Published online May 16, 2024. doi: 10.4253/wjge.v16.i5.250
Revised: February 28, 2024
Accepted: April 25, 2024
Published online: May 16, 2024
Processing time: 121 Days and 19.5 Hours
Most endoscopic anti-reflux interventions for gastroesophageal reflux disease (GERD) management are technically challenging to practice with inadequate data to support it utility. Therefore, this study was carried to evaluate the effectiveness and safety newer endoscopic full-thickness fundoplication (EFTP) device along with Argon Plasma Coagulation to treat individuals with GERD.
To evaluate the effectiveness and safety newer EFTP device along with Argon Plasma Coagulation to treat individuals with GERD.
This study was a single-center comparative analysis conducted on patients treated at a Noble Institute of Gastroenterology, Ahmedabad, hospital between 2020 and 2022. The research aimed to retrospectively analyze patient data on GERD symptoms and proton pump inhibitor (PPI) dependence who underwent EFTP using the GERD-X system along with argon plasma coagulation (APC). The primary endpoint was the mean change in the total gastroesophageal reflux disease health-related quality of life (GERD-HRQL) score compared to the baseline measurement at the 3-month follow-up. Secondary endpoints encompassed enhancements in the overall GERD-HRQL score, improvements in GERD symptom scores at the 3 and changes in PPI usage at the 3 and 12-month time points.
In this study, patients most were in Hill Class II, and over half had ineffective esophageal motility. Following the EFTP procedure, there were significant improvements in heartburn and regurgitation scores, as well as GERD-HRQL scores (P < 0.001). PPI use significantly decreased, with 82.6% not needing PPIs or prokinetics at end of 1 year. No significant adverse events related to the procedures were observed in either group.
The EFTP along with APC procedure shows promise in addressing GERD symptoms and improving patients' quality of life, particularly for suitable candidates. Moreover, the application of a lone clip with APC yielded superior outcomes and exhibited greater cost-effectiveness.
Core Tip: The findings of this study are clinically relevant as they demonstrate that the endoscopic full-thickness fund
- Citation: Harwani Y, Butala S, More B, Shukla V, Patel A. Endoscopic full-thickness plication along with argon plasma coagulation for treatment of proton pump inhibitor dependent gastroesophageal reflux disease. World J Gastrointest Endosc 2024; 16(5): 250-258
- URL: https://www.wjgnet.com/1948-5190/full/v16/i5/250.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i5.250
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal ailment worldwide, causing significant morbidity[1]. It has a global prevalence of 8%-33%[2]. It is associated with the retrograde movement of gastric contents into the esophagus. This results in symptoms such as heartburn, regurgitation dysphagia, odynophagia, water brash, globus sensation, chronic cough, hoarseness, and wheezing and atypical chest pain. As a result there is energy and sleep disturbances and anxiety leading to adverse impact on patients’ health-related quality of life[3].
In the past the key initial treatment of GERD was medical management with lifestyle modification and anti-secretory agents remains the mainstay of GERD treatment[1]. Medication used were antacids for mild symptoms, changing over to histamine-2 receptor antagonists or proton pump inhibitors (PPIs) in non-responsive symptoms[1].
While proton pump inhibitors (PPIs) have demonstrated efficacy in managing GERD symptoms, patients may develop dependence on them for symptom relief, necessitating prolonged treatment[4]. Moreover long-term PPI intake is associated with increased the risk of renal disease, hypomagnesaemia, osteoporotic fractures and infection with Clostridium difficile[5]. Also some PPI are contraindicated or are not tolerated for long by certain patients. These patients are typically managed by surgical anti-reflux procedures such as hiatal hernia repair with Nissen fundoplication. However laparoscopic anti-reflux surgery have shown to have a higher incidence of postoperative gas/bloat, dysphagia resulting in adverse impact on quality of life[6].
Recently various endoscopic anti-reflux therapies have emerged which seem to be promising in managing medically resistant GERD as they are less invasive, reduce heartburn, regurgitation and PPI usage[1,4]. During endoscopic full-thickness plication (EFTP) or endoscopic transmural fundoplication, sutures are utilized at the gastro-esophageal junction to reshape the structure of the gastric cardia. This results in the reinforcement of the valvular mechanism, consequently diminishing gastro-esophageal reflux[7,8].
The Food and Drug Administration in the United States has approved various endoscopic devices for managing GERD, including Stretta® for radiofrequency therapy, Esophyx-Z® for transoral incisionless fundoplication (TIF), and Overstitch® for endoscopic suturing, among others. Of these, Esophyx device has ample effectiveness evidence in EFTP. It has demonstrated a 70% effectiveness in reducing GERD symptoms, with an adverse event rate of 2%[9]. However, the device is cumbersome to use requiring training, general anesthesia and long duration (45–100 min) of procedure.
GERD-X (G-SURG, Germany) is an endoscopic plication device introduced in 2014 which is relatively simple, easy to use and with a shorter procedure time and safer. Clinical studies have reported to GERD-X device effective and safe in management of patients with PPIs resistant GERD[4,10]. However, there are events of clip dehiscence when used alone argon plasma coagulation (APC) along with clip may prevent dislodgement of clip/suture.
APC is a method of achieving thermal hemostasis without contact. It uses high-frequency current, which is delivered to the target tissue via an argon plasma jet. This process induces hemostasis and results in a uniform surface coagulation with a limited depth of penetration. In the luminal digestive tract, APC is widely used for non-contact, targeted thermal injury to achieve mucosal ablation. This application may facilitate a more substantial submucosal healing component between tissue plications, potentially leading to increased durability of the gastroplasty[11].
Introducing enhanced fibrosis along the site of endoscopic plications through the addition of APC, there is the potential to reduce the occurrence of GERD symptoms in patients undergoing Endoscopic plication. This approach aims to capitalize on the reported metabolic benefits of gastric mucosal revitalization associated with APC alone. Nonetheless, this approached of endoscopic plications and argon plasma coagulation has not been studied so far in GERD manage
This was a single center retrospective study to evaluate the effectiveness and safety newer EFTP device to treat patients with GERD. The study cohort was derived from the patients treated at Noble Institute of Gastroenterology, Ahmedabad hospital from 2020 to 2022. The study conducted a retrospective analysis of patient data exhibiting GERD symptoms and relying on PPI therapy, who underwent EFTP (GERD-X system). The study's inclusion criteria comprised individuals aged between 18 to 60 years, who experienced classic reflux symptoms such as heartburn and regurgitation and had been dependent on PPI therapy for a minimum of 6 months. Patients were considered eligible for inclusion if their medical records satisfied the following criteria: Gastro-oesophageal flap valve grade I–II (according to Hill’s classification); pathological oesophageal acid exposure, indicated by a percentage of time with oesophageal pH < 4 over 24 h exceeding 6%; abnormal DeMeester score equal to or greater than 14.7, or a total number of reflux episodes surpassing 80 and lower oesophageal sphincter pressure (LESP) within the range of 5 to 15 mm Hg. Patients whose data did not conform to the following criteria were excluded from the study: Having an ASA physical status greater than II; a history of previous oesophageal or gastric surgery; a large hiatal hernia exceeding 3 cm in size; having Los Angeles grade C/D oesophagitis; presence of a para-oesophageal hernia; a diagnosis of Barrett's oesophagus and women with pregnancy.
High-resolution Manometry (HRM) was used to establish evidence of oesophageal dysmotility. The initial screening process encompassed the documentation of the duration of reflux symptoms and the specifics regarding the dosage and duration of PPI usage. Subsequent to a 7-d discontinuation of PPI therapy, patients underwent an Esophagogastroduodenoscopy to screen for oesophagitis and to evaluate the Hill's grade of the gastro-oesophageal junction. Furthermore, the study evaluated patients' responses to the GERD HRQL questionnaire. On the eighth day following the cessation of PPIs, oesophageal HRM was conducted, accompanied by 24-h pH impedance monitoring. Various parameters were evaluated during the baseline assessment, including the oesophageal motility pattern, LESP, the percentage of time with oeso
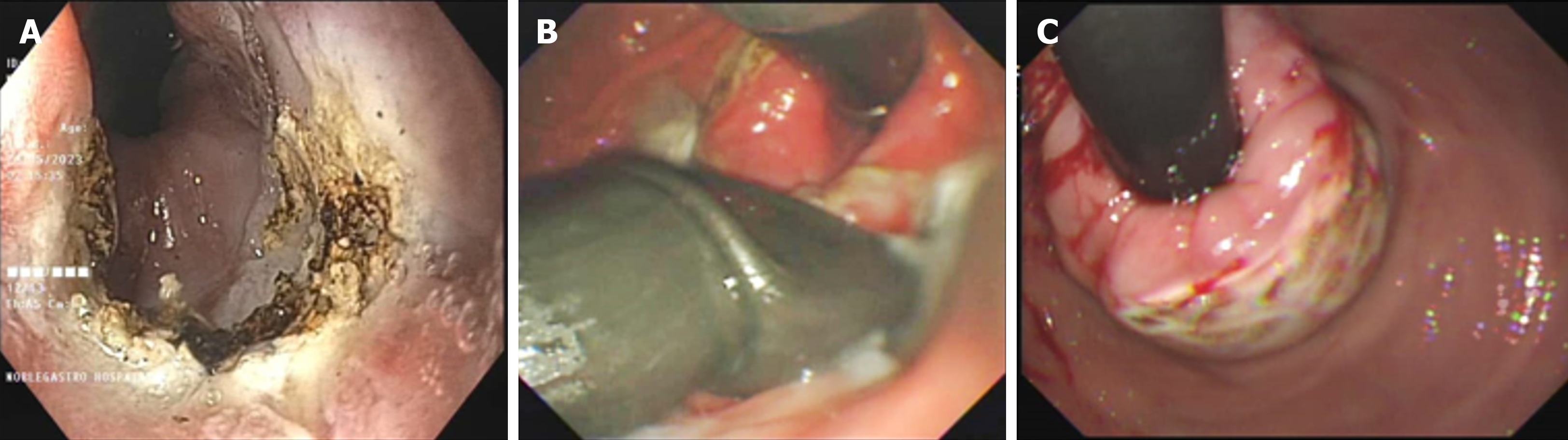
The investigators thoroughly explained the procedural steps to the patients and obtained written informed consent before the procedure. On the day of the procedure, all patients underwent the prescribed intervention, known as endoscopic fundoplication (EFTP), while under general anesthesia and endotracheal intubation, following an overnight fasting period. Patients received intravenous antibiotics just before the endoscopy procedure. An adult gastroscope was carefully inserted into the stomach, and a metallic guidewire was then passed through the biopsy channel of the endoscope. Subsequently, the gastroscope was withdrawn, and the EFTP device was introduced, guided by the wire (Figure 1). Utilizing an ultraslim endoscope for direct visual monitoring, the distal end of the device was retroflexed to the anterior gastric cardia, positioning it approximately 1 cm below the gastro-oesophageal junction. The arms of the device were opened, and the endoscopic tissue retractor was advanced deep into the gastric cardia mucosa. This retraction maneuver pulled the gastric wall into the open arms of the EFTP device. Once the tissue was appropriately positioned, the device's arms were closed, and following the study protocol, either one or two pre-tied transmural pledget sutures were deployed. This ensured a complete full-thickness plication and a secure closure around the gastro-oesophageal junction[10].
Subsequently, both the EFTP system and the ultraslim endoscope were carefully withdrawn and straightened. The gastroscope was then reintroduced to evaluate the gastro-oesophageal junction post-plication. Importantly, no additional treatments or retreatments were allowed.
Following the intervention, patients were regularly monitored and instructed to visit the hospital for check-ups at the 3-month, 6-month, and 12-month marks. If a patient reported experiencing reflux symptoms more than twice a week, they were prescribed PPI medication equivalent to either 20 mg of rabeprazole or 40 mg of pantoprazole per day over the phone. These prescriptions were documented and tracked. In cases where symptom control was insufficient after 4 wk of starting PPI therapy, the dosage was increased as necessary.
Over-the-counter use of PPIs was restricted, although patients were allowed to use antacids as needed, and such usage was documented. Additionally, the patients' responses to the GERD HRQL questionnaire were assessed at the end of 3 months. For patients who resumed taking PPIs after the assigned intervention, these assessments were conducted after they had stopped PPI therapy for at least 3 d. The primary objective of this study was to determine the mean change in the total GERD-HRQL score compared to the baseline measurement at the 3-month follow-up. Secondary endpoints of the study encompassed various aspects, including enhancements in the overall GERD-HRQL score, improvements in GERD symptom scores at the 3, 6, and 12-month follow-up intervals, changes in PPI usage. Adverse events within the study were identified and categorized using the terminology outlined in the lexicon for endoscopic adverse events provided by the American Society of Gastrointestinal Endoscopy[12]. Incidents classified as adverse events included minor instances of intra-procedural bleeding, as well as mild post-procedural pain that did not necessitate any medical intervention. Additionally, situations such as the premature termination of the procedure and instances where patients required an extended hospital stay were also considered as adverse events.
This study was approved by the appropriate ethics committee and has performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The data obtained was entered in a Microsoft Excel sheet, and statistical analysis was performed using statistical package for the social sciences (Version 26). Data was presented as mean ± SD, frequency and percentages. Categorical variables were presented as proportions. Paired data i.e. before and after treatment data were compared using Wilcoxon signed rank test. For comparison of PPI usage before and after procedure Chi Square for R by C Table was applied using OpenEpi, Version 3, open source calculator. Statistical significance was established at a threshold of P < 0.05.
The study included patients with an average age of 36.78 years (± 15.53 SD), comprising 34.8% females and 65.2% males. Table 1 presents an overview of the distribution of various patient characteristics. The largest proportion of patients (34%) fell within the 21-30 age group, with 21.7% in the 31-40 age group. Notably, all patients in the study were receiving PPIs. The baseline mean lower esophageal sphincter pressure was recorded at 9.52 mm Hg. In Table 2, we provide a summary of the mean baseline DeMeester Score, AET, reflux episodes, SI, and DCI.
| Basic characteristics | No. of patients | Percentage |
| Age | ||
| ≤ 20 | 2 | 8.7 |
| 21–30 | 8 | 34.8 |
| 31–40 | 5 | 21.7 |
| 41–50 | 4 | 17.4 |
| 51–60 | 1 | 4.3 |
| 61+ | 3 | 13.0 |
| Gender | ||
| Female | 8 | 34.8 |
| Male | 15 | 65.2 |
| Hill’s classification | ||
| I | 3 | 13.0 |
| II | 17 | 73.9 |
| III | 3 | 13.0 |
| Manometry findings | ||
| Absent contractility | 4 | 17.3 |
| Fragmented peristalsis | 1 | 4.3 |
| Esophageal motility ineffective | 12 | 52.2 |
| Normal | 6 | 26.1 |
| Argon plasma coagulation | ||
| No | 14 | 60.9 |
| Yes | 9 | 39.1 |
| Number of clips | ||
| 1 | 13 | 56.5 |
| 2 | 10 | 43.5 |
| Complications | ||
| No complications | 22 | 95.7 |
| Hematemesis, dysphagia | 1 | 4.3 |
| Total | 23 | 100 |
| n | Minimum | Maximum | Mean | SD | |
| Age | 23 | 17 | 73 | 36.78 | 15.53 |
| Lower oesophageal sphincter pressure (mm Hg) | 23 | 3.8 | 20.0 | 9.52 | 0 |
| DeMeester score | 23 | 7.90 | 129.00 | 48.32 | 34.59 |
| Acid exposure time | 23 | 5.00 | 39.30 | 15.03 | 10.47 |
| Reflux episodes | 23 | 21 | 332 | 88.61 | 68.91 |
| Symptom index | 23 | 0.0 | 100.0 | 60.11 | 29.30 |
| Distal contractile integral | 23 | 0.0 | 1732.0 | 456.40 | 510.54 |
The majority of patients, constituting 73.9%, were classified as Class II according to the Hill classification. Manometry findings indicated that a significant proportion, specifically 52.2% of patients, exhibited ineffective esophageal motility. Additionally, Argon plasma coagulation was performed in 39.1% of patients. Among the cases, a single clip was utilized in 56.5% of patients at lesser curvature, while double clips were employed in 43.5% of patients. It's noteworthy that only one patient experienced an adverse event in single chip, which manifested as hematemesis and dysphagia. Both single clip and double clip methods were effective. However single clip with APC offered better outcome and cost effectiveness. Importantly, no other adverse events related to the procedure were reported in the study.
Before the procedure, the average heartburn score was 17.96 (± 10.38), and this significantly decreased to 3.52 (± 5.17) after EFTP, marking a statistically significant improvement (P < 0.001). Likewise, the mean regurgitation score prior to the procedure was 18.26 (± 6.66), and it notably dropped to 2.04 (± 3.37) post-EFTP, also demonstrating a statistically significant improvement (P < 0.001). In the evaluation of health-related quality of life before and after the endoscopic full-thickness plication (EFTP; GERDx) procedure, a statistically significant change was observed, with the pre-procedure score at 39.26 (± 13.95) and post-procedure score at 5.83 (± 8.38), as presented in Table 3. This change was statistically significant.
The need for PPIs at the 12-month mark following the intervention showed a significant reduction post-procedure. Prior to the procedure, the highest requirement was for the combined use of PPIs and prokinetics, with a prevalence of 78.3%. After the procedure, at 3, 6, and 12 months, there was a substantial decrease in the need for combined PPI and prokinetic therapy, and these reductions were statistically significant (P < 0.005). Impressively, at the 12-month post-procedure milestone, 82.6% of patients no longer required either PPIs or prokinetics, and this change was also statistically significant. Figure 1 illustrates the GERDx system and Argon Plasma coagulation procedure.
Argon plasma coagulation finds application across a wide spectrum of surgical specialties, including general and visceral surgery, urology, gynecology, gastroenterology, bronchological endoscopy, and otorhinolaryngology. Its primary utility lies in areas such as hemostasis and tissue devitalization, with a specific emphasis on endoscopic procedures[13]. We attempted to analyze the effectiveness Endoscopic Full-Thickness Plication along with Argon Plasma Coagulation for the treatment of PPI-dependent GERD.
APC triggers an inflammatory reaction at the lower esophageal sphincter, initiating an inflammatory response healing cascade. This process promotes the formation of vascular-rich collagen, resulting in increased tightness at the gastroesophageal (GE) junction. This phenomenon can be described as tissue remodeling. In cases where the GE junction acts as a high-pressure zone, there is a risk of suture dehiscence following full-thickness fundoplication. APC, through its fibrosis-inducing action during the healing phase, also facilitates suture adherence.
In this study there was statistically significant improvement in GERD-HRQL total score in the patient subjected to the EFTP. In the study by Kalapala et al[4] there was 50% or more improvement in GERD-HRQL total score in the EFTP group (65.7%) in comparison to the sham group (3%) 3 months post intervention (P < 0.001). Another randomised clinical trials (RCT) TIF also demonstrated similar improvement GERD-HRQL score at 3 months[14]. Based on similar outcome measure, Witteman et al[15] showed betterment in the GERD-HRQL score in 55% of patients 6 months post TIF. The GERD-X device demonstrated a notable enhancement in mean reflux-specific symptom scores after three months in another prospective, non-comparative trial[10]. However, similar to our study, this study did not include a control group, and its results did not provide information on efficacy beyond three months.
Improvement in GERD-HRQL, as observed in this study, may have greater significance from a patient-centered viewpoint compared to the relatively infrequent attainment of normalized esophageal acid exposure associated with existing devices. The current study highlights substantial improvements in symptoms, with less conspicuous enhancements in objective measures, especially among patients diagnosed with non-erosive esophagitis, mirroring the findings of the study conducted by Kalapala et al[4].
In alignment with our own findings, numerous studies assessing the efficacy of laparoscopic anti-reflux (LAR) surgery consistently demonstrate a significant reduction in the number of patients experiencing reflux symptoms during the initial year following surgery. These rates declined from an initial range of 80% to 90% at baseline to as minimal as 2% to 4% by the end of the first year[16,17].
Studies conducting long-term follow-ups on LAR surgery have highlighted a remarkable and sustained reduction in symptom severity at both the 6-month and 1-year marks. However, it's crucial to emphasize that a significant portion of patients encounter symptom relapses, with prevalence rates ranging from 14% to 18% after 5 years and increasing to 30% to 35% after a decade[17]. Studies assessing the long-term effectiveness of TIF have reported comparable trends[18,19]. Stefanidis et al[20] demonstrated a meaning improvement in regurgitation and chest pain scores at a year compared to baseline and even at half year post-TIF using the Esophyx device.
In a subset of open-label randomized controlled trials comparing TIF with PPIs, a significant drop in the mean GERD-HRQL score was observed at the one-year mark[16,21]. Specifically, scores decreased from an initial range of 27 to 32 at baseline to a lower range of 7 to 10 at 12 months in the TIF-treated group[4]. These findings are consistent with the notion that the effects of both surgical and endoscopic fundoplication tend to show progressive improvement over the course of a year or two after the procedure, with a subsequent gradual decline.
Given that the EFTP device used in our study was a novel approach and that short-term data on its efficacy were limited, we conducted a comprehensive analysis of outcomes at regular intervals, commencing from 3 months after intervention. Notably, the 12-month outcomes in our study significantly outperformed those reported in prior studies that evaluated a similar plicator device[22-24].
In this study at the 12-month follow-up post intervention, there was a noteworthy reduction in the necessity for PPIs. Prior to the procedure, the highest demand was for a combination of PPIs and prokinetics, with a prevalence of 78.3%. Subsequent to the procedure, at 3, 6, and 12 months, a substantial decline in the need for combined PPI and prokinetic therapy was evident, and these reductions were statistically significant (P < 0.005). Remarkably, at the 12-month post-procedure mark, as many as 82.6% of patients no longer required either PPIs or prokinetics, and this change was also statistically significant. In GERDx study by Kalapala et al[4], out of the 13 patients who resumed taking PPIs in the EFTP group, 11 (85%) did so within the first 3 months following the intervention. Similar outcomes have been observed in studies assessing TIF procedures utilizing the Esophyx device. In a prospective trial without a comparative control group, it was found that 80% of patients who relied on PPIs at the 12-month mark reintroduced PPI usage within 6 months after undergoing TIF[19]. In most of clinical trials examining the effectiveness of an older plicator device in PPI-dependent patients, approximately 60% to 70% of patients were no longer using PPIs at both 6 and 12 months after the intervention[22,25]. However, it's important to note that many of these studies lacked randomization and a control arm for comparison.
Improvements in GERD-HRQL may hold more relevance from a patient-centered perspective compared to the infrequent achievement of normalized oesophageal acid exposure seen with currently available devices. The present study demonstrates significant symptom improvement, with less pronounced enhancements in objective parameters, particularly in patients diagnosed with non-erosive oesophagitis[4].
In contrast to other endoscopic antireflux surgical techniques, the novel EFTP device offers the distinct advantage of a relatively brief operating time, which can be a valuable attribute. A shorter operating duration serves as one of the surrogate indicators of the procedure's technical simplicity. With the exception of a few adverse events linked to suture characteristics, EFTP has demonstrated a favorable safety profile and does not necessitate extended hospital stays. Furthermore, it is presumed that the procedure has a shallow learning curve, and acquiring sufficient experience may only require the performance of an initial 10 to 15 procedures under supervision.
Anti-reflux mucosectomy could serve as an efficient procedure for managing reflux, offering the additional benefit of not necessitating supplementary devices and avoiding the presence of artificial prostheses[26]. Mucosal scarring, inherently uncontrollable, introduces an element of capriciousness to the efficacy of treatment, with outcomes varying among patients ranging from ineffectiveness in some cases to excessive scarring that predisposes individuals to stenosis. Additionally, the decision to leave the mucosectomy scar unsealed heightens the likelihood of delayed complications, including bleeding or perforation. Certain studies have illuminated the impact of implementing closure, specifically through the application of a clip[27]. Mucosectomy at the esophagogastric junction coupled with the closure of resected mucosa holds promise in mitigating the risk of delayed bleeding, postendoscopy pain, and perforation. The utilization of clips in the closure process also affords a potential enhancement in the precision of tissue retraction. There are events of clip dehiscence when used alone APC along with clip may prevent dislodgement of clip/suture. A recent investigation has linked endoscopic mucosal resection to suture-plication using the Overstitch system, thereby underscoring the evolving strategies in this domain[28].
In the study, a solitary clip was applied in 56.5% of patients at the lesser curvature, whereas double clips were utilized in 43.5% of cases. It is worth highlighting that only one patient encountered an adverse event, presenting as hematemesis and dysphagia. Both the single-clip and double-clip approaches demonstrated effectiveness. Nevertheless, the employment of a single clip with APC yielded superior outcomes and proved to be more cost-effective.
This study has several notable limitations. Firstly, the study sample was small, and the follow-up period was comparatively short. Moreover, there was a lack of an appropriate control group that underwent laparoscopic Nissen fund
The utilization of an innovative endoscopic full-thickness fundoplication procedure such as GERDx along with Argon Plasma Coagulation has proven effective in relieving GERD symptoms and enhancing the quality of life for patients, albeit without incorporating objective 24-h pH impedance data. Moreover, the application of a lone clip with APC yielded superior outcomes and exhibited greater cost-effectiveness. This endoluminal approach shows promise as a viable alternative to surgery, particularly for appropriately selected patients who wish to avoid long-term reliance on PPI medications. The ideal candidates for EFTP include individuals with PPI dependency, abnormal acid or non-acid reflux, and small hiatus hernias (Hills I and II). The procedure is characterized by its brevity and minimal side effects. However, to establish the long-term advantages of this procedure, comprehensive prospective trials with extended follow-up periods exceeding one year are imperative.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s classification
Scientific Quality: Grade C, Grade D
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Cabezuelo AS, Spain S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Lee DP, Chang KJ. Endoscopic Management of GERD. Dig Dis Sci. 2022;67:1455-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1263] [Article Influence: 114.8] [Reference Citation Analysis (2)] |
| 3. | Wiklund I. Review of the quality of life and burden of illness in gastroesophageal reflux disease. Dig Dis. 2004;22:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Kalapala R, Karyampudi A, Nabi Z, Darisetty S, Jagtap N, Ramchandani M, Gupta R, Lakhtakia S, Goud R, Venkat Rao G, Sharma P, Reddy DN. Endoscopic full-thickness plication for the treatment of PPI-dependent GERD: results from a randomised, sham controlled trial. Gut. 2022;71:686-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Kinoshita Y, Ishimura N, Ishihara S. Advantages and Disadvantages of Long-term Proton Pump Inhibitor Use. J Neurogastroenterol Motil. 2018;24:182-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 6. | Bazerbachi F, Krishnan K, Abu Dayyeh BK. Endoscopic GERD therapy: a primer for the transoral incisionless fundoplication procedure. Gastrointest Endosc. 2019;90:370-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Antoniou SA, Koch OO, Kaindlstorfer A, Asche KU, Berger J, Granderath FA, Pointner R. Endoscopic full-thickness plication versus laparoscopic fundoplication: a prospective study on quality of life and symptom control. Surg Endosc. 2012;26:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Chen YK, Raijman I, Ben-Menachem T, Starpoli AA, Liu J, Pazwash H, Weiland S, Shahrier M, Fortajada E, Saltzman JR, Carr-Locke DL. Long-term outcomes of endoluminal gastroplication: a U.S. multicenter trial. Gastrointest Endosc. 2005;61:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | McCarty TR, Itidiare M, Njei B, Rustagi T. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy. 2018;50:708-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Weitzendorfer M, Spaun GO, Antoniou SA, Witzel K, Emmanuel K, Koch OO. Clinical feasibility of a new full-thickness endoscopic plication device (GERDx™) for patients with GERD: results of a prospective trial. Surg Endosc. 2018;32:2541-2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Zenker M. Argon plasma coagulation. GMS Krankenhhyg Interdiszip. 2008;3:Doc15. [PubMed] |
| 12. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1841] [Article Influence: 122.7] [Reference Citation Analysis (1)] |
| 13. | Ginsberg GG, Barkun AN, Bosco JJ, Burdick JS, Isenberg GA, Nakao NL, Petersen BT, Silverman WB, Slivka A, Kelsey PB; American Society for Gastrointestinal Endoscopy. The argon plasma coagulator: February 2002. Gastrointest Endosc. 2002;55:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Håkansson B, Montgomery M, Cadiere GB, Rajan A, Bruley des Varannes S, Lerhun M, Coron E, Tack J, Bischops R, Thorell A, Arnelo U, Lundell L. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther. 2015;42:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Witteman BP, Conchillo JM, Rinsma NF, Betzel B, Peeters A, Koek GH, Stassen LP, Bouvy ND. Randomized controlled trial of transoral incisionless fundoplication vs. proton pump inhibitors for treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2015;110:531-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Långström G, Lind T, Lundell L; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 17. | Lundell L, Bell M, Ruth M. Systematic review: laparoscopic fundoplication for gastroesophageal reflux disease in partial responders to proton pump inhibitors. World J Gastroenterol. 2014;20:804-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Wilson EB, Barnes WE, Mavrelis PG, Carter BJ, Bell RC, Sewell RW, Ihde GM, Dargis D, Hoddinott KM, Shughoury AB, Gill BD, Fox MA, Turgeon DG, Freeman KD, Gunsberger T, Hausmann MG, Leblanc KA, Deljkich E, Trad KS. The effects of transoral incisionless fundoplication on chronic GERD patients: 12-month prospective multicenter experience. Surg Laparosc Endosc Percutan Tech. 2014;24:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Testoni PA, Testoni S, Mazzoleni G, Vailati C, Passaretti S. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc. 2015;29:2770-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Stefanidis G, Viazis N, Kotsikoros N, Tsoukalas N, Lala E, Theocharis L, Fassaris A, Manolakopoulos S. Long-term benefit of transoral incisionless fundoplication using the esophyx device for the management of gastroesophageal reflux disease responsive to medical therapy. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Trad KS, Fox MA, Simoni G, Shughoury AB, Mavrelis PG, Raza M, Heise JA, Barnes WE. Transoral fundoplication offers durable symptom control for chronic GERD: 3-year report from the TEMPO randomized trial with a crossover arm. Surg Endosc. 2017;31:2498-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | von Renteln D, Schiefke I, Fuchs KH, Raczynski S, Philipper M, Breithaupt W, Caca K, Neuhaus H. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease using multiple Plicator implants: 12-month multicenter study results. Surg Endosc. 2009;23:1866-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Chuttani R, Sud R, Sachdev G, Puri R, Kozarek R, Haber G, Pleskow D, Zaman M, Lembo A. A novel endoscopic full-thickness plicator for the treatment of GERD: A pilot study. Gastrointest Endosc. 2003;58:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Pleskow D, Rothstein R, Lo S, Hawes R, Kozarek R, Haber G, Gostout C, Lembo A. Endoscopic full-thickness plication for the treatment of GERD: a multicenter trial. Gastrointest Endosc. 2004;59:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Koch OO, Kaindlstorfer A, Antoniou SA, Spaun G, Pointner R, Swanstrom LL. Subjective and objective data on esophageal manometry and impedance pH monitoring 1 year after endoscopic full-thickness plication for the treatment of GERD by using multiple plication implants. Gastrointest Endosc. 2013;77:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Inoue H, Ito H, Ikeda H, Sato C, Sato H, Phalanusitthepha C, Hayee B, Eleftheriadis N, Kudo SE. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol. 2014;27:346-351. [PubMed] |
| 27. | Laquière A, Trottier-Tellier F, Urena-Campos R, Lienne P, Lecomte L, Katsogiannou M, Penaranda G, Boustière C. Evaluation of Antireflux Mucosectomy for Severe Gastroesophageal Reflux Disease: Medium-Term Results of a Pilot Study. Gastroenterol Res Pract. 2022;2022:1606944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Benias PC, D'Souza L, Lan G, Gluckman C, Inamdar S, Trindade AJ, Miller LS, Carr-Locke DL. Initial experience with a novel resection and plication (RAP) method for acid reflux: a pilot study. Endosc Int Open. 2018;6:E443-E449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |