S, Reinisch W, Schreiber S, Travis S, Peyrin-Biroulet L. Development and validation of the Nancy histological index for UC. Gut. 2017;66:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Gui X, Bazarova A, Del Amor R, Vieth M, de Hertogh G, Villanacci V, Zardo D, Parigi TL, Røyset ES, Shivaji UN, Monica MAT, Mandelli G, Bhandari P, Danese S, Ferraz JG, Hayee B, Lazarev M, Parra-Blanco A, Pastorelli L, Panaccione R, Rath T, Tontini GE, Kiesslich R, Bisschops R, Grisan E, Naranjo V, Ghosh S, Iacucci M. PICaSSO Histologic Remission Index (PHRI) in ulcerative colitis: development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut. 2022;71:889-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |