Published online Jun 16, 2024. doi: 10.4253/wjge.v16.i6.368
Revised: April 30, 2024
Accepted: May 21, 2024
Published online: June 16, 2024
Processing time: 69 Days and 3.4 Hours
Duodenal Brunner's gland hyperplasia (BGH) is a therapeutic target when complications such as bleeding or gastrointestinal obstruction occur or when malignancy cannot be ruled out. Herein, we present a case of large BGH treated with endoscopic mucosal resection (EMR).
An 83-year-old woman presented at our hospital with dizziness. Blood tests revealed severe anemia, esophagogastroduodenoscopy showed a 6.5 cm lesion protruding from the anterior wall of the duodenal bulb, and biopsy revealed the presence of glandular epithelium. Endoscopic ultrasonography (EUS) demonstr
Large duodenal lesions with good endoscopic maneuverability and no evident muscular layer involvement on EUS may be resectable via EMR.
Core Tip: This is a report of a large Brunner’s gland hyperplasia in an older female patient with anemia that was successfully resected en bloc using endoscopic mucosal resection (EMR). After obtaining information on the base of the lesion as well as on the internal characteristics using endoscopic ultrasonography, we chose EMR as a minimally invasive treatment.
- Citation: Makazu M, Sasaki A, Ichita C, Sumida C, Nishino T, Nagayama M, Teshima S. Giant Brunner's gland hyperplasia of the duodenum successfully resected en bloc by endoscopic mucosal resection: A case report. World J Gastrointest Endosc 2024; 16(6): 368-375
- URL: https://www.wjgnet.com/1948-5190/full/v16/i6/368.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i6.368
Brunner's glands are exocrine glands located in the duodenum, extending from the mucosal to the submucosal layer, which secrete alkaline mucus. Brunner’s gland hyperplasia (BGH) is characterized by the proliferation of Brunner’s gland without histological atypia. There are proposals to classify relatively large proliferative disorders of Brunner's glands as Brunner’s gland hamartomas and smaller ones as BGH; however, there is no established consensus[1,2]. Since the term “hamartoma” is traditionally used for congenital disorders, we have used the term “Brunner’s gland hyperplasia” in our study.
BGH is targeted for treatment when complications such as bleeding or gastrointestinal obstruction occur or when the existence of a malignant component cannot be ruled out. Although surgery is often the preferred treatment for large lesions, several recent reports have described endoscopic submucosal dissection (ESD)[3]. However, ESD for duodenal lesions involves longer treatment time and a higher complication rate[4].
To the best of our knowledge, few reports on the criteria for large BGHs suitable for endoscopic mucosal resection (EMR) have been published. This report aims to present a case of large BGH treated with EMR, as well as to illustrate the criteria for determining the appropriateness of EMR.
An 83-year-old woman presented at our hospital with dizziness and anemia.
She had been complaining of dizziness for approximately two weeks.
Her medical history included dementia, diabetes mellitus, dyslipidemia, hypertension, and an appendectomy. The patient was not taking any antithrombotic drugs.
The personal and family history of the patient was unremarkable.
The patient’s vital signs were stable.
Laboratory tests revealed normocytic normochromic anemia with a hemoglobin level of 5.6 g/dL.
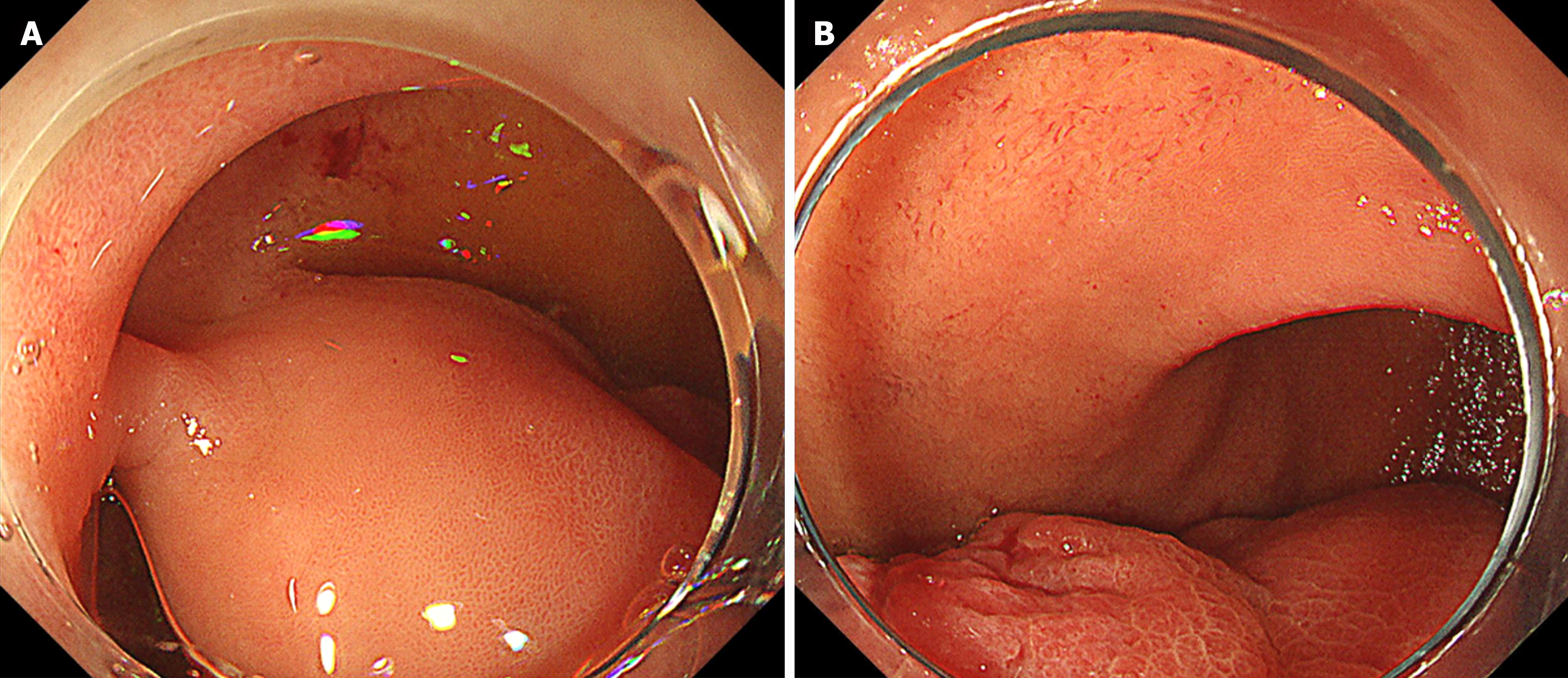
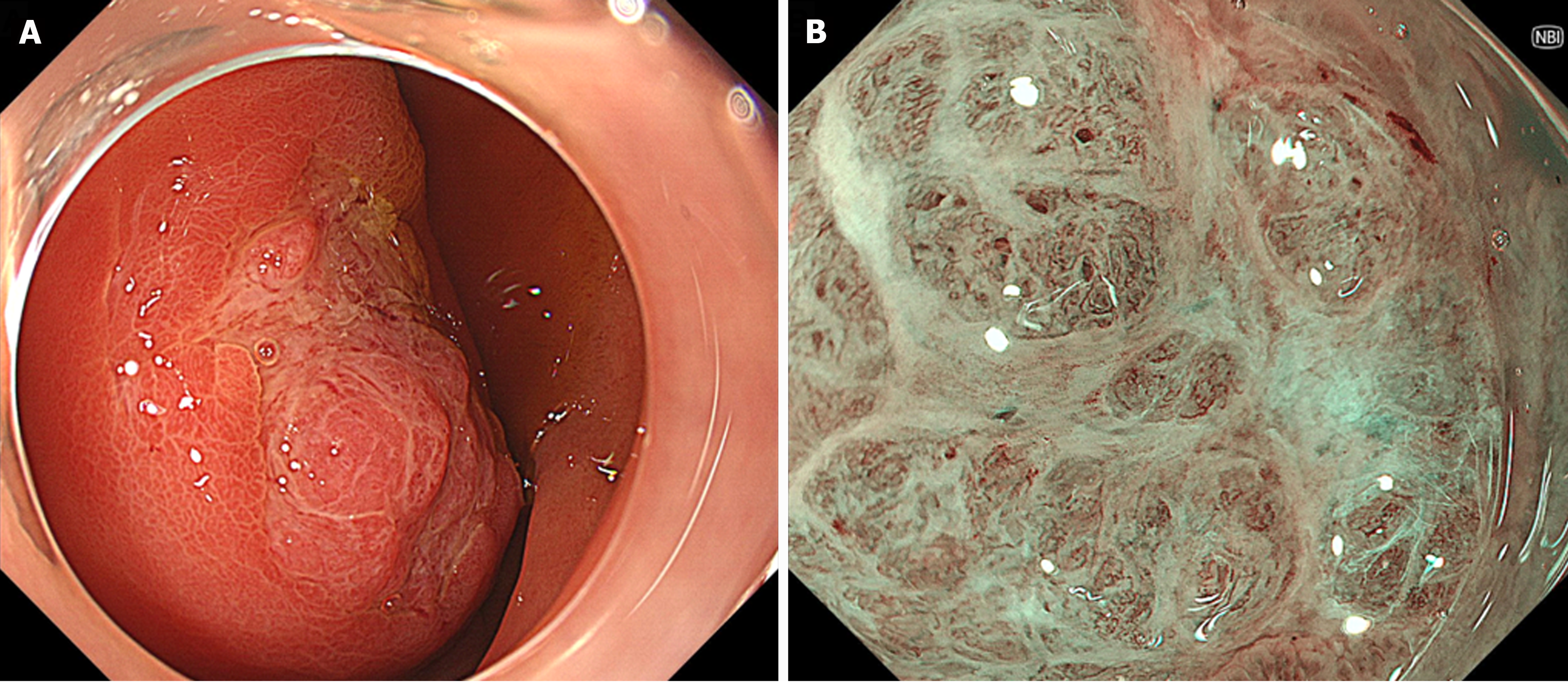
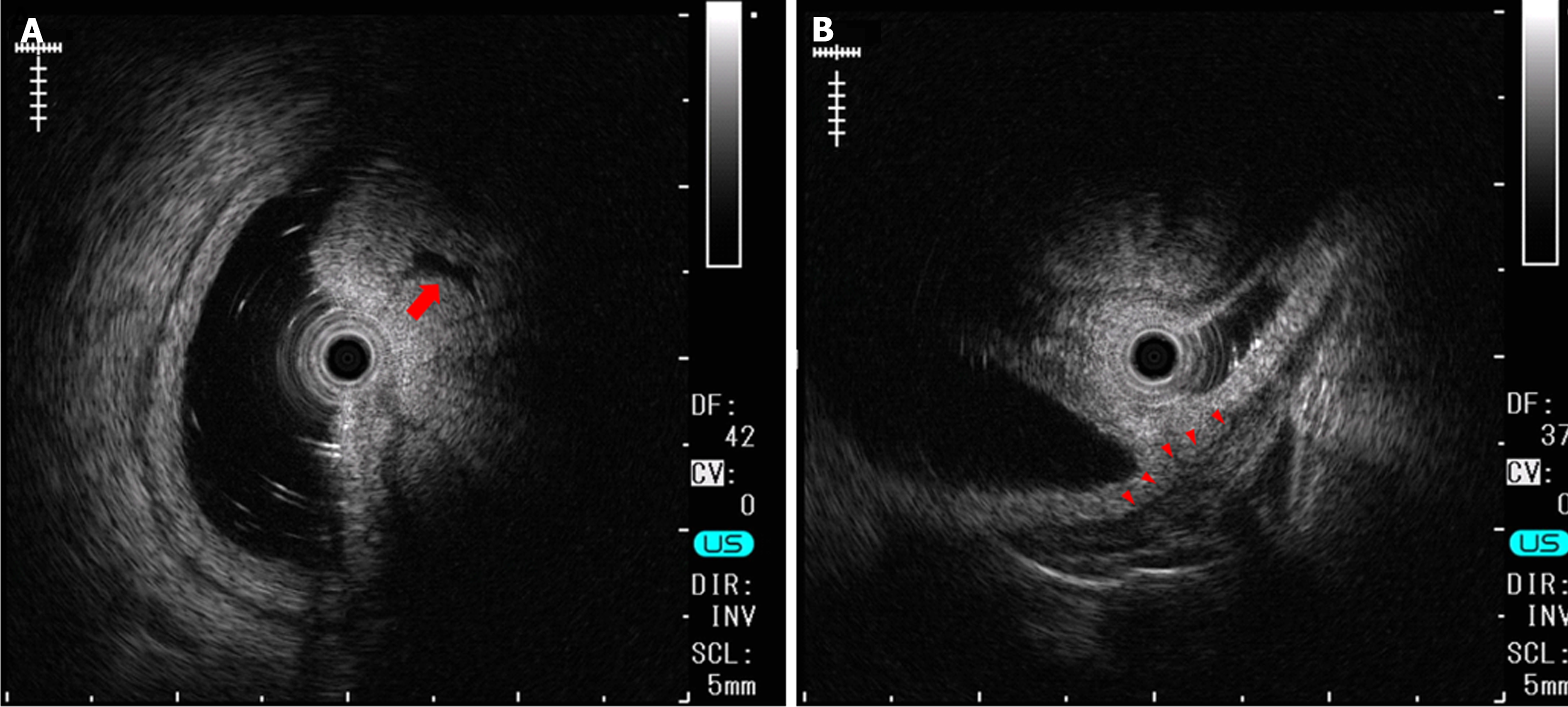
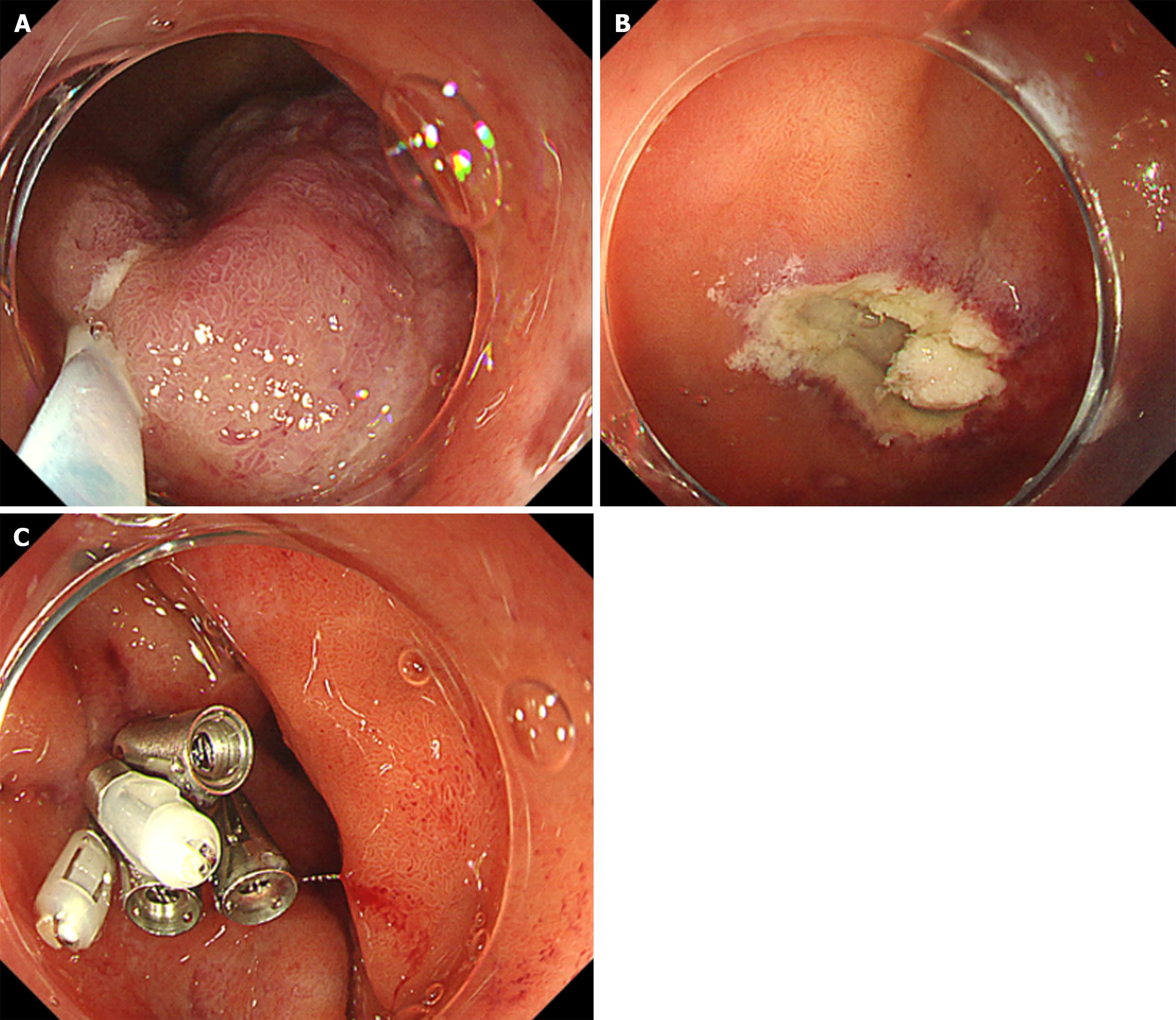
Esophagogastroduodenoscopy (EGD) identified a 6.5 cm lesion protruding from the anterior wall of the duodenal bulb (Figure 1A and B). The lesion surface was mostly covered with normal mucosa; however, a depression with a mucosal defect was observed in a portion of the lesion (Figure 2A). Magnified narrowband imaging of this depressed area revealed structures resembling small round glandular openings and band-like septa suggestive of fibrous components (Figure 2B). A boring biopsy of the depressed area revealed the presence of glandular epithelium. Endoscopic ultrasonography (EUS) revealed that the lesion had a relatively high echogenicity with an internal cystic component (Figure 3A). At the base of the lesion, a slight elevation of a low-echogenicity layer, suggestive of the muscular layer, was observed (Figure 3B).
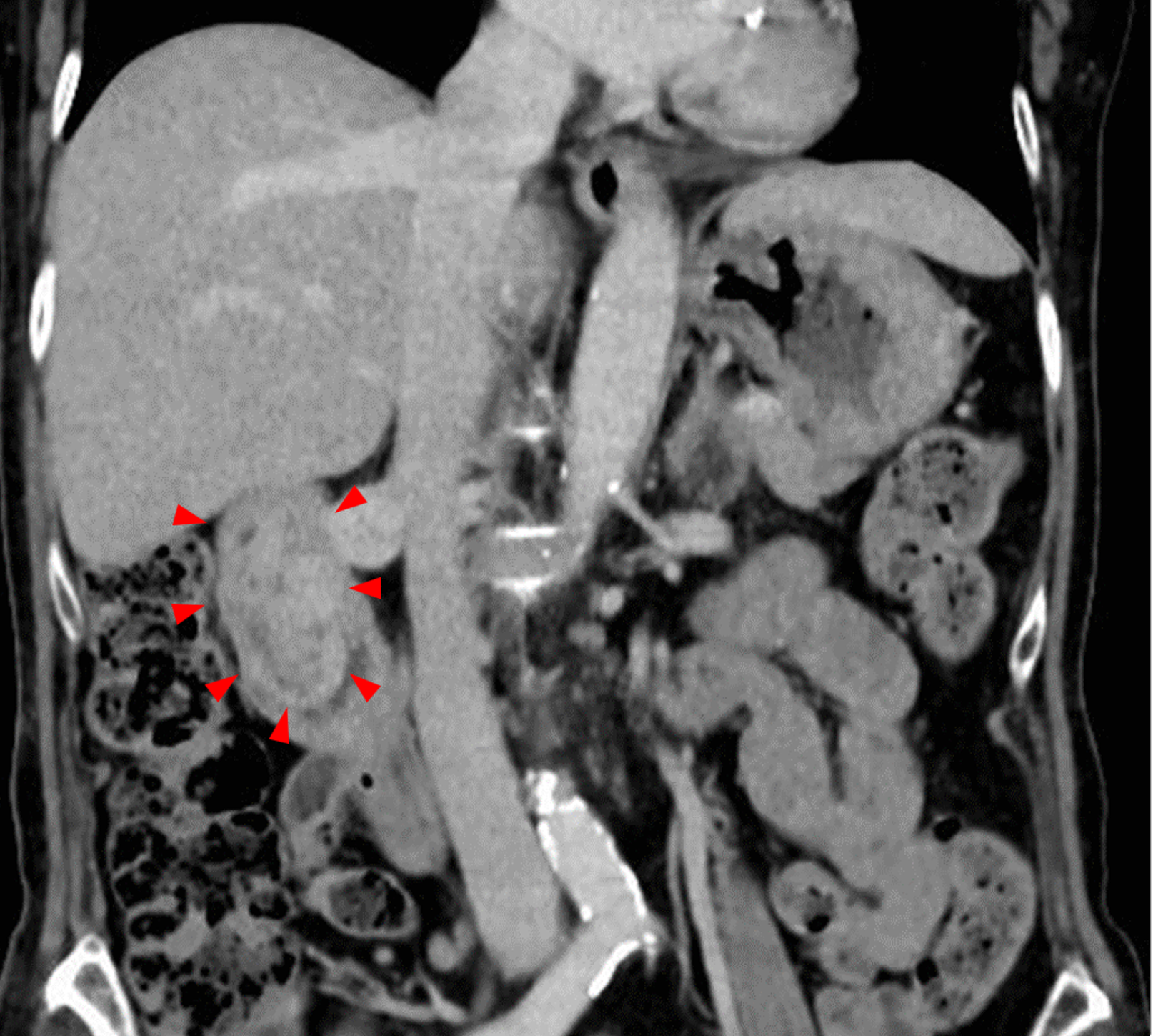
Abdominal contrast-enhanced computed tomography (CT) revealed a 7 cm protruding lesion extending from the bulb to the descending portion of the duodenum (Figure 4). The lesion showed minimal enhancement in the arterial phase and a mosaic-like enhancement pattern in the venous phase.
Differential diagnoses included BGH, gastrointestinal stromal tumor (GIST), and cancer. However, considering endoscopic morphology, biopsy results, and EUS findings, the likelihood of malignancy was low.
Upon considering the following conditions associated with the present case, we chose EMR as the treatment method: preference for a non-invasive approach due to the patient's advanced age; EUS and biopsy findings suggesting a low probability of malignancy; the relatively favorable maneuverability of the endoscope; the lesion having a wide base but being thin, with visibility across its entire circumference upon endoscopic examination; and minimal retraction of the muscle layer into the lesion as observed on EUS.
We injected hypertonic saline epinephrine solution at the base of the lesion and grasped it with a snare measuring 33 mm in diameter (Captivator II; Boston Scientific, Tokyo, Japan). We attempted to excise the lesion using the ENDO CUT Q mode (effect, 3; cut duration, 2; cut interval, 4) of the high-frequency generator VIO300D (Erbe Elektromedizin, Tübingen, Germany); however, there was no electrical activation. We relocated the constriction site with the snare slightly away from the base. We attempted to resect the lesion again in the same ENDO CUT Q mode as before (Figure 5A). At this time, satisfactory electrical conduction was achieved, and the lesion was excised entirely. There were no macroscopic residual tumors on the ulcerated surface after lesion excision and no muscle layer injuries or perforations (Figure 5B). The excised lesion was grasped using a snare and extracted orally. The ulceration after excision was completely closed using endoclips (Figure 5C).
EGD on the day following treatment showed that the resection site was maintained with clip suturing, with no bleeding or perforation. The patient resumed diet on the second day after treatment and was discharged without any problems on the third day. Thereafter, the patient experienced no recurrence of anemia.
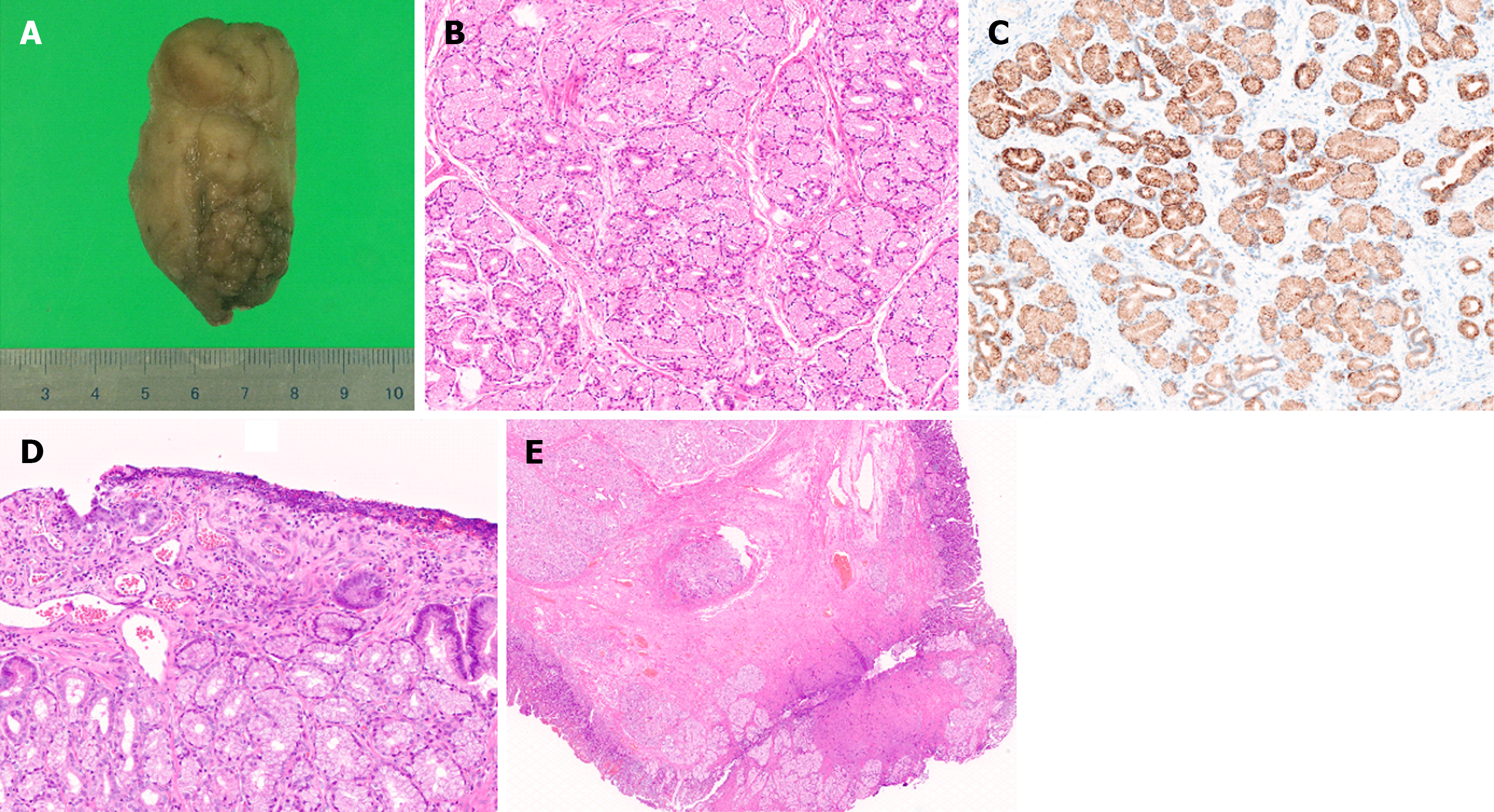
The size of the lesion after formalin fixation was 6 cm × 3.5 cm × 3 cm (Figure 6A). Histopathological examination revealed nodular proliferation of the Brunner's glands without atypia, partitioned by fibrous septa (Figure 6B). This site was positive for MUC6 (Figure 6C). The pathological diagnosis was BGH. The area observed as a depressed region during EGD showed superficial erosion and a regenerating epithelium (Figure 6D). At the resection margin, thickened muscularis mucosa, fibrosis in the submucosal layer, and normal Brunner's glands were observed, whereas the Brunner’s gland hyperplastic area was completely excised (Figure 6E).
We encountered a large BGH in an older female patient with anemia and successfully resected it en bloc using EMR. After obtaining information about the internal characteristics of the lesion and its base using EUS, we chose EMR as a minimally invasive treatment.
Several differential diagnoses should be considered when a subepithelial lesion (SEL) is identified in the duodenum. Malignant conditions include cancer and GIST, whereas benign conditions include lipomas and BGH. The diagnostic rate of boring biopsy for gastrointestinal SEL has been reported to be approximately 25%–65%[5,6]. EUS-guided sampling has been reported to account for approximately 60%–70% of EUS-guided fine-needle aspiration (FNA) and 80%–90% of EUS-guided fine–needle biopsy (FNB)[7,8]. In the present case, we did not perform EUS-FNA/FNB after obtaining the pathological result of "glandular epithelium" on a boring biopsy. This is because the EUS findings inside the lesion did not strongly suggest the presence of a malignant disease.
EUS can delineate the origin layer and the internal characteristics of SELs. This information helps hypothesize the nature of such SEL. BGH originating from the third layer is often depicted on EUS. In the present case, the lesion originated in the third layer. In terms of the EUS image of BGH, Hizawa et al[9] described, in a summary of six cases of BGH, that the EUS features of Brunner’s gland hamartoma are of a heterogeneous solid or cystic mass within the submucosa. Additionally, in their study of EUS findings in 10 cases of BGH, Lee et al[10] classified them into three types: (1) Heterogeneous appearance with large cysts; (2) heterogeneous echogenicity without cystic lesions; and (3) heterogeneous mixed echogenicity with very small cysts. They stated that these findings reflect diverse histological images of the BGH. In the present case, the lesion exhibited relatively high echogenicity on EUS with internal cystic components. This finding is consistent with previously reported EUS findings of BGH.
Methods of BGH resection include surgery and endoscopic treatment. Although numerous cases of BGH resection have been reported, there are no established guidelines for selecting the appropriate resection method. When the possibility of concomitant malignancy cannot be ruled out or when endoscopic maneuverability is poor, surgery is considered the preferred approach to BGH treatment. Factors such as the patient's overall condition, comorbidities, and preferences also influenced the treatment choice. Historically, large BGHs have primarily been resected surgically[11]. In older adult patients, as in the present case, surgery may be excessively invasive. Compared to surgery, endoscopic treatment of duodenal SELs offers advantages such as less invasiveness, shorter hospital stays, and lower complication rates[12]. In recent years, there have been several reports of ESD for BGH[13]. ESD for non-ampullary duodenal epithelial tumors has a higher en bloc resection rate than EMR but is associated with longer treatment times and higher complication rates[14]. In the present case, we chose EMR for a large BGH. One of the bases for this decision was EUS finding of the lesion. Based on the EUS findings, it was possible to assess that the involvement of the muscle layer in the lesion was minimal. From this finding, we concluded that it was possible to safely perform resection with a snare by temporarily increasing the volume of the submucosal layer with hypertonic saline without causing thermal damage to the muscle layer. In practice, during the first snaring attempt, we were unable to achieve satisfactory electrical conduction; however, on the second attempt after repositioning the snare, we successfully achieved effective electrical conduction and were able to cut the lesion. The pathological specimen showed normal Brunner’s glands, submucosal fibrosis, and thickening of the muscularis mucosae at the resection margin. The "hardness" of the tissue due to these factors may have prevented adequate tissue strangulation by the snare, resulting in the inability to achieve effective electrical conduction on the first try. Based on this experience, we believe that if prior EUS confirms the absence of obvious muscular layer involvement, even if effective electrical conduction cannot be achieved with initial strangulation by the snare, it may be possible to resect the lesion by changing the snare position, considering the influence of duodenal specific structures such as Brunner's glands.
According to a search in MEDLINE, there were seven reported cases in which EMR or hot snare polypectomy was performed for BGH > 6 cm[15-21] (Table 1). Some of these were described as Brunner’s gland hamartomas or adenomas; however, based on the histopathological findings, they were all considered synonymous with BGH. Although some cases only reported the longest axis, the images suggested that the diameter of the base or neck of the lesion was less than 3 cm. EUS was performed in only one other case besides the present case, where the absence of muscle layer invasion was used to aid in the choice of treatment[20]. In cases meeting conditions such as relatively good endoscopic maneuverability, non-broad-based lesions, and lack of significant findings of muscular layer involvement on EUS, EMR could be a viable option even for large lesions.
| Ref. | Age | Sex | Location | EUS | Size (mm) | Interventional approach |
| Tai et al[15], 2001 | 62 | M | 1st | - | 105 | HSP |
| Stermer et al[16], 2006 | 20 | F | 2nd | - | 70 | HSP |
| Itaba et al[17], 2006 | 75 | F | 2nd-3rd | - | 60 × 9 | HSP |
| Chen et al[18], 2006 | 78 | F | 1st | - | 60 × 4 × 2 | HSP following endoclip |
| Kitagawa et al[19], 2018 | 64 | F | 1st | - | 70 × 20 | EMR following endoclip and endoloop |
| Pasetti et al[20], 2018 | 67 | M | 1st | + | 60 × 30 × 20 | HSP |
| Yi et al[21], 2019 | 63 | F | 1st | - | 70 × 30 × 16 | HSP following endoloop |
| Our case | 83 | F | 1st | + | 60 × 35 × 30 | EMR |
We encountered a large BGH in an older female patient with anemia that was successfully resected en bloc using EMR. By obtaining information about the internal characteristics of the lesion as well as its base through EUS, we were able to select a minimally invasive treatment option. Because BGH is a benign lesion, the possibility of minimally invasive treatments through imaging diagnosis, such as EUS, should be explored, especially in older adult patients.
We would like to thank Takashi Yao, Professor at Department of Human Pathology, Juntendo University Graduate School of Medicine, for his valuable comments on the pathological evaluation of this case.
| 1. | Collins K, Ligato S. Duodenal Epithelial Polyps: A Clinicopathologic Review. Arch Pathol Lab Med. 2019;143:370-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Patel ND, Levy AD, Mehrotra AK, Sobin LH. Brunner's gland hyperplasia and hamartoma: imaging features with clinicopathologic correlation. AJR Am J Roentgenol. 2006;187:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Atsumi K, Fujimasa I, Imachi K, Nakajima M, Mabuchi K, Tsukagoshi S, Chinzei T, Abe Y. Research and development on total artificial heart in University of Tokyo. Med Prog Technol. 1987;12:5-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501-12508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Ji JS, Lee BI, Choi KY, Kim BW, Choi H, Huh M, Chung WC, Chae HS, Chung IS. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV, Kim MK. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Inoue T, Okumura F, Sano H, Mizushima T, Tsukamoto H, Fujita Y, Ibusuki M, Kitano R, Kobayashi Y, Ishii N, Ito K, Yoneda M. Impact of endoscopic ultrasound-guided fine-needle biopsy on the diagnosis of subepithelial tumors: A propensity score-matching analysis. Dig Endosc. 2019;31:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Osoegawa T, Minoda Y, Ihara E, Komori K, Aso A, Goto A, Itaba S, Ogino H, Nakamura K, Harada N, Makihara K, Tsuruta S, Yamamoto H, Ogawa Y. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: A randomized cross-over study. Dig Endosc. 2019;31:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Hizawa K, Iwai K, Esaki M, Suekane H, Inuzuka S, Matsumoto T, Yao T, Iida M. Endosonographic features of Brunner's gland hamartomas which were subsequently resected endoscopically. Endoscopy. 2002;34:956-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Lee KJ, Park B, Kim HM. Endoscopic Ultrasonography Findings for Brunner's Gland Hamartoma in the Duodenum. Clin Endosc. 2022;55:305-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Okutomi Y, Kato T, Aizawa H, Endo Y, Kasahara N, Watanabe F, Noda H, Rikiyama T. Large Brunner's Gland Hyperplasia with Bleeding: A Case Report. Case Rep Surg. 2021;2021:8861308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Li C, Liang C, Wang X, Le M, Liu D, Tan Y. Safety and efficacy of surgical and endoscopic resection in the treatment of duodenal subepithelial lesions. Surg Endosc. 2022;36:4145-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Xiang Y, Liu J, Wang NY, Tang D, Wang L, Zou PX, Xu G, Huang Q. The Characteristics and Treatment Outcomes of 71 Duodenal Brunner's Gland Adenomas with Endoscopic Submucosal Dissection. Dig Dis. 2023;41:852-859. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Hoteya S, Furuhata T, Takahito T, Fukuma Y, Suzuki Y, Kikuchi D, Mitani T, Matsui A, Yamashita S, Nomura K, Kuribayashi Y, Iizuka T, Kaise M. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion. 2017;95:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Tai M, Yoshikawa I, Kume K, Murata I, Otsuki M. A large Brunner's gland hamartoma resected by endoscopic polypectomy. Gastrointest Endosc. 2001;53:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Stermer E, Elias N, Keren D, Rainis T, Goldstein O, Lavy A. Acute pancreatitis and upper gastrointestinal bleeding as presenting symptoms of duodenal Brunner's gland hamartoma. Can J Gastroenterol. 2006;20:541-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Itaba S, Iwasa T, Sadamoto Y, Nasu T, Misawa T, Nakamura K. An unusual duodenal polyp: pedunculated Brunner's glands hyperplasia. Gastrointest Endosc. 2006;63:1070-1; discussion 1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Chen YY, Su WW, Soon MS, Yen HH. Hemoclip-assisted polypectomy of large duodenal Brunner's gland hamartoma. Dig Dis Sci. 2006;51:1670-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kitagawa Y, Osumi H, Kawachi H, Yoshio T, Yoshimizu S, Horiuchi Y, Ishiyama A, Hirasawa T, Tsuchida T, Fujisaki J. Giant duodenal Brunner's gland hamartoma successfully treated via endoscopic mucosal resection. Arab J Gastroenterol. 2018;19:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Pasetti A, Capoferro E, Querzoli G, Montanari R, Bocus P. En Bloc Endoscopic Resection of Large Pedunculated Brunner's Gland Hamartoma: A Case Report. Case Rep Gastroenterol. 2018;12:344-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Yi L, Cheng Z, Qiu H, Yang J, Wang T, Liu K. A giant Brunner's gland hamartoma being treated as a pedunculated polyp: a case report. BMC Gastroenterol. 2019;19:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |