Published online Jul 16, 2024. doi: 10.4253/wjge.v16.i7.396
Revised: May 17, 2024
Accepted: June 13, 2024
Published online: July 16, 2024
Processing time: 132 Days and 8 Hours
The functional lumen imaging probe (FLIP) is a Food and Drug Administration approved tool to aid the diagnosis and management of esophageal disorders. However, widespread adoption of FLIP remains limited and its utility in high-volume practices remains unclear.
To analyze large sample data on clinical use of FLIP and provide insight on seve
We conducted a retrospective comparative and descriptive analysis of FLIP proce
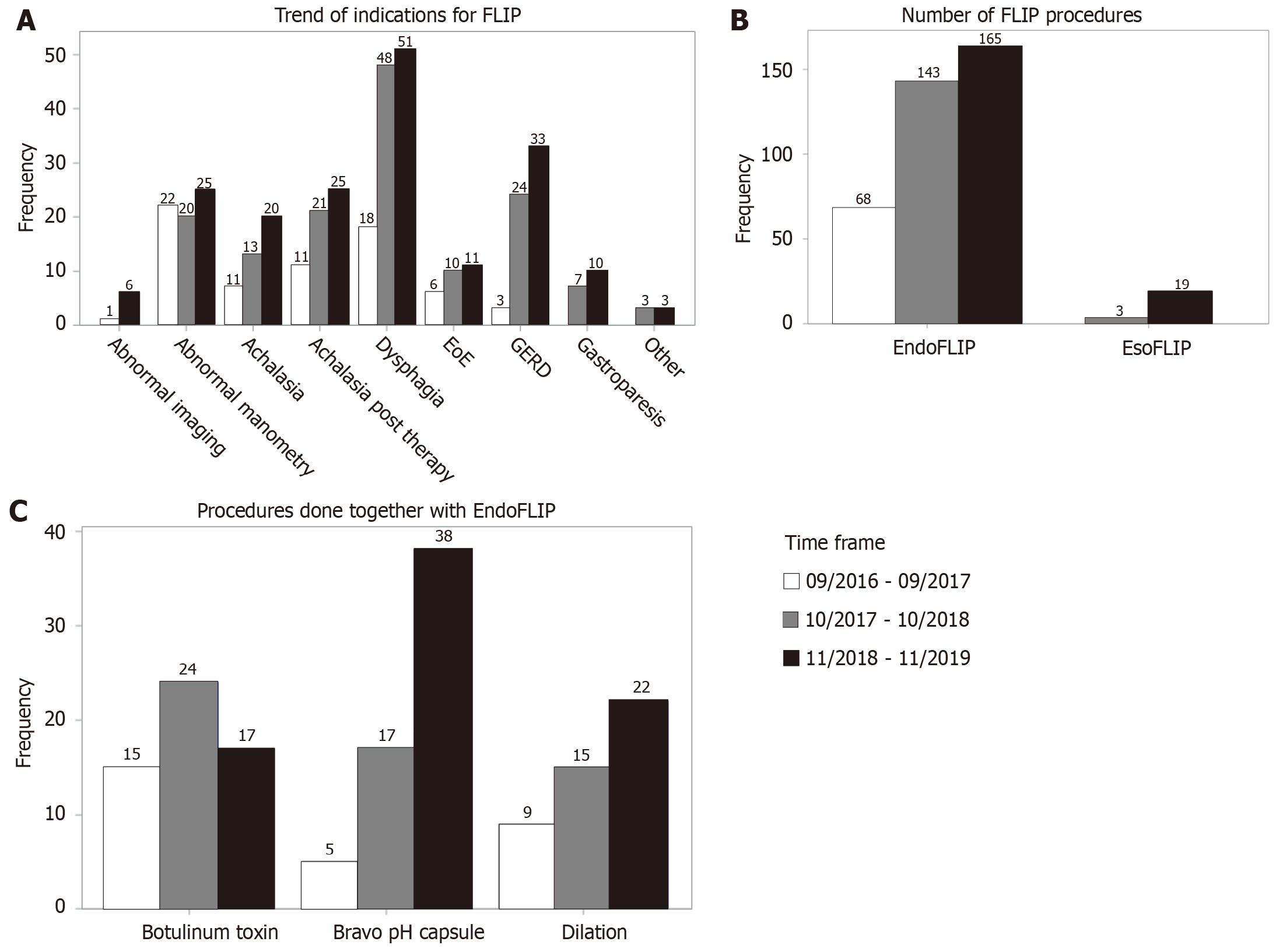
There was an increase in FLIP cases with each successive time period of 13 mon
FLIP cases have increased in our practice with expanding indications for its use. Given limited normative data, providers should be aware of several potential technical issues, including the possible impact of sedation choice when assessing esophageal motility patterns.
Core Tip: In this study, we provide both a large sample analysis on functional lumen imaging probe (FLIP) use in our practi
- Citation: Jiang Y, Vazquez-Reyes R, Kamal A, Zikos T, Triadafilopoulos G, Clarke JO. Functional lumen imaging probe use in a high-volume practice: Practical and technical implications. World J Gastrointest Endosc 2024; 16(7): 396-405
- URL: https://www.wjgnet.com/1948-5190/full/v16/i7/396.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i7.396
The functional lumen imaging probe (FLIP) is a Food and Drug Administration approved tool to aid the diagnosis and management of esophageal disorders by measuring luminal diameter, cross sectional area (CSA), distensibility and motility. The American Gastroenterological Association Clinical Practice Committee concluded that FLIP assessment is a “complimentary tool to assess esophagogastric junction (EGJ) opening dynamics and the stiffness of the esophageal wall[1].” ACG clinical guidelines on use of esophageal physiology testing offered similar recommendations for FLIP in diagnosis of esophageal motility disorders[2].
The most useful FLIP metric has been the EGJ distensibility index (EGJ-DI), which is calculated by dividing the CSA at the site of interest by the intra-balloon pressure[3]. The EGJ-DI can be a useful measure of LES relaxation in a variety of scenarios including confirmation of EGJ outflow obstruction[4,5]. In addition, by visualizing esophageal diameter chan
We aim to provide large sample descriptive data on clinical use of FLIP in a high-volume academic practice. In addi
The study was approved by the Stanford University Institutional Review Board: 53329. The patient population consisted of patients who had FLIP as part of routine clinical care at Stanford Healthcare. There was a total of 398 FLIP procedures identified between September 2016 through November 2019. If a patient received another FLIP on subsequent occasions, these were counted as separate procedures/data points.
Patients received FLIP for a variety of indications. If multiple indications were listed, then the primary one was determined by the proceduralist upon chart review. If an indication was ambiguous based on symptoms, then chart review would determine which was the primary one. The primary indications were abnormal imaging, abnormal manometry, achalasia, achalasia post therapy, dysphagia, EoE, GERD, and gastroparesis. The indication was listed as “other” if it did not clearly fit into primary categories (e.g., these were amyloidosis, bloating, dissection, diverticulum, scleroderma). If a symptom was listed for indication, then it would be categorized into an appropriate indication if no disease process was also mentioned.
FLIP was performed during upper endoscopy by a single provider. Standard FLIP procedures were performed to record diameter, pressure and cross-sectional area at 10 mL intervals up to 70 mL. Similar procedure protocols have been published previously[3,13,20]. Sedation type for the procedure was at the discretion of the proceduralist and consisted of either conscious sedation (e.g., midazolam and fentanyl) vs monitored anesthesia care (e.g., propofol). Subsequently, a retrospective chart review of these procedures was done for analysis. Patient medical records were reviewed and data regarding demographics and procedural details were collected. Statistical tests, including χ2 tests, t-test, and multivariable logistic and linear regression, were performed using SAS (version 9.4).
For a descriptive measure, FLIP was separated as either diagnostic or therapeutic (EsoFLIP). For diagnostic procedures, all FLIP measurements recorded for analysis purposes were of the gastroesophageal junction. For those with indications of gastroparesis, pyloric FLIP was done but measurements and motility patterns were not included in comparative analyses given the different anatomical sites. Additional upper GI procedures done in conjunction with diagnostic FLIP on the same procedure encounter were also recorded, including wireless pH monitoring, non-EsoFLIP dilation and botulinum toxin injection.
For each procedure, the FLIP balloon type used was identified. There are two available diagnostic balloons, EF-325 (8 cm in length, 50 mL max volume distention) and EF-322 (16 cm in length, 72 mL max volume distention). The catheter used (16 cm vs 8 cm) was chosen at the discretion of the proceduralist. FLIP motility patterns, when available, were also collected from procedure reports. These patterns were initially recorded intra-procedure by endoscopist. Patients were classified as having recurrent antegrade contractions, isolated antegrade contractions, retrograde contractions or absent motility.
We identified 398 FLIP procedures over the course of 3 years. The mean age of patients was 55 years and the majority were Caucasian females (Table 1). A third of patients had prior history of foregut surgery and 16.3% were on opioid medications at the time of the procedure. The type of sedation was almost split equally amongst the procedures with 52.5% of the procedures using anesthesia. Among those who received moderate sedation, the median medication doses were midazolam 5 mg and fentanyl 125 mcg. Average procedure time was 21.4 min with anesthesia and 17.0 min with moderate sedation (P < 0.01).
| Characteristics | Patient details (n = 398) |
| Age (years) | 55.1 ± 16.3 |
| Sex | |
| Male | 148 (37.2) |
| Female | 250 (62.8) |
| Race | |
| White | 240 (60.3) |
| Black | 14 (3.5) |
| Hispanic | 40 (10.1) |
| Asian | 47 (11.8) |
| Other | 49 (12.3) |
| Unknown | 8 (2.0) |
| Opioid use | 65 (16.3) |
| Prior foregut surgery | 132 (33.2) |
| Anesthesia use | 209 (52.5) |
| Moderate sedation | 189 (47.5) |
| Midazolam dose (median) (mg) | 5 |
| Fentanyl dose (median) (mcg) | 125 |
| Procedure time (minutes)a | 21.4 ± 8.8 |
| Moderate sedation | 17.0 ± 6.3 |
| Major complications1 | 0 |
With each successive time period of 13 months, there was a rise in number of FLIP procedures, with notable rises specifically for indications of dysphagia and GERD (Figure 1A and B). This pattern was in conjunction with an increase in outside referrals. There were 22 EsoFLIP dilations done during this time period. Many procedures (42.8%) were perfor
For the 381 esophageal FLIP procedures, the rates of sedation type were similar (51.4% anesthesia vs 48.6% moderate sedation; Table 2). Median moderate sedation doses employed were 5 mg midazolam and 125 mcg fentanyl. There were differences in procedure indication between groups (P = 0.01), with more patients with EoE having moderate sedation. More patients on baseline opioids underwent anesthesia (P < 0.01). After adjusting for indication and balloon type, procedure time was 4.4 minutes longer, on average, in the anesthesia group (P < 0.01). All but one EsoFLIP dilation were done with anesthesia support.
| Anesthesia 196 (51.4) | Moderate sedation 185 (48.6) | |
| Midazolam dose (median), 5 mg | ||
| Fentanyl dose (median), 125 mcg | ||
| Patient age | 56.6 ± 16.5 | 54.0 ± 16.0 |
| Sex | ||
| Male | 64 (32.7) | 77 (41.6) |
| Female | 132 (67.3) | 108 (58.4) |
| Race | ||
| White | 124 (63.3) | 106 (57.3) |
| Black | 5 (2.6) | 9 (4.9) |
| Hispanic | 22 (11.2) | 16 (8.7) |
| Asian | 20 (10.2) | 25 (13.5) |
| Other | 21 (10.7) | 25 (13.5) |
| Unknown | 4 (2.0) | 4 (2.2) |
| Procedure indicationa | ||
| Abnormal imaging | 5 (2.6) | 2 (1.1) |
| Abnormal manometry | 31 (15.8) | 36 (19.5) |
| Achalasia | 26 (13.3) | 14 (7.6) |
| Achalasia post therapy | 29 (14.8) | 28 (15.1) |
| Dysphagia | 63 (32.1) | 54 (29.2) |
| EoE | 7 (3.6) | 20 (10.8) |
| GERD | 29 (14.8) | 31 (16.8) |
| Other | 6 (3.1) | 0 (0) |
| Patient opioid usea | 46 (23.5) | 17 (9.2) |
| Prior foregut surgery | 73 (37.2) | 53 (28.7) |
| Procedure time (mean min)a | 21.4 ± 8.8 | 17.0 ± 6.4 |
| Procedure typea | ||
| EndoFLIP | 175 (89.3) | 184 (99.5) |
| EsoFLIP | 21 (10.7) | 1 (0.05) |
There was a slight statistical difference in diameter measurement at 60 cc balloon distention between groups (11.5 mm vs 12.3 mm), but this trend was not seen in any other static measurements or at other distention volumes (Table 3). After adjusting for procedural indication, opioids on medication list and type of balloon, those who had anesthesia were less likely to have recurrent antegrade contractions [odds ratio (OR) = 0.4, 95%CI: 0.3-0.8]. They were also more likely to have absent contractility (OR = 2.4, 95%CI: 1.3-4.4).
| Measurement at distention volume | Anesthesia | Moderate sedation |
| DI (mm2/mmHg) | ||
| 30 | 2.3 ± 2.0 | 2.1 ± 1.5 |
| 40 | 2.6 ± 2.2 | 2.5 ± 2.0 |
| 50 | 3.2 ± 2.4 | 3.0 ± 2.2 |
| 60 | 4.0 ± 2.9 | 3.6 ± 2.3 |
| 70 | 4.2 ± 2.4 | 3.9 ± 2.1 |
| Diameter (mm) | ||
| 30 | 6.8 ± 2.4 | 6.9 ± 2.0 |
| 40 | 8.1 ± 3.1 | 8.4 ± 3.0 |
| 50 | 10.2 ± 4.1 | 10.7 ± 3.8 |
| 60a | 11.5 ± 3.3 | 12.3 ± 3.4 |
| 70 | 14.6 ± 3.3 | 15.1 ± 3.6 |
| Motility | ||
| RAC1 | 65 (42.5) | 88 (57.5) |
| IAC | 10 (6.8) | 9 (6.4) |
| RC | 19 (12.9) | 16 (11.4) |
| Absent2 | 63 (42.9) | 38 (27.0) |
Most diagnostic FLIP procedures (70.4%) were done using the EF-322 balloon (Table 4). There was an increase in EF-322 procedures over time with a decrease in EF-325 procedures (P < 0.01). There were differences in procedure indication, with more GERD evaluations performed using EF-322 and more post therapy achalasia and prior foregut surgery evaluations done with EF-325 (49.5% vs 26.4%, P < 0.01). Procedure sedation type and moderate sedation dosages were similar between the two groups. After adjusting for sedation type, indication, prior foregut surgery and opiate use, balloon type did not affect procedure length (P = 0.49). The variation in FLIP parameters, by balloon type, are shown in Table 5.
| Balloon | EF-322 (n = 254, 70.4%) | EF-325 (n = 107, 29.6%) |
| Procedure datea | ||
| 9/2017-9/2018 | 10 (3.9) | 58 (54.2) |
| 10/2017-10/2018 | 97 (38.2) | 39 (36.5) |
| 11/2018-11/2019 | 147 (57.9) | 10 (9.4) |
| Patient details | ||
| Age | 54.7 ± 16.0 | 56.0 ± 16.9 |
| Sex | ||
| Male | 92 (36.2) | 41 (38.3) |
| Female | 162 (63.8) | 66 (61.7) |
| Race | ||
| White | 144 (56.7) | 73 (68.2) |
| Black | 10 (3.9) | 4 (3.7) |
| Hispanic | 28 (11.0) | 8 (7.5) |
| Asian | 35 (13.8) | 7 (6.5) |
| Other | 31 (12.2) | 13 (12.2) |
| Unknown | 6 (2.4) | 2 (1.9) |
| Indicationa | ||
| Abnormal imaging | 6 (2.4) | 1 (0.9) |
| Abnormal manometry | 38 (15.0) | 29 (27.1) |
| Achalasia pre therapy | 17 (6.7) | 10 (9.4) |
| Achalasia post therapy | 26 (10.2) | 25 (23.4) |
| Dysphagia | 86 (33.9) | 30 (28.0) |
| EoE | 22 (8.7) | 5 (4.7) |
| GERD | 53 (20.9) | 7 (6.5) |
| Other | 6 (2.4) | 0 (0) |
| Opioid use | 43 (16.9) | 18 (16.8) |
| Prior foregut surgerya | 67 (26.4) | 53 (49.5) |
| Procedure details | ||
| Anesthesia use | 127 (50) | 49 (45.8) |
| Moderate sedation | 127 (50) | 58 (54.2) |
| Midazolam dose (mg), (median) | 5 | 6 |
| Fentanyl dose (mcg), (median) | 125 | 125 |
| Procedure time (minute) | ||
| Anesthesia | 22.3 ± 7.6 | 22.0 ± 11.4 |
| Moderate sedation | 17.5 ± 5.7 | 15.9 ± 7.6 |
| Distention volume (mL) | Balloon used | |
| EF-322 | EF-325 | |
| DI (mm2/mmHg) | ||
| 30 | 1.93 ± 1.18a | 2.66 ± 2.24 |
| 40 | 2.32 ± 1.65a | 3.17 ± 2.83b |
| 50 | 3.05 ± 2.27a | 3.27 ± 2.41b |
| 60 | 3.79 ± 2.58a | |
| 70 | 4.03 ± 2.25a | |
| Diameter (mm)c | ||
| 30 | 6.07 ± 1.62 | 8.01 ± 2.51 |
| 40 | 7.23 ± 2.42 | 10.58 ± 3.15 |
| 50 | 9.29 ± 3.20 | 13.53 ± 4.13 |
| 60 | 11.92 ± 3.36 | |
| 70 | 14.84 ± 3.45 | |
| CSA (mm2)c | ||
| 30 | 30.97 ± 18.24 | 55.26 ± 35.13 |
| 40 | 45.71 ± 30.82 | 95.57 ± 53.83 |
| 50 | 75.78 ± 48.13 | 157.19 ± 86.11 |
| 60 | 120.18 ± 61.24 | |
| 70 | 182.75 ± 75.45 | |
FLIP has been a key component of our esophageal practice and its use has grown steadily from 2016 through 2019, with increased outside referrals representing more awareness of FLIP in the our community. Indications have changed with time, with earlier studies performed more for patients with achalasia and follow-up of abnormal esophageal manometries, whereas more recently, there has been an increase in use for patients with dysphagia and GERD. Especially with addition of real time panometry, FLIP can theoretically be a more valuable tool for initial evaluation of dysphagia as it can be done at time of endoscopy. Prospective studies are needed to determine if FLIP performed with index endos
In our current clinical practice, FLIP is often performed in conjunction with other tests or treatments, with 42.8% percent of FLIP being done in the same endoscopy as wireless pH testing, botulinum toxin injection and/or esophageal dilation in our study. The addition of FLIP does not add much time to endoscopy, as previously demonstrated[20]. Its safety has been excellent, with no major complications. Of the 398 procedures, there were 3 post procedure visits to the emergency department, with complications ruled out at that time.
Given the large sample size of this study’s FLIP cohort, we aimed to examine two technical issues which have had limited published data so far. Given the potential impact of endoscopic sedation on esophageal motility, we examined the differences in FLIP parameters based on sedation type. At our institution, this is most commonly propofol when anesthesia is utilized vs midazolam/fentanyl when conscious sedation is used. After adjusting for procedure indication, patient opioid use, and type of balloon used in our regression algorithm, sedation type did not seem to have a meaningful impact on measurements of distensibility or diameter. However, interestingly, even when these factors were adjusted, we noted that those who underwent anesthesia compared to moderate sedation were less likely to exhibit normal motility patterns on FLIP. Though not looking directly at the same issue, this is seemingly in contrast to the study by Carlson et. al in which 21% of cases performed with monitored anesthesia care in which differences between patterns of esophageal manometry peristalsis and FLIP panometry were not observed related to sedation type[6]. Though we did try to statistically control for some confounders in our data, it is possible that the increase in abnormal motility in patients undergoing anesthesia represents a selection bias wherein patients with increased disease burden were selectively referred for anesthesia over conscious sedation. However, there is no evidence from our data that dysmotility was more likely be seen in patients receiving periprocedural opiates (in the form of conscious sedation), which was our initial concern leading to this analysis.
We also found that EF-322 use has increased over time. Though there is some selection bias, this is likely reflective of increasing adoption of esophageal peristalsis measurements. The EF-325 catheter was still used limitedly for some indications such as symptoms after achalasia therapy and follow-up from prior abnormal studies incorporating the shorter balloon. The EF-325 catheter was also used exclusively for pyloric evaluation; however, that data was not includ
Though our study includes both descriptive and comparative analyses of practical and technical aspects of FLIP in a large cohort, there are still several limitations. First, this is a retrospective study and is subject to selection bias as discussed previously. Though having a single provider performing FLIP procedures reduces variability and increases internal validity, the patterns of FLIP use we present may vary from other high-volume practices. We also do not have manometric data available to correlate with our FLIP panometry patterns. Though we do control for certain potential confounders in our regression model, having correlation with manometry findings would strengthen our findings of altered contractile patterns based on sedation type. Regardless, we do feel this is a potentially important finding and warrants further evaluation.
In conclusion, FLIP use has increased over the past several years with evaluation of dysphagia becoming increasingly more common among other expanding indications. Given limited normative data, providers should be aware of several potential technical issues, including the possible impact of sedation choice when assessing esophageal motility patterns on FLIP. As this technology gains more awareness in the community, more outcome-based studies will be needed to evaluate both its utility in early evaluation of dysphagia as well as expanding indications for its use.
| 1. | Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Gyawali CP, Carlson DA, Chen JW, Patel A, Wong RJ, Yadlapati RH. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol. 2020;115:1412-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 3. | Savarino E, di Pietro M, Bredenoord AJ, Carlson DA, Clarke JO, Khan A, Vela MF, Yadlapati R, Pohl D, Pandolfino JE, Roman S, Gyawali CP. Use of the Functional Lumen Imaging Probe in Clinical Esophagology. Am J Gastroenterol. 2020;115:1786-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Ponds FA, Bredenoord AJ, Kessing BF, Smout AJ. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, Babaei A, Mittal RK, Rommel N, Savarino E, Sifrim D, Smout A, Vaezi MF, Zerbib F, Akiyama J, Bhatia S, Bor S, Carlson DA, Chen JW, Cisternas D, Cock C, Coss-Adame E, de Bortoli N, Defilippi C, Fass R, Ghoshal UC, Gonlachanvit S, Hani A, Hebbard GS, Wook Jung K, Katz P, Katzka DA, Khan A, Kohn GP, Lazarescu A, Lengliner J, Mittal SK, Omari T, Park MI, Penagini R, Pohl D, Richter JE, Serra J, Sweis R, Tack J, Tatum RP, Tutuian R, Vela MF, Wong RK, Wu JC, Xiao Y, Pandolfino JE. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil. 2021;33:e14058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 575] [Article Influence: 143.8] [Reference Citation Analysis (1)] |
| 6. | Carlson DA, Baumann AJ, Prescott JE, Donnan EN, Yadlapati R, Khan A, Gyawali CP, Kou W, Kahrilas PJ, Pandolfino JE. Validation of secondary peristalsis classification using FLIP panometry in 741 subjects undergoing manometry. Neurogastroenterol Motil. 2022;34:e14192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Carlson DA, Gyawali CP, Khan A, Yadlapati R, Chen J, Chokshi RV, Clarke JO, Garza JM, Jain AS, Katz P, Konda V, Lynch K, Schnoll-Sussman FH, Spechler SJ, Vela MF, Prescott JE, Baumann AJ, Donnan EN, Kou W, Kahrilas PJ, Pandolfino JE. Classifying Esophageal Motility by FLIP Panometry: A Study of 722 Subjects With Manometry. Am J Gastroenterol. 2021;116:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Donnan EN, Pandolfino JE. EndoFLIP in the Esophagus: Assessing Sphincter Function, Wall Stiffness, and Motility to Guide Treatment. Gastroenterol Clin North Am. 2020;49:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Carlson DA, Lin Z, Kahrilas PJ, Sternbach J, Donnan EN, Friesen L, Listernick Z, Mogni B, Pandolfino JE. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015;149:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 10. | Carlson DA, Kahrilas PJ, Lin Z, Hirano I, Gonsalves N, Listernick Z, Ritter K, Tye M, Ponds FA, Wong I, Pandolfino JE. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol. 2016;111:1726-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Carlson DA, Gyawali CP, Kahrilas PJ, Triggs JR, Falmagne S, Prescott J, Dorian E, Kou W, Lin Z, Pandolfino JE. Esophageal motility classification can be established at the time of endoscopy: a study evaluating real-time functional luminal imaging probe panometry. Gastrointest Endosc. 2019;90:915-923.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Su B, Callahan ZM, Kuchta K, Linn JG, Haggerty SP, Denham W, Ujiki MB. Use of Impedance Planimetry (Endoflip) in Foregut Surgery Practice: Experience of More than 400 Cases. J Am Coll Surg. 2020;231:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Carlson DA, Kou W, Lin Z, Hinchcliff M, Thakrar A, Falmagne S, Prescott J, Dorian E, Kahrilas PJ, Pandolfino JE. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clin Gastroenterol Hepatol. 2019;17:674-681.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Desprez C, Roman S, Leroi AM, Gourcerol G. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP® ) in the gastrointestinal tract: A systematic review. Neurogastroenterol Motil. 2020;32:e13980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Kwiatek MA, Kahrilas K, Soper NJ, Bulsiewicz WJ, McMahon BP, Gregersen H, Pandolfino JE. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. 2010;14:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 18. | Tucker E, Sweis R, Anggiansah A, Wong T, Telakis E, Knowles K, Wright J, Fox M. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013;25:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Ahuja NK, Agnihotri A, Lynch KL, Hoo-Fatt D, Onyimba F, McKnight M, Okeke FC, Garcia P, Dhalla S, Stein E, Pasricha PJ, Clarke JO. Esophageal distensibility measurement: impact on clinical management and procedure length. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |