Published online Jun 16, 2024. doi: 10.4253/wjge.v16.i6.326
Revised: February 24, 2024
Accepted: May 8, 2024
Published online: June 16, 2024
Processing time: 145 Days and 0.7 Hours
Endoscopic submucosal dissection (ESD) for over 2 cm in size undifferentiated type (UD type) early gastric cancer (EGC) confined to the mucosa is not only challenging, but also long-term outcomes are not well known.
To evaluate the long-term outcomes of ESD done for UD type EGCs confined to the mucosa over 2 cm in size and compare the results with those where the lesions were less than 2 cm.
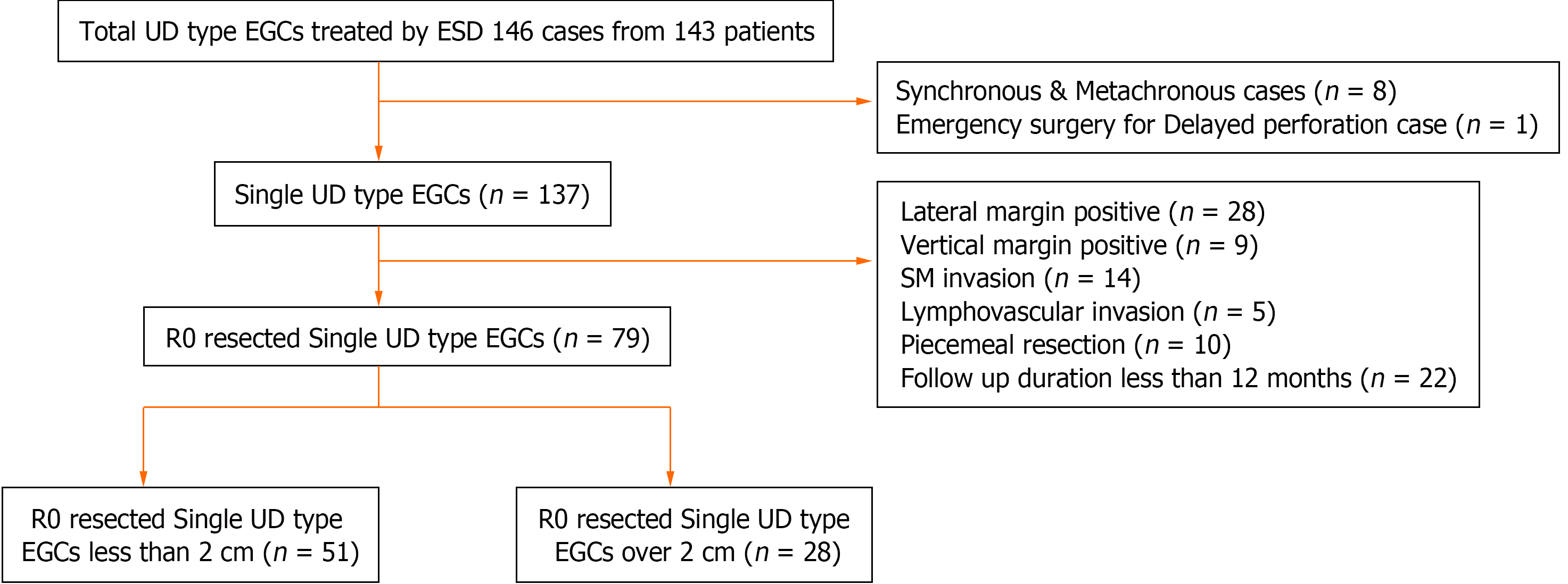
143 patients with UD type EGC confirmed on histology after ESD at a tertiary hospital were reviewed. Cases with synchronous and metachronous lesions and a case with emergency surgery after ESD were excluded. A total of 137 cases were enrolled. 79 cases who underwent R0 resection were divided into 2 cm or less (group A) and over 2 cm (group B) in size.
Among 79 patients who underwent R0 resection, the number in group A and B were 51 and 28, respectively. The mean follow-up period (SD) was 79.71 ± 45.42 months. There was a local recurrence in group A (1/51, 2%) and group B (1/28, 3.6%) respectively. This patient in group A underwent surgery while the patient in group B underwent repeated ESD with no further recurrences in both patients. There was no regional lymph node metastasis, distant metastasis, and deaths in both groups. With R0 resection strategy for ESD on lesions over 2 cm, 20.4% (28/137) of patients were able to avoid surgery compared with expanded indication.
If R0 resection is achieved by ESD, UD type EGCs over 2 cm also showed good and similar clinical outcomes as compared to lesions less than 2 cm when followed for over 5 years. With R0 resection strategy, several patients can avoid surgery.
Core Tip: The long-term outcomes of endoscopic submucosal dissection (ESD) in undifferentiated (UD) type early gastric cancer (EGC) over 2 cm confined to the mucosa are not well known. In our study, R0 resection is achieved by ESD in these patients also showed good long-term clinical outcomes similar to lesions if less than 2 cm. With R0 resection strategy, 20.4% of patients were able to avoid surgery compared with expanded indication. Further prospective randomized studies are recommended to determine which UD type EGCs over 2 cm are suitable for endoscopic treatment.
- Citation: Bae JY, Ryu CB, Lee MS, Dua KS. Long-term outcomes of endoscopic submucosal dissection for undifferentiated type early gastric cancer over 2 cm with R0 resection. World J Gastrointest Endosc 2024; 16(6): 326-334
- URL: https://www.wjgnet.com/1948-5190/full/v16/i6/326.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i6.326
Endoscopic submucosal dissection (ESD) was developed for treatment of early gastric cancer (EGC) during the 1990s[1,2]. As the experience in performing ESD grew, the indications for ESD have evolved from absolute indication to expanded indication[3-5]. In differentiated type (D type) EGC, ESD showed favorable and effective long-term clinical outcomes compared to surgery[6]. A recent study also showed that ESD is an effective treatment for undifferentiated (UD) type EGCs confined to the mucosa that is less than 2 cm in size[7]. ESD can be challenging in UD type EGCs confined to the mucosa over 2 cm in size, and evidence of its long-term outcomes has been limited. The objective of this study was to evaluate the safety and long-term efficacy of ESD in treating UD type EGC confined to the mucosa that were over 2 cm in size.
This is a retrospective review of a cohort of patients who underwent gastric ESD for EGD at one tertiary-care center. This study was approved by the ethical committee of the Medical Center (IRB number 2018-07-028). The study was conducted in accordance with the guidelines of the Declaration of Helsinki.
From May 2005 to February 2021, 146 ESDs were performed on 143 patients. All these patients were confirmed to have UD type EGC confined to the mucosa after ESD on histology. From the study, those with synchronous, metachronous lesions and one patient who had emergency surgery for delayed perforation were excluded. 137 patients with single UD type EGCs were reviewed. Excluded from the study were also those with: (1) Patients with a follow-up period of less than 12 months; and (2) those who did not meet the R0 resection such as positive lateral margins, positive vertical margins, submucosal invasion, and piecemeal resection. For the reasons described above, 58 cases were excluded. Finally, 79 cases who underwent R0 resection were divided into two groups; Group A: Those with UD type EGCs less than 2 cm in size, and Group B: those with UD type EGCs over 2 cm in size (Figure 1).
All patients underwent staging workup through upper gastrointestinal (GI) endoscopy, chest and abdominal computed tomography (CT), and endoscopic ultrasonography before ESD. During upper endoscopy, chromoendoscopy using indigo carmine and magnifying endoscopy (GIF-H260Z, GIF-HQ290, Olympus, Tokyo, Japan) using narrow band image were performed. The location and margin of the lesions (size) were determined. Histology was performed on the resected ESD specimens.
Patients diagnosed with UD type EGC less than 2 cm were informed about ESD in accordance with the expanded indication for ESD and informed consent was obtained. Patients with UD type EGC over 2 cm in size were informed the advantages and disadvantages of surgery and ESD. The advantage of surgery explained was that it is generally a classic curative treatment for EGC and the results of recurrence after surgery were well known. Disadvantages of surgery were explained as the possibility of adverse events from surgery and not being an organ-preserving approach that may affect quality of life. An advantage of ESD explained was that it is an organ-preserving approach. Hence after ESD, quality of life could be maintained. However, the disadvantages of ESD explained were that it might not be a curative treatment and can also be associated with adverse events. Patients then selected the surgical or ESD option according to their wishes, considering their age, comorbidities, and preferences.
In all patients, ESD was performed by endoscopists with more than 2 years of experience. ESDs were performed using standard methods as described before[8]. A single-channel water jet endoscope (GIF-Q260J, HQ290; Olympus, Tokyo, Japan) or a 2-channel multi-bending endoscope (GIF-2TQ260M; Olympus, Tokyo, Japan) were used. Several spots were marked with the Needle knife (Olympus, Tokyo, Japan), Hook knife (Olympus, Tokyo, Japan) or argon plasma coagulation 5 to 10 mm outside the margin of the lesion. After injection of normal saline solution with diluted epinephrine into the submucosa, an initial incision hole was made with the Needle knife (Olympus, Tokyo, Japan) or Hook knife (Olympus, Tokyo, Japan) outside the line of spots. The insulated-tipped (IT) knife (Olympus, Tokyo, Japan), Hook knife (Olympus, Tokyo, Japan), Flex knife ( Olympus, Tokyo, Japan), or Dual knife (Olympus, Tokyo, Japan) was then inserted into the initial incision, and electrosurgical current was applied with the use of an electrosurgical generator (ICC, VIO 200D or VIO 300D, Endocut mode effect: 2, duration: 4, interval: 1, Erbe, Tübingen, Germany) to complete the circumferential mucosal incision around the lesion. After a circumferential cut was completed, saline solution with diluted epinephrine was injected into the submucosa underneath the lesion whenever needed during dissection. The IT knife, Hook knife, Flex knife or Dual knife was then used to dissect the submucosa (swift effect: 3, 40 Watts or Forced effect: 2, 50 Watts).
After ESD, the specimen was spread out on the board using pins and then fixed in formalin. The specimen was then embedded in paraffin block and cut into 2 mm sections. Histological evaluation of all lesions followed the classification of the Japanese Gastric Cancer Association[9]. UD type EGC was defined as poorly differentiated adenocarcinoma, signet ring cell carcinoma or mucinous adenocarcinoma. If the histological findings were mixed in the lesion, it was classified as the predominant histology type, accounting for more than 50% of the whole lesion. The gross morphology, presence of ulcers, tumor size, distance from the tumor to the lateral and vertical margin, depth of invasion, and lymphovascular invasion were also evaluated and documented.
Curative resection of UD type EGC was defined as intramucosal cancer confined to the mucosa less than 2 cm in size with negative submucosal invasion, negative lateral and vertical margin, negative lymphovascular invasion, en bloc resection and no lymphadenopathy on computed tomography and endoscopic ultrasonography.
We defined R0 resection of UD type EGC as intramucosal cancer confined to the mucosa regardless of size with negative submucosal invasion, negative lateral and vertical margin, negative lymphovascular invasion, en bloc resection and no lymphadenopathy on computed tomography and endoscopic ultrasonography.
Once final tissue diagnosis was made on histology, patients diagnosed with UD type EGC after ESD were informed the results. Regular follow-up examinations were scheduled as per standard guidelines for those with UD type EGC less than 2 cm in size. Patients with UD type EGC over 2 cm in size were informed about the advantages and disadvantages of additional surgery versus careful follow-up examinations. The advantages of careful follow-up examinations were that complications of surgery and anesthesia could be avoided and a good quality of life could be maintained as ESD is organ-preserving. However, the disadvantages of careful follow-up examinations were that it might not be a curative treatment method and might be associated with recurrence. Patients selected the additional surgery or careful follow-up examinations according to their preferences.
Follow-up examinations after ESD were performed every 6 to 12 months for patients with a size of less than 2 cm for the first 2 years. However, for patients over 2 cm in size, follow-up examinations were performed every 6 months for the first 3 years. After the first 3 years, follow-up examinations were performed every year. For follow-up examinations included upper GI endoscopy and abdominal CT scans.
Our aim was to evaluate recurrence free survival (RFS), disease specific survival (DSS), and overall survival (OS) of enrolled patients. RFS was defined as the time from receiving ESD to recurrence. DSS was defined as the time from ESD to death from gastric cancer. OS was defined as the time from receiving ESD to death from any cause.
For continuous variables, Student's t test or Mann-Whitney U test was performed. For categorical variables, Pearson's chi-square test or Fisher's exact test was performed. Survival data were analyzed using the Kaplan-Meier method, and the differences between the two groups were compared by the log-lank test. Statistical analysis was performed using SPSS (version 25.0; SPSS Inc., Chicago, IL, United States), and when the P value was 0.05 or higher, it was considered to be statistically significant.
A total of 137 patients with single UD type EGC were reviewed. Of these, 79 patients who underwent R0 resection with a follow-up period of 12 months or more were enrolled and were classified as Group A and Group B as described earlier (Figure 1). There were no statistically significant differences in mean age, gender, location of tumor, gross endoscopic appearance, tumor size, histology, en bloc resection and follow-up periods between the groups (Table 1). However, there were statistical differences in curative resection, submucosal invasion, positive lateral margin, positive vertical margin, lymphovascular invasion and R0 resection between single and R0 resected UD type EGCs. Most of UD type EGCs were seen in the middle part of the stomach (single versus R0 resected UD type EGCs, 51.8% and 59.5%, respectively) and were of the depressed type on endoscopic appearance (single vs R0 resected UD type EGCs, 51.8% and 59.5%, respectively). The basic characteristics are summarized in Table 1. Overall R0 resection rate was 69.3% (96/137). It was 69.9% (51/73) for those less than 2 cm in size as compared to 43.7% (28/64) for those over 2 cm, in size (P value 0.003). For lesions less than 2 cm in size, curative resection rate was 69.9% (51/73).
| Single UD (n = 137) | R0 resected UD (n = 79) | P value | |
| Age, yr, mean (SD) | 54.75 ± 14.20 | 51.47 ± 12.31 | 0.088 |
| Male sex | 70 (51.1) | 42 (53.2) | 0.779 |
| Location of tumor | 0.563 | ||
| Upper | 9 (6.6) | 4 (5.1) | |
| Middle | 71 (51.8) | 47 (59.5) | |
| Lower | 57 (41.6) | 28 (35.4) | |
| Gross endoscopic appearance | 0.382 | ||
| Elevated | 31 (22.6) | 12 (15.2) | |
| Flat | 35 (25.6) | 20 (25.3) | |
| Depressed | 71 (51.8) | 47 (59.5) | |
| Tumor size, cm, mean (SD) | 2.04 ± 1.19 | 1.71 ± 1.06 | 0.266 |
| ≤ 2 cm | 73 (53.3) | 51 (64.6) | |
| 2.1-3 cm | 42 (30.7) | 19 (24.0) | |
| > 3 cm | 22 (16.0) | 9 (11.4) | |
| Depth of invasion | 0.043 | ||
| M1 | 0 (0) | 0 (0) | |
| M2 | 56 (40.9) | 40 (50.6) | |
| M3 | 66 (48.2) | 39 (49.4) | |
| SM1 | 4 (2.9) | 0 (0) | |
| SM2 | 7 (5.1) | 0 (0) | |
| SM3 | 4 (2.9) | 0 (0) | |
| Histology | > 0.999 | ||
| Poorly differentiated adenocarcinoma | 89 (65.0) | 51 (64.6) | |
| Signet ring cell carcinoma | 48 (35.0) | 28 (35.4) | |
| Enbolc resection | 127 (92.7) | 79 (100) | 0.060 |
| Curative resection | 60 (43.8) | 51 (64.6) | 0.005 |
| Non curative resection | 77 (56.2) | 28 (35.4) | |
| Over 2 cm | 64 (46.7) | 28 (35.4) | 0.118 |
| Submucosal invasion | 15 (10.9) | 0 (0) | 0.004 |
| Positive lateral margin | 29 (21.1) | 0 (0) | < 0.001 |
| Positive vertical margin | 9 (6.6) | 0 (0) | 0.028 |
| Lymphovascular invasion | 5 (3.7) | 0 (0) | 0.274 |
| R0 resection | 95 (69.3) | 79 (100) | < 0.001 |
| Follow-up periods, months, mean (SD) | 68.83 ± 52.10 | 79.71 ± 45.42 | 0.123 |
79 patients with R0 resection were followed for over 12 months. Table 2 summarizes the comparison between group A and group B. The Kaplan-Meier curve and cox regression analysis could not be done due to the low frequency of recurrence.
| Group A | Group B | P value | |
| Less than 2 cm (n = 51) | Over 2 cm (n = 28) | ||
| Age, yr, mean (SD) | 49.84 ± 11.90 | 54.43 ± 12.71 | 0.114 |
| Male sex | 28 (54.9) | 14 (50) | 0.814 |
| Location of tumor | 0.567 | ||
| Upper | 3 (5.9) | 1 (3.6) | |
| Middle | 28 (54.9) | 19 (67.8) | |
| Lower | 20 (39.2) | 8 (28.6) | |
| Gross endoscopic appearance | 0.148 | ||
| Elevated | 10 (19.6) | 2 (7.1) | |
| Flat | 10 (19.6) | 10 (35.7) | |
| Depressed | 31 (60.8) | 16 (57.2) | |
| Tumor size, cm, mean (SD) | 1.06 ± 0.60 | 2.89 ± 0.58 | < 0.001 |
| ≤ 2 cm | 51 (100) | 0 (0) | |
| 2.1-3 cm | 0 (0) | 19 (67.9) | |
| > 3 cm | 0 (0) | 9 (32.1) | |
| Depth of invasion | 0.062 | ||
| M1 | 0 (0) | 0 (0) | |
| M2 | 30 (58.8) | 10 (35.7) | |
| M3 | 21 (41.2) | 18 (64.3) | |
| Histology | 0.630 | ||
| Poorly differentiated adenocarcinoma | 34 (66.7) | 17 (60.7) | |
| Signet ring cell carcinoma | 17 (33.3) | 11 (39.3) | |
| Follow-up periods, months, mean (SD) | 82.57 ± 43.62 | 74.51 ± 48.92 | 0.454 |
The mortality and cause of death of 22 patients with a follow-up period of less than 12 months were reviewed through the Central Cancer Registry of the Ministry of Health and Welfare of the Republic of Korea. The number of confirmed deaths were 6 in 22 patients (2 of 8 patients in Group A and 4 of 14 patients in Group B). All confirmed deaths were not related to gastric cancer. The mean time (SD) to death after ESD in these patients was 58.90 ± 55.49 months.
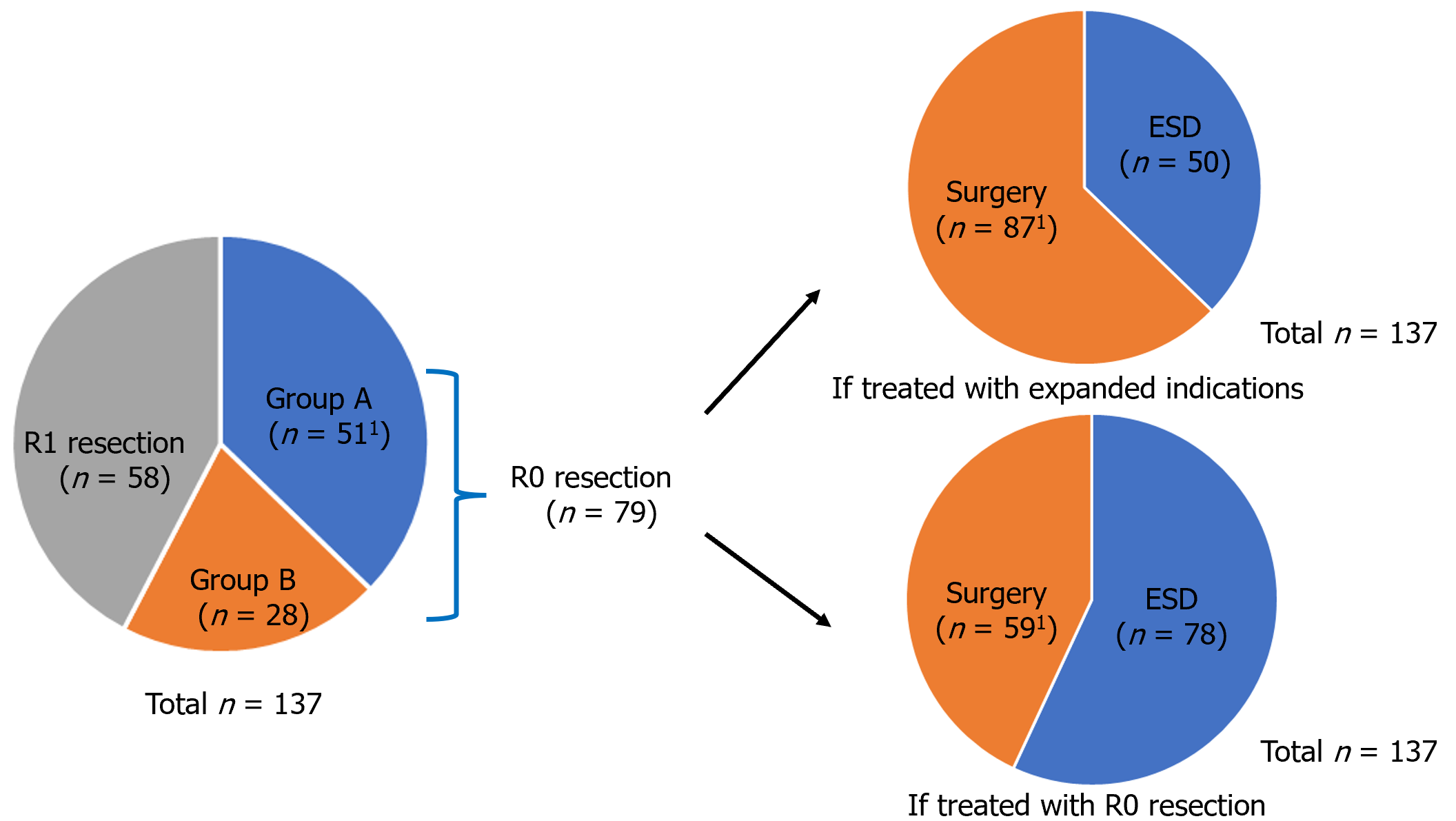
There was one local recurrence each in group A (1/51, 2%) and group B (1/28, 3.6%, P value 1.000). In group A, a recurrence case was detected 13.4 months after ESD. The patient underwent surgery and there was no evidence of recurrence during a follow-up period of 158 months. In group B, a recurrence case was detected 7.6 months after ESD. This patient underwent ESD again and no further recurrence was identified during a follow-up period of 85.4 months. There was no regional lymph node metastasis, distant metastasis and deaths during the follow-up period in both groups. Table 3 summarizes the clinical results. If these patient group had been treated with expanded indication, only 50 (36.5%) patients would have undergone ESD and avoided surgery. However, if these patients were treated with R0 resection strategy, 78 (56.9%) patients could have undergone ESD and avoided surgery. This means that an additional 28 (20.4%) patients were able to avoid surgery (Figure 2).
| | Group A | Group B | P value |
| Less than 2 cm (n = 51) | Over 2 cm (n = 28) | ||
| Recurrence, n (%) | 1 (2) | 1 (3.6) | > 0.999 |
| Local recurrence | 1 (2) | 1 (3.6) | |
| Regional lymph node | 0 (0) | 0 (0) | |
| Distant metastasis | 0 (0) | 0 (0) | |
| Regional lymph node metastasis | 0 (0) | 0 (0) | |
| Distant metastasis | 0 (0) | 0 (0) | |
| Death | 0 (0) | 0 (0) | |
| Recurrence free survival, months, mean (SD) | 204.12 ± 3.77 | 178.82 ± 9.03 | 0.7501 |
Through retrospective analysis of surgical specimens obtained after surgery, indications for ESD were decided according to the possibilities of lymph node involvement with the depth of invasion, ulceration, the size of the lesion, lympho
Over time, ESD techniques for EGC were developed and experience was also accumulating on its safety and efficacy. ESD became possible even for larger and more complex lesions. As a result, endoscopists who currently practice ESD are gaining experience on its indications and limitations in a variety of clinical situations. Hence, ESD is now evolving from its absolute indication to expanded indication. However, there is a need for long-term follow-up data, especially when ESD is used for indications such as for UD type EGC that are over 2 cm in size. Moreover, like was done in this study, post-ESD specimens should be evaluated on histology with 2 mm sections as against the traditional 5 mm sections on specimens obtained after surgery. This is important since 5 mm histologic sections may not accurately reflect depth and lymphovascular invasion as compared to 2 mm sections.
Several studies have shown that ESD is a safe and effective way to resect UD type EGC that are less than 2 cm in size[7,10-12]. However, the role of using ESD for UD type EGC over 2 cm in size is unclear[9]. According to the guidelines of the Japanese Gastric Cancer Society, fourth edition, surgery is recommended for these patients[13], Later this was revised and classified as a relative indication in the fifth edition[9]. Although, still surgery is considered standard of care for curative treatment in these patients. However, unlike ESD, surgery is not an organ-preserving approach and may adversely affect the patient’s quality of life. Moreover, if the UD type EGC is slightly over 2 cm by a few millimeters, it is questionable if one should subject these patients to surgery for curative treatment especially, as it is not easy to accurately gauge the size of a few millimeters during endoscopy. Lesions tend to look bigger when the gastric wall is stretched with air insufflation and the excised specimen looks smaller with contraction following fixation in formalin. This study was done to evaluate the safety and efficacy of doing ESD on patients with lesions over 2 cm and showed that the results were comparable to those with lesions less than 2 cm.
Many patients and physicians are still apprehensive about doing less invasive procedures like ESD on patients with large UD type EGC where surgery is preferred. This is because larger tumor size of UD type gastric cancer were associated with higher depth of invasion, higher lymph node metastasis, and higher local tumor stage based on studies that included both EGCs and advanced gastric cancers[14]. However, in a large study of EGCs after ESD, the risk of lymph node metastasis was not significantly higher in UD type EGCs than in D type EGCs (Hazard ratio for recurrence of UD type EGCs were 1.11 in univariate analysis 1.64 in multivariate analysis, Odds ratio for lymph node metastasis of UD type EGCs was 1.22)[15,16].
Recently, there was a large multicenter retrospective study with long-term follow-up on UD type EGCs over 2 cm comparing to surgery and observation after ESD[17]. The median tumor size (IQR) was 2.8 (2.4–3.6) cm and the follow-up period was 90.1 (62.7–108.5) months in the observation group after ESD. In the ESD group, there were 4 patients (2.3%) with local recurrence, and 2 patients (1.1%) regional lymph node or distant metastases, 3 patients (1.7%) with synch
It is well known that Helicobacter pylori eradication is effective in the prevention of metachronous gastric cancer[18]. There is a study suggesting that somatic alterations and mutational burden are associated with metachronous gastric cancer[19]. Other factors may also be involved in the development of synchronous and metachronous gastric cancer. In many studies, synchronous and metachronous gastric cancers are usually classified as recurrences. However, in terms of the therapeutic outcomes of ESD, it is questionable whether synchronous and metachronous gastric cancers can be regarded as recurrences. Hence, we excluded synchronous and metachronous lesions patients in our study so as to avoid the above factors confounding the results.
A limitation of this study is that it was conducted at a single tertiary-care center with physicians experienced in the techniques of ESD. The study was retrospective and the number of patients reviewed was small. However, the long-term outcomes of using ESD for UD type EGCs over 2 cm in size was encouraging. Additionally, over 20% of patients were able to avoid surgery. Still, there is lack of sufficient data to generalize ESD to all patients with UD type EGC over 2 cm solely based on this study. Although it is true that data on recurrences and survival did not differ significantly between ESD and surgery in several studies, including this study, recurrences were not negligible[17,20,21].
Similar to other studies, this study also showed that ESD is safe and effective in treating patients with UD type EGCs less than 2 cm[7,10-12]. This study showed that ESD is also safe and effective for UD type EGCs over 2 cm in size if R0 resection was achieved. In this study, ESD saved over 20% of patients with lesions over 2 cm in size from having surgery and long-term follow-up showed encouraging results. Further prospective, randomized studies with long-term follow-up are needed before ESD can be adopted as the standard approach in these patients with UD type EGCs over 2 cm in size and R0 resection.
| 1. | Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, Sasako M, Sano T, Ochiai A, Yoshida S. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 3. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1326] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 4. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 5. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Abdelfatah MM, Barakat M, Ahmad D, Ibrahim M, Ahmed Y, Kurdi Y, Grimm IS, Othman MO. Long-term outcomes of endoscopic submucosal dissection vs surgery in early gastric cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M; Gastrointestinal Endoscopy Group (GIESG) and the Stomach Cancer Study Group (SCSG) of Japan Clinical Oncology Group. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer. 2021;24:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Conlin A, Kaltenbach T, Kusano C, Matsuda T, Oda I, Gotoda T. Endoscopic resection of gastrointestinal lesions: advancement in the application of endoscopic submucosal dissection. J Gastroenterol Hepatol. 2010;25:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1332] [Article Influence: 333.0] [Reference Citation Analysis (2)] |
| 10. | Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T, Chino A, Inamori M, Yamamoto Y, Yamamoto N, Tsuchida T, Tamegai Y, Nakajima A, Hoshino E, Igarashi M. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2018;32:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1913] [Article Influence: 239.1] [Reference Citation Analysis (1)] |
| 14. | Feng F, Liu J, Wang F, Zheng G, Wang Q, Liu S, Xu G, Guo M, Lian X, Zhang H. Prognostic value of differentiation status in gastric cancer. BMC Cancer. 2018;18:865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Shimosegawa T. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: "eCura system". Am J Gastroenterol. 2017;112:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 17. | Yang HJ, Nam SY, Min BH, Ahn JY, Jang JY, Kim J, Kim JH, Lee WS, Lee BE, Joo MK, Park JM, Shin WG, Lee HL, Gweon TG, Park MI, Choi J, Tae CH, Kim YI, Choi IJ. Clinical outcomes of endoscopic resection for undifferentiated intramucosal early gastric cancer larger than 2 cm. Gastric Cancer. 2021;24:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med. 2018;378:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 19. | Sakuta K, Sasaki Y, Abe Y, Sato H, Shoji M, Yaoita T, Yagi M, Mizumoto N, Onozato Y, Kon T, Koseki A, Sato S, Murakami R, Miyano Y, Ueno Y. Somatic alterations and mutational burden are potential predictive factors for metachronous development of early gastric cancer. Sci Rep. 2020;10:22071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Jeon HK, Kim GH, Lee BE, Park DY, Song GA, Kim DH, Jeon TY. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: a propensity-matched analysis. Gastric Cancer. 2018;21:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Lim JH, Kim J, Kim SG, Chung H. Long-term clinical outcomes of endoscopic vs. surgical resection for early gastric cancer with undifferentiated histology. Surg Endosc. 2019;33:3589-3599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |