Published online Jan 16, 2025. doi: 10.4253/wjge.v17.i1.100904
Revised: November 6, 2024
Accepted: December 12, 2024
Published online: January 16, 2025
Processing time: 139 Days and 15.7 Hours
Endoscopic therapy is the primary approach for treating Mallory-Weiss syndro
A 52-year-old patient was admitted with symptoms of hematemesis and melena, and an endoscopy revealed a gastric fundus tear approximately 4 cm × 5 cm in size. The lesion was considered unsuitable for endoscopic repair by the attending endoscopist. Despite conservative measures, including fasting and acid sup
For patients with gastric tears presenting with active hemorrhage, HBOT might offer an effective alternative when conventional endoscopic therapies are not viable or have been unsuccessful.
Core Tip: This case highlights the rapid healing of a gastric laceration using hyperbaric oxygen therapy, an approach not previously reported for treating gastric mucosal tears. Our findings suggest that hyperbaric oxygen therapy can be a viable treatment option for managing severe gastric lacerations with active bleeding, especially in cases where conservative or endoscopic therapies have failed and surgical intervention is not indicated.
- Citation: Chen JL, Zhi HX, Pan JY, Chen ZH, Huang JL, Yao J. Hyperbaric oxygen therapy in the treatment of severe gastric laceration with active bleeding: A case report. World J Gastrointest Endosc 2025; 17(1): 100904
- URL: https://www.wjgnet.com/1948-5190/full/v17/i1/100904.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i1.100904
Mallory-Weiss syndrome (MWS) is marked by tears in the lower esophagus or at the gastroesophageal junction and gastric mucosa, typically resulting from a sudden surge in intra-abdominal pressure caused by severe vomiting, intense coughing, or blunt abdominal trauma[1]. This syndrome’s primary clinical manifestation is upper gastrointestinal bleeding, with epidemiological data indicating that esophageal and gastric mucosal tears contribute to 5%-15% of all cases of upper gastrointestinal bleeding[2]. Most MWS patients experience minor bleeding, and small tears often heal spontaneously within 72 hours under conservative management. However, in cases with active bleeding, endoscopy is generally the most effective and preferred treatment approach[3]. For patients unable to undergo endoscopic therapy or those for whom it has proven ineffective, options such as arterial embolization or emergency surgery are typically considered.
Hyperbaric oxygen therapy (HBOT) is widely recognized as an effective treatment for ischemic and hypoxic conditions, including decompression sickness, carbon monoxide poisoning, and gas gangrene, due to its rapid therapeutic effects. Its application has expanded to various medical conditions[4,5]. Within the gastrointestinal domain, HBOT is primarily used for managing radiation esophagitis, ulcerative colitis, Crohn’s disease, and ischemic anastomotic healing[6-8]. However, there are currently no reports of HBOT being employed for hemorrhagic gastric lacerations. This case report will document the first successful use of HBOT to treat a significant gastric laceration with active bleeding, showcasing its potential as a novel therapeutic approach in such cases.
A 52-year-old female presented with a 1-day history of vomiting and hematemesis.
The patient initially experienced vomiting of gastric contents after consuming chicken soup, which was followed by the vomiting of approximately 80 mL of bright red blood and a single episode of melena. Upon admission, gastroscopy revealed a 4 cm × 5 cm laceration in the gastric fundus with active bleeding, accompanied by a hematoma at the esophagogastric junction. The laceration extended nearly through the full thickness of the muscle layer, and a substantial amount of dark red blood was present in the stomach.
The patient had a history of long-term aspirin and atorvastatin use for hyperlipidemia, a previous episode of gastric bleeding 3 years ago, and a history of uterine fibroid surgery.
The patient’s other personal and family histories were unremarkable.
Upon admission, the patient’s vital signs were stable, with a temperature of 36.6 °C, pulse rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and blood pressure of 132/84 mmHg. Abdominal examination showed a non-distended, soft, and non-tender abdomen. Mild abdominal tenderness and rebound pain emerged on the 3rd day of hospitalization but had largely resolved by the 7th day.
Initial laboratory tests indicated a hemoglobin concentration of 110 g/L (normal range: 115-150 g/L). During the first 3 days of hospitalization, the patient experienced black, watery stools, with a daily volume ranging from 200 mL to 550 mL, resulting in a hemoglobin decrease to 83 g/L by the 3rd day.
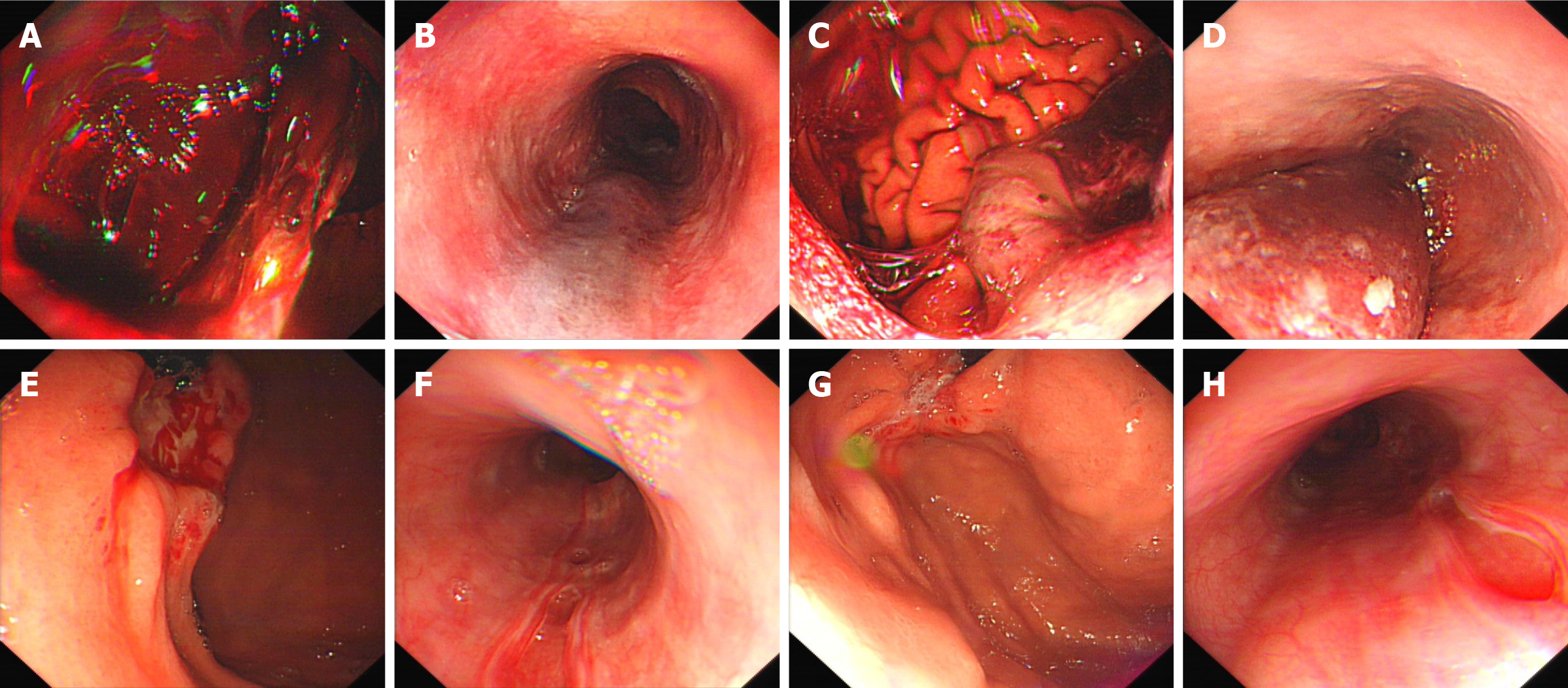
On admission, chest and abdominal computed tomography scans revealed a small amount of air within the cervical esophagus and surrounding soft tissues, with no signs of gastric perforation in the abdominal cavity. An emergency endoscopy performed on the day of admission identified a hematoma at the esophagogastric junction and a large volume of dark red blood in the stomach (Figure 1A and B, Video 1). A tear in the gastric fundus, approximately 4 cm × 5 cm in size, was observed with blood clots, though perforation could not be definitively determined. On the 3rd day, a follow-up gastroscopy showed prolapse of the gastric fundal mucosa and muscular layer covered by a hematoma, along with congestion, edema, and erosion at the esophagus and gastroesophageal junction (Figure 1C and D). By the 11th day, repeat endoscopy indicated notable improvement in the gastric fundus, with ulcer formation and no visible hematoma in the esophagus. Multiple mucosal defects in the esophagus showed signs of improvement (Figure 1E and F). On the 16th day, further endoscopic examination demonstrated a marked reduction in the gastric fundal ulcer size and continued healing of the esophageal mucosal defects (Figure 1G and H).
A multidisciplinary consultation was conducted, involving specialists from thoracic surgery, gastrointestinal surgery, and the intensive care unit.
The final diagnosis was MWS.
Due to the extensive tear and severe mucosal erosion, the endoscopist deemed endoscopic hemostasis inappropriate. The patient was initially managed conservatively with fasting, proton-pump inhibitor and hemostatic agent. Despite these measures, the patient continued to pass black, watery stools, ranging from 200 mL to 550 mL per day. Urgent follow-up endoscopy revealed worsening erosion and bleeding at the gastric fundus tear site, along with an aggravated esophageal hematoma. Given the patient’s stable vital signs and the absence of esophageal or gastric perforation, the gastrointestinal and thoracic surgery teams recommended ongoing conservative treatment. On the 5th day, HBOT was initiated, involving mask oxygen inhalation at a pressure of 1.6-2.2 atmospheres absolute for 60-70 minutes per session, and was administered over nine consecutive days.
By the 11th day of hospitalization, the patient’s stool color returned to normal. She was discharged in stable condition after a 16-day hospital stay, and no signs of bleeding were observed at the 30-day follow-up.
This case was diagnosed as MWS, notable for the extensive tear in the gastric fundus, involving a near full-thickness rupture of the muscular layer. Endoscopic treatment was considered unsuitable as sufficient inflation to visualize the wound could have worsened the tear and increased the risk of perforation. Additionally, the wound’s mucosal edges were highly congested and swollen, posing a risk of further damage when using hemostatic clips, which could potentially shift and fail to achieve a secure closure. Other closure devices, such as over-the-scope clips, were also deemed ineffective due to the elongated nature of the wound. Unlike small circular defects, this type of injury could result in the detached gastric wall folding, preventing effective closure.
Given the ongoing bleeding despite conservative treatment and the lack of surgical indication, the patient proceeded with HBOT alongside proton-pump inhibitors. A follow-up endoscopy on the 7th day of HBOT revealed an almost complete absence of white coating on the ulcer surface, indicating substantial healing progress. In a study involving 70 patients who underwent endoscopic submucosal dissection, the artificial ulcers measured an average of 34.7 mm and were all in the active stage 7 days after treatment with proton pump inhibitors[9]. In contrast, the laceration wound in this case was both larger and deeper than the postoperative wound described in the aforementioned literature. However, it is noteworthy that the ulcer had entered the healing stage after a period of 7 days. Therefore, the combination of HBOT with conservative treatment proved to be more effective, this integrated approach facilitated a rapid cessation of bleeding and significantly accelerated the healing process of wounds.
Existing studies indicate that HBOT can enhance coagulation at wound sites by activating and recruiting platelets and upregulating tissue factor expression, thus supporting the clotting cascade[10,11]. Additionally, HBOT promotes wound healing by stimulating tissue formation, angiogenesis, and re-epithelialization[12,13]. This evidence supports the potential efficacy of HBOT in managing gastric tears with active bleeding. Nevertheless, as this report is based on a single case, further research is needed to establish its precise therapeutic value. Reviewing this patient’s treatment pathway suggests that for patients with gastric lacerations, particularly those who cannot undergo endoscopic intervention yet exhibit ongoing bleeding, integrating HBOT with conventional treatments offers a promising strategy. This combined approach can notably accelerate the healing of extensive ulcerative wounds and prevent further blood loss.
HBOT presents a promising alternative for managing patients with gastric tears and active bleeding, particularly when endoscopic intervention is unfeasible or has proven ineffective.
| 1. | Ansari A. Mallory-Weiss syndrome--revisted. Am J Gastroenterol. 1975;64:460-466. [PubMed] |
| 2. | Sugawa C, Benishek D, Walt AJ. Mallory-Weiss syndrome. A study of 224 patients. Am J Surg. 1983;145:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Yin A, Li Y, Jiang Y, Liu J, Luo H. Mallory-Weiss syndrome: clinical and endoscopic characteristics. Eur J Intern Med. 2012;23:e92-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Sen S, Sen S. Therapeutic effects of hyperbaric oxygen: integrated review. Med Gas Res. 2021;11:30-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 555] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 6. | Wu X, Liang TY, Wang Z, Chen G. The role of hyperbaric oxygen therapy in inflammatory bowel disease: a narrative review. Med Gas Res. 2021;11:66-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Yuan JH, Song LM, Liu Y, Li MW, Lin Q, Wang R, Zhang CS, Dong J. The Effects of Hyperbaric Oxygen Therapy on Pelvic Radiation Induced Gastrointestinal Complications (Rectal Bleeding, Diarrhea, and Pain): A Meta-Analysis. Front Oncol. 2020;10:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Boersema GSA, Wu Z, Kroese LF, Vennix S, Bastiaansen-Jenniskens YM, van Neck JW, Lam KH, Kleinrensink GJ, Jeekel J, Lange JF. Hyperbaric oxygen therapy improves colorectal anastomotic healing. Int J Colorectal Dis. 2016;31:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kakushima N, Yahagi N, Fujishiro M, Iguchi M, Oka M, Kobayashi K, Hashimoto T, Omata M. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc. 2004;16:327-331. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | André-Lévigne D, Modarressi A, Pepper MS, Pittet-Cuénod B. Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair. Int J Mol Sci. 2017;18:2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Imperatore F, Cuzzocrea S, De Lucia D, Sessa M, Rinaldi B, Capuano A, Liguori G, Filippelli A, Rossi F. Hyperbaric oxygen therapy prevents coagulation disorders in an experimental model of multiple organ failure syndrome. Intensive Care Med. 2006;32:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Lindenmann J, Kamolz L, Graier W, Smolle J, Smolle-Juettner FM. Hyperbaric Oxygen Therapy and Tissue Regeneration: A Literature Survey. Biomedicines. 2022;10:3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Huang X, Liang P, Jiang B, Zhang P, Yu W, Duan M, Guo L, Cui X, Huang M, Huang X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020;259:118246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |