Published online Feb 16, 2024. doi: 10.4253/wjge.v16.i2.72
Peer-review started: November 22, 2023
First decision: November 30, 2023
Revised: December 19, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: February 16, 2024
Processing time: 69 Days and 20.5 Hours
Endoscopic submucosal dissection (ESD) and surgical resection are the standard of care for cT1N0M0 esophageal cancer (EC), whereas definitive chemoradiotherapy (d-CRT) is a treatment option. Nevertheless, the comparative efficiency and safety of ESD, surgery and d-CRT for cT1N0M0 EC remain unclear.
To compare the efficiency and safety of ESD, surgery and d-CRT for cT1N0M0 EC.
We retrospectively analyzed the hospitalized data of a total of 472 consecutive patients with cT1N0M0 EC treated at Sun Yat-sen University Cancer center between 2017-2019 and followed up until October 30th, 2022. We analyzed demographic, medical recorded, histopathologic characteristics, imaging and endoscopic, and follow-up data. The Kaplan-Meier method and Cox proportional hazards modeling were used to analyze the difference of survival outcome by treatments. Inverse probability of treatment weighting (IPTW) was used to minimize potential confounding factors.
We retrospectively analyzed patients who underwent ESD (n = 99) or surgery (n = 220) or d-CRT (n = 16) at the Sun Yat-sen University Cancer Center from 2017 to 2019. The median follow-up time for the ESD group, the surgery group, and the d-CRT group was 42.0 mo (95%CI: 35.0-60.2), 45.0 mo (95%CI: 34.0-61.75) and 32.5 mo (95%CI: 28.3-40.0), respectively. After adjusting for background factors using IPTW, the highest 3-year overall survival (OS) rate and 3-year recurrence-free survival (RFS) rate were observed in the ESD group (3-year OS: 99.7% and 94.7% and 79.1%; and 3-year RFS: 98.3%, 87.4% and 79.1%, in the ESD, surgical, and d-CRT groups, respectively). There was no difference of severe complications occurring between the three groups (P ≥ 0.05). Multivariate analysis showed that treatment method, histology and depth of infiltration were independently associated with OS and RFS.
For cT1N0M0 EC, ESD had better long-term survival and lower hospitalization costs than those who underwent d-CRT and surgery, with a similar rate of severe complications occurring.
Core Tip: This is a first retrospective study to compare overall survival, recurrence-free survival and complication rates of endoscopic submucosal dissection (ESD), surgery and definitive chemoradiotherapy (d-CRT). In this study, we found that ESD attained better survival benefits and lower hospitalization costs than surgery and d-CRT, and they had similar complication rates. This study provides a more comprehensive analysis of the efficacy and safety of current cT1N0M0 esophageal cancer (EC) treatment patterns and provides new evidence for the use of ESD in cT1N0M0 EC.
- Citation: Luo SA, Sun YY, Zeng YT, Huang CY. Comparative efficacy and safety between endoscopic submucosal dissection, surgery and definitive chemoradiotherapy in patients with cT1N0M0 esophageal cancer. World J Gastrointest Endosc 2024; 16(2): 72-82
- URL: https://www.wjgnet.com/1948-5190/full/v16/i2/72.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i2.72
Esophageal cancer (EC) is an aggressive and poorly prognostic gastrointestinal tumor and one of the common causes of cancer death[1]. Over the past few decades, the proportion of patients with cT1N0M0 EC has increased due to improvements in endoscopic techniques and increased awareness of disease prevention. Approximately 90% of EC are squamous cell carcinoma (SCC) and vary by geographical region, with SCC being more common in Central Asia and China[2]. According to the depth of infiltration, cT1N0M0 EC is classified as mucosal carcinoma (T1a) and submucosal carcinoma (T1b), regardless of lymph node status.
In the European Society of Endoscientific Oncology and the National Comprehensive Cancer Network guidelines, endoscopic resection is recommended for mucosal (T1a) lesions, surgical resection is recommended for patients with submucosal (T1b) lesions, and definitive chemoradiotherapy (d-CRT) is recommended for patients who are unable or unwilling to undergo surgery[3,4]. Endoscopic resection can accurately stage the patients, reduce the surgical complications, and achieve the effect of curative resection[5-7], but carries a higher risk of recurrence (especially for large lesions). And radical esophagectomy is usually associated with postoperative complications, including anastomotic fistula, vocal cord paralysis, and pneumonia[8].
d-CRT is the standard treatment for patients with locally unresectable esophageal squamous cell carcinoma (ESCC) and an alternative treatment option for locally resectable ESCC[9-13]. However, in clinical practice, d-CRT is often selected as an alternative therapy for cT1N0M0 EC patients, depending on the comorbidities, tumor localization, and widespread expansion. Few reports have described the use of d-CRT in patients with stage I ESCC. A parallel group controlled trial conducted in Japan found that the survival of CRT in cT1bN0M0 ESCC was comparable to surgery and had acceptable toxicity[14]. However, the trial was conducted in Japan, and it was not clear about the generalizability of the evidence to different countries, while elderly patients and those not medically fit for surgery were excluded or underrepresented in the trial, and the study mainly included thoracic EC, thus questioning the generalizability of the results. Given the lack of sufficient evidence for the comparative efficacy of different treatments in cT1N0M0 EC, especially the role of d-CRT, we conducted this first retrospective study to compare the efficacy and complications of endoscopic submucosal dissection (ESD), surgery and d-CRT.
We retrospectively analyzed patients with cT1N0M0 EC treated with ESD, surgery and d-CRT between January 2017 and December 2019 at the Cancer Center of Sun Yat-sen University. The inclusion criteria were as follows: (1) All patients met the diagnostic criteria for cT1N0M0 EC: the tumor tissue was limited to the esophageal mucosa or submucosa without lymph node or distant metastasis (cT1N0M0), and the diagnosis was made by endoscopy, pathological biopsy and imaging evaluation; (2) Patients with histology of SCC or precancerous lesions; (3) Patients without other concomitant malignancies; and (4) Patients with complete clinical medical records.
The study was performed in accordance with the guidelines of the Declaration of Helsinki and was approved by the institutional review board of the Sun Yat-sen University Cancer Center.
The preoperative and postoperative evaluations mainly included endoscopic, imaging and histopathological examinations. Endoscopic examinations were performed by physicians with more than 6 years of endoscopic experience in the Department of Endoscopy of Sun Yat-sen University Cancer Center. Endoscopic examinations generally included conventional endoscopy with white light imaging for all lesions; magnifying endoscopy with narrow-band or blue laser imaging (commonly referred to as ME-NBI/BLI) using a GIF-H260Z (Olympus Corporation, Tokyo, Japan) or EG-L590ZW gastroscope (Fujifilm Corporation, Tokyo, Japan) for suspicious lesions; ultrasound endoscopy utilizing 7.5 MHz, 10 MHz, or 12 MHz radical scanning probes (SU 9000, EG-530UR2, Fujifilm; EU-ME2, Olympus) or a 20-MHz miniature probe (UM-DP20-25R, Olympus) was applied for identifying the depth of tumor infiltration or metastasis of lymph nodes. Preoperative enhanced computed tomography (CT), magnetic resonance or positron emission tomography/CT were performed to assist in the diagnosis of esophageal carcinoma. Postoperative follow-up examinations were started 1-2 mo after the end of treatment, once every 3 mo during the initial 2 years, once every 6 mo from 2 to 5 years, and once a year after 5 years. R0 resection was defined as complete resection of the tumor, and histopathology showed a negative resection margin and no tumor residue after ESD or surgery therapy. Complete response (CR) was defined as the disappearance of the primary tumor and the absence of irregular erosive lesions, ulcerative lesions, or apparently elevated lesions as observed during endoscopy and/or the absence of malignant cells in biopsy specimens after d-CRT therapy[15].
ESD was performed by endoscopists with extensive experience. All inpatients were placed in the left lateral position with general anesthesia under tracheal intubation. The esophageal lesion was stained with Lugol solution, and the resection margin was marked with adenomatous polyposis coli or high-frequency electrocoagulation. A mixed liquid (0.9% NS: Sodium hyaluronate = 4:1) was injected into the submucosa, the submucosa was dissected on the surface of the intrinsic muscular layer after circumferential incision outside the marked points, and the lesion was completely excised. Finally, the specimen was laid flat, fixed on a cork board with pins, soaked in formalin and sent for pathological examination. The intrinsic muscular layer of the esophagus was carefully examined endoscopically for any additional damage or residual tumor at the resection margin. The decision to add other additional treatments was made after a thorough evaluation based on the pathological findings and the therapeutic wishes of the patient and their family. In this study, 2 patients underwent radical EC resection after ESD because of the positive resection margin of the pathological specimen.
Surgery was performed by experienced surgeons in our hospital. After general anesthesia was stabilized, the patients were placed in a supine position. After routine disinfection, surgeons removed the esophageal tumors, dissected the peripheral lymph nodes via thoracotomy or thoracoscopy and reconstructed the digestive tract using a laparotomy or laparoscopic approach. In this study, the pathological examination of 1 patient after esophagectomy indicated that tumor cells were visible at the resection margin of the specimen, so an extended surgical procedure was implemented.
The d-CRT regimen was discussed and decided by physicians with extensive experience in the medical oncology and radiotherapy departments of our hospital. d-CRT consisted of 5 courses of albumin paclitaxel (45-60 mg/m2) and cisplatin (20-25 mg/m2) on Day 1 every week along with concurrent radiotherapy by the intensity-modulated radiotherapy technique. Radiation therapy was delivered using megavoltage equipment (≥ 6 MV). The patients were treated 5 d per week at 1.8 to 2.0 Gy/d for a total dose of 60 Gy. The target volume of radiotherapy was individualized according to the primary tumor site and metastasis. The clinical target volume of mid-thoracic EC was defined as the gross tumor volume with a 3 cm margin for upper and lower extents and the lymph node target volume (gross tumor volume-nd) with a 0.5 to 1 cm margin for three-dimensional extents. The planned target volume was decided according to the actual positional error and was generally formed by a 0.5 cm margin for three-dimensional outward extents based on the clinical target volume and a 0.3 cm margin for cervical or upper thoracic EC fixed by head, neck and shoulder mesh.
The statistical methods used in this study included Student's t test (or Mann-Whitney U test) and Fisher's exact test (or Pearson's chi-square test). The mean ± standard deviation for normally distributed measures was expressed by t test and the median and interquartile range [M (P25, P75)] for nonnormally distributed measures were expressed by rank sum test; the count data were expressed as percentages (%) and compared by chi-square test (χ2 test). To account for selection bias and potential cofounding factors between groups in comparisons of outcome, we performed weighted propensity score analysis to control for differences in baseline characteristics between patients who underwent ESD, surgery and d-CRT. The propensity model was generated using the inverse probability treatment weighting (IPTW) method. Each patient was weighted by inverse probability with the goal of balancing observable features. The Bonferroni correction was needed as a conservative method for probability thresholding to control the occurrence of false positives. The 3-year overall survival (OS) and recurrence-free survival (RFS) were calculated and expressed as months. OS was right censored if the patient was alive at study termination or was lost to follow-up, and patient death was considered an event. In RFS analysis, the recurrence of EC after eradication therapy was considered an event. The follow-up period was calculated from treatment, and the cutoff date was October 30, 2022. Follow-up ended when patients died or were lost to follow-up and cause of death and cause of loss analysis was analyzed. Time to recurrence was calculated from the time of treatment to the time of the most recent endoscopic evaluation at our facility or another hospital. The survival curves were plotted using the Kaplan-Meier method, and OS and RFS rates of therapeutic groups were compared by log-rank test. Cox proportional hazards modeling was used to assess the hazard ratios (HRs) and 95% confidence intervals (CIs). All data were analyzed by SPSS version 25.0 (IBM Corp., Armonk, NY, United States) and R version 4.3.1. All tests were two-sided with a significance level of P < 0.05.
A total of 3911 patients with EC were treated in our hospital between January 2017 and December 2019, including 75 patients with precancerous esophageal lesions and 472 patients with cT1N0M0 EC. After exclusion, we retrospectively analyzed cT1N0M0 EC patients who underwent ESD (n = 99) or surgery (n = 220) or d-CRT (n = 16) at our hospital. Tables 1 and 2 show the baseline characteristics and complications of patients in the ESD, surgery and d-CRT groups before and after IPTW adjustment.
| Characteristic | Unmatched | IPTW | |||||||
| d-CRT | ESD | Surgery | P value | d-CRT | ESD | Surgery | P value | ||
| 16 | 99 | 220 | 9 | 157 | 207 | ||||
| Sex | Female | 5 (31.2) | 39 (39.4) | 53 (24.1) | 0.02 | 1 (11.1) | 24 (15.3) | 61 (29.5) | 0.228 |
| Male | 11 (68.8) | 60 (60.6) | 167 (75.9) | 8 (88.9) | 133 (84.7) | 146 (70.5) | |||
| Age | < 60 | 11 (68.8) | 44 (44.4) | 79 (35.9) | 0.02 | 8 (88.9) | 102 (65.0) | 29 (38.2) | 0.17 |
| ≥ 60 | 5 (31.2) | 55 (55.6) | 141 (64.1) | 1 (11.1) | 55 (35.0) | 128 (61.8) | |||
| Tumor location | Cervical | 0 | 3 (3.0) | 0 | < 0.001 | 0 | 1 (0.6) | 0 | 0.047 |
| Upper thoracic | 4 (25.0) | 13 (13.1) | 14 (6.4) | 0 | 5 (3.2) | 12 (5.8) | |||
| Middle thoracic | 4 (25.0) | 42 (42.4) | 111 (50.5) | 1 (10.5) | 27 (17.2) | 99 (47.8) | |||
| Lower thoracic | 5 (31.2) | 39 (39.4) | 87 (39.5) | 7 (77.4) | 121 (77.1) | 87 (42.0) | |||
| Multiple sources | 3 (18.8) | 2 (2.0) | 8 (3.6) | 1 (8.5) | 4 (2.5) | 9 (4.4) | |||
| Tumor's longest diameter in cm | < 3 | 5 (31.2) | 66 (66.7) | 93 (42.3) | < 0.001 | 1 (11.1) | 31 (19.7) | 93 (44.9) | 0.077 |
| ≥ 3 | 11 (68.8) | 33 (33.3) | 127 (57.7) | 8 (88.9) | 126 (80.3) | 114 (55.1) | |||
| Circumference ratio | < 3/4 | 12 (75.0) | 97 (98.0) | 178 (80.9) | < 0.001 | 8 (88.9) | 136 (86.6) | 176 (85.0) | 0.902 |
| ≥ 3/4 | 4 (25.0) | 2 (2.0) | 42 (19.1) | 1 (11.1) | 21 (13.4) | 31 (15.0) | |||
| Depth of infiltration | M1 | 4 (25.0) | 87 (87.9) | 49 (22.3) | < 0.001 | 2 (22.2) | 59 (37.6) | 85 (41.1) | 0.072 |
| M2 | 2 (12.5) | 4 (4.0) | 9 (4.1) | 6 (66.7) | 3 (1.9) | 9 (4.3) | |||
| M3-SM1 | 2 (12.5) | 3 (3.0) | 18 (8.2) | 0 | 8 (5.1) | 14 (6.8) | |||
| SM2-3 | 8 (50.0) | 5 (5.1) | 144 (65.5) | 1 (11.1) | 87 (55.4) | 99 (47.8) | |||
| Histology | Precancerous | 11 (68.8) | 89 (89.9) | 51 (23.2) | < 0.001 | 3 (33.3) | 61 (38.9) | 88 (42.5) | 0.422 |
| Well-differentiated SCC | 0 | 1 (1.0) | 7 (3.2) | 0 | 0 | 5 (2.4) | |||
| Moderately-differentiated SCC | 5 (31.2) | 6 (6.1) | 108 (49.1) | 6 (66.7) | 88 (56.1) | 77 (37.2) | |||
| Poorly-differentiated SCC | 0 | 3 (3.0) | 54 (24.5) | 0 | 8 (5.0) | 37 (17.9) | |||
| R0 resection or CR | No | 4 (25.0) | 8 (8.1) | 147 (66.8) | < 0.001 | 1 (11.1) | 88 (55.9) | 101 (48.8) | 0.544 |
| Yes | 12 (75.0) | 91 (91.9) | 73 (33.2) | 8 (88.9) | 69 (44.1) | 106 (51.2) | |||
| Complication | No | 9 (56.2) | 93 (93.9) | 143 (65.0) | < 0.001 | 7 (77.8) | 54 (34.4) | 151 (72.9) | 0.039 |
| Yes | 7 (43.8) | 6 (6.1) | 77 (35.0) | 2 (22.2) | 103 (65.6) | 56 (27.1) | |||
| Lymph node metastasis | N0 | 14 (87.5) | 96 (97.0) | 196 (89.1) | 0.059 | 9 (100.0) | 155 (98.7) | 189 (91.3) | 0.002 |
| N1-2 | 2 (12.5) | 3 (3.0) | 24 (10.9) | 0 | 2 (1.3) | 18 (8.7) |
| Complication | Unmatched | IPTW | |||||||
| d-CRT | ESD | Surgery | P value | d-CRT | ESD | Surgery | P value | ||
| 16 | 99 | 220 | 9 | 157 | 207 | ||||
| Radiation pneumonitis | No | 15 (93.8) | 99 (100.0) | 220 (100.0) | < 0.001 | 9 (100.0) | 157 (100.0) | 207 (100.0) | 0.783 |
| Yes | 1 (6.2) | 0 | 0 | 0 | 0 | 0 | |||
| Aphonia | No | 16 (100.0) | 99 (100.0) | 218 (99.1) | 0.591 | 9 (100.0) | 157 (100.0) | 206 (99.5) | 0.745 |
| Yes | 0 | 0 | 2 (0.9) | 0 | 0 | 1 (0.5) | |||
| Pneumothorax | No | 16 (100.0) | 99 (100.0) | 214 (97.3) | 0.203 | 9 (100.0) | 157 (100.0) | 203 (98.1) | 0.537 |
| Yes | 0 | 0 | 6 (2.7) | 0 | 0 | 4 (1.9) | |||
| Dysphagia | No | 15 (93.8) | 99 (100.0) | 219 (99.5) | 0.01 | 9 (100.0) | 157 (100.0) | 206 (99.5) | 0.439 |
| Yes | 1 (6.2) | 0 | 1 (0.5) | 0 | 0 | 1 (0.5) | |||
| Anastomotic ulcer | No | 16 (100.0) | 99 (100.0) | 215 (97.7) | 0.265 | 9 (100.0) | 157 (100.0) | 204 (98.5) | 0.58 |
| Yes | 0 | 0 | 5 (2.3) | 0 | 0 | 3 (1.5) | |||
| Pulmonary infection | No | 16 (100.0) | 99 (100.0) | 206 (93.6) | 0.022 | 9 (100.0) | 157 (100.0) | 197 (95.2) | 0.308 |
| Yes | 0 | 0 | 14 (6.4) | 0 | 0 | 10 (4.8) | |||
| Anastomotic fistula | No | 16 (100.0) | 99 (100.0) | 192 (87.3) | < 0.001 | 9 (100.0) | 157 (100.0) | 187 (90.3) | 0.133 |
| Yes | 0 | 0 | 28 (12.7) | 0 | 0 | 20 (9.7) | |||
| Anastomotic or esophageal stenosis | No | 13 (81.2) | 95 (96.0) | 181 (82.3) | 0.004 | 9 (100.0) | 132 (84.1) | 178 (86.0) | 0.734 |
| Yes | 3 (18.8) | 4 (4.0) | 39 (17.7) | 0 | 25 (15.9) | 29 (14.0) | |||
| MOF | No | 16 (100.0) | 99 (100.0) | 213 (96.8) | 0.154 | 9 (100.0) | 157 (100.0) | 202 (97.6) | 0.489 |
| Yes | 0 | 0 | 7 (3.2) | 0 | 0 | 5 (2.4) |
Before IPTW adjustment, patients in the d-CRT group were older than those in the ESD and surgery groups. In the d-CRT group, there were 6 patients with clinical stage T1a (cT1a: M1-2), of whom 4 (66.7%) achieved CR, and 10 patients with clinical stage T1b (cT1b: M3-SM1-3), of whom 8 (80.0%) achieved CR. Among the cT1a patients, an 87-year-old patient developed more serious radiotherapy toxic side effects such as radiation pneumonia and finally died despite achieving CR, while 1 patient who did not achieve CR died of EC and severe complications of radiotherapy. Among the cT1b patients, severe complications were observed in 4 patients who achieved CR and survived, including 3 patients with esophageal stricture and 2 patients with radiation pneumonia. In contrast, 1 patient who did not achieve CR died after receiving additional treatments because of lymph node metastasis. Patients in the surgery and d-CRT groups had more complications than those in the ESD group. Esophageal stricture was the main postoperative complication (surgery vs ESD vs d-CRT: 17.7% vs 4.1% vs 18.8%, P = 0.004).
While after IPTW adjustment, the covariate balance in the three groups was improved; the number of background factors with P value above 0.05 was increased from 1 to 7. Complication rates were similar in the three groups, with all P values > 0.05.
Table 3 shows the hospitalization costs and remedies after recurrence or metastasis in the ESD, surgery and d-CRT groups. The median follow-up time was 42.0 mo (95%CI: 35.0-60.2) in the ESD group, 45.0 mo (95%CI: 34.0-61.75) in the surgery group and 32.5 mo (95%CI: 28.3-40.0) in the d-CRT group. The ESD group had the lowest hospitalization costs, while the d-CRT group had the highest hospitalization costs among the three groups. One patient died of EC in the ESD group. In the surgery group, 16 patients died of progression or metastasis of EC (84.2%), 2 patients died of postoperative multiorgan failure, and 2 patient died of severe respiratory disease. Two patients died of EC, and one patient died of severe complications of radiation therapy in the d-CRT group. During the follow-up period, 4 patients developed recurrence or metastasis (4.0%), and 3 patients underwent surgical resection of the lesions in the ESD group, while 38 patients in the surgery group developed recurrence or metastasis (17.3%), and 31 patients underwent salvage treatments. Additionally, 1 patient in the d-CRT group was treated with palliative chemoradiotherapy after recurrence or multiple metastases.
| Parameter | ESD, n = 98 | Surgery, n = 220 | d-CRT, n = 16 |
| Follow up time | 40.0 (35.0-48.0) | 43.0 (34.0-53.75) | 32.5 (28.3-40.0) |
| Total cost | 25 | 100 | 130 |
| Death toll | 1 (1.0) | 19 (8.6) | 3 (18.8) |
| Recurrence | 4 (4.0) | 3 8(17.3) | 4 (25.0) |
| Salvage measures after recurrence or metastasis | Surgery (n = 3) | Surgery (n = 5) | Neoadjuvant chemo-radiotherapy (n = 1) |
| No treatment (n = 1) | Neoadjuvant radiotherapy (n = 4) | ||
| Neoadjuvant chemotherapy (n = 8) | |||
| Neoadjuvant chemo-radiotherapy (n = 15) | |||
| No treatment (n = 7) |
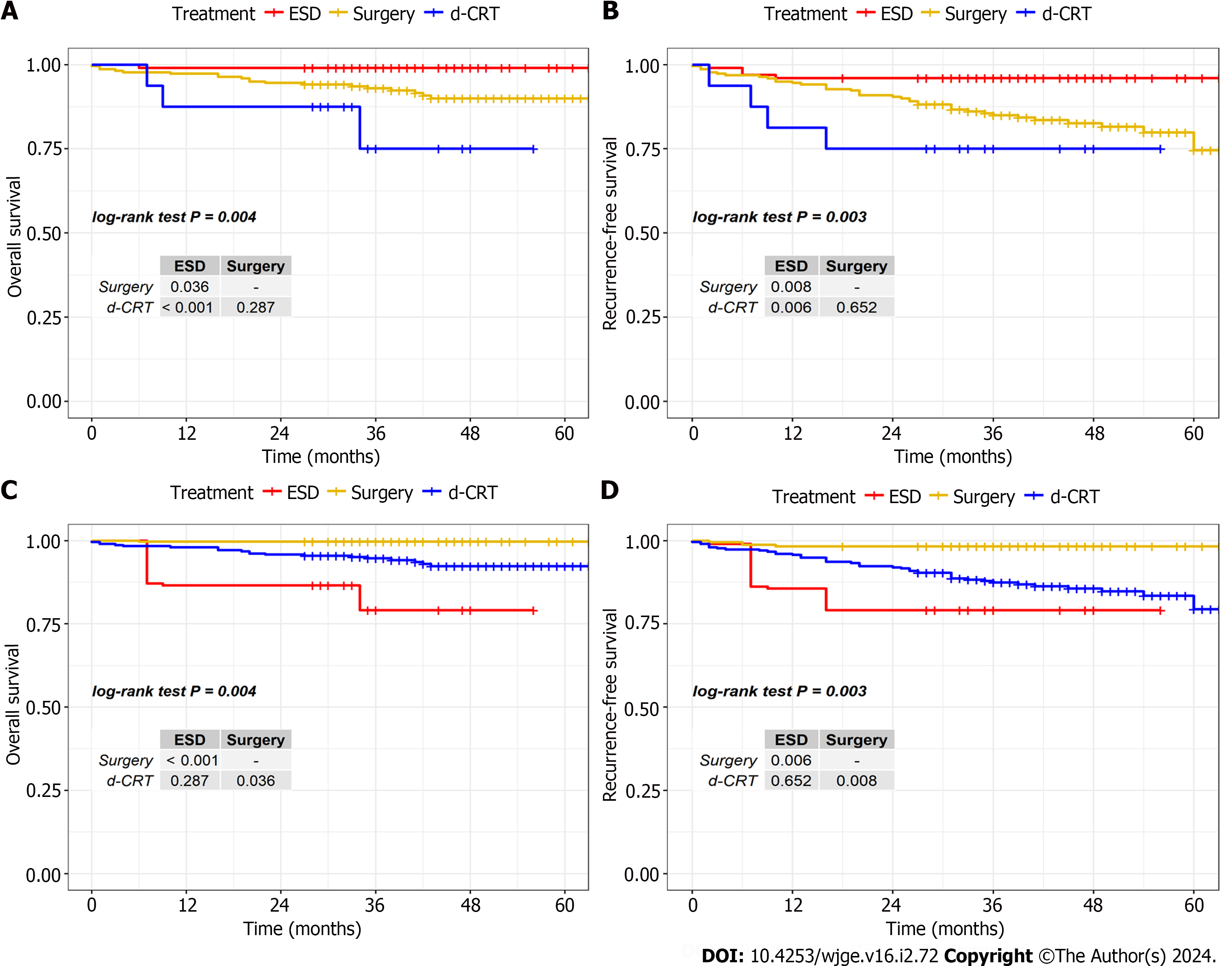
To compare 3-year OS and RFS in the ESD, surgery and d-CRT groups, the survival analysis was performed with IPTW adjustment and using Bonferroni correction to control the occurrence of false positives. Figure 1 shows the Kaplan-Meier survival curves before and after IPTW adjustment. The 3-year OS and RFS of ESD were superior to those of surgery and d-CRT (OS: ESD: 99.7%, surgery: 94.7%, d-CRT: 79.1%; RFS: ESD 98.3%, surgery: 87.4%, d-CRT: 79.1%).
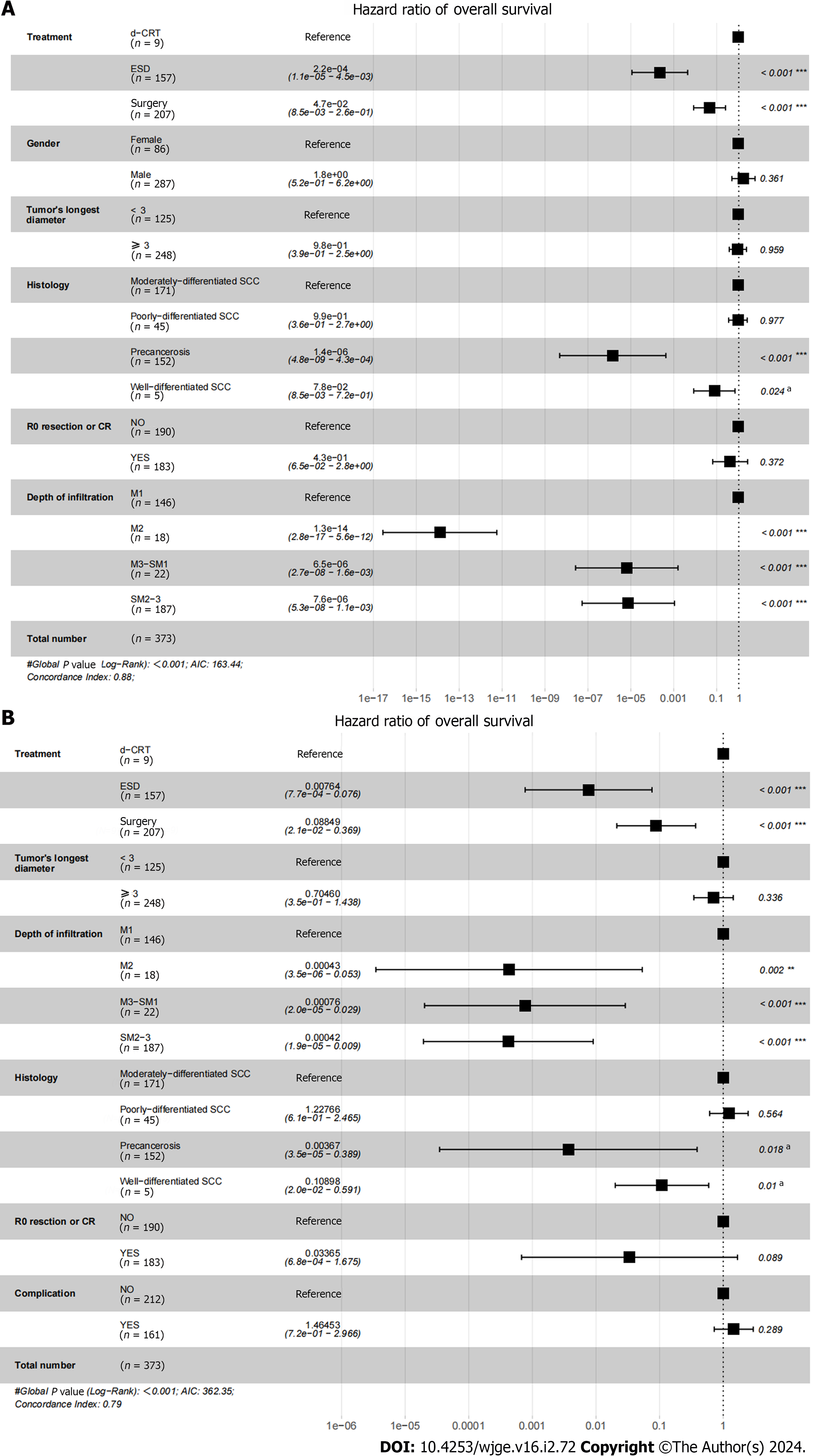
We further investigated the risk factors for OS and RFS in the different treatment modalities. Figure 2 shows the results of the Cox proportional hazards model for OS and RFS after IPTW adjustment. Multivariate analysis showed that treatment method, histology and depth of infiltration were independently associated with OS and RFS.
Many studies have compared the outcomes of ESD and surgery, or surgery and CRT in the treatment of cT1N0M0 EC, but there is a lack of studies directly comparing the efficacy and safety between ESD, surgery and d-CRT. After analyzing our included patients’ clinical data before and after IPTW adjustment, we found that ESD yielded better OS, RFS rates and lower hospitalized costs than surgery and d-CRT. Multivariate analysis showed that treatment method, histology and depth of infiltration were independently associated with OS and RFS, and it was similar to the previous study[16].
We explored the reasons for the difference in 3-year OS and RFS between the three treatments. We found that the depth of infiltration was more superficial in patients in the ESD group and that the local oncological control rate with ESD or surgery was higher, so patients could achieve tumor-free status to a greater extent. Patients in the d-CRT group were generally older and had high-grade and larger tumors, so EC was more likely to progress to an advanced stage and could not achieve CR. Our study showed that the rates of CR and severe complications for patients who received d-CRT were 75.0% and 43.8%, respectively, a relatively lower curative rate and higher complication rate than ESD and surgery. Those factors were perhaps associated with the significant difference in 3-year OS and RFS between the ESD, surgery and d-CRT groups.
Besides, the severe complications were similar among patients treated by these therapies. But we found that esophageal stricture was the major complication of the three treatment methods. Several previous studies have shown that the circumferential extent of the tumor and infiltration depth were independent risk factors for esophageal stricture[17-19]. Currently, clinical measures for the prevention and treatment of postoperative esophageal stenosis include esophageal dilatation, esophageal stent placement, mucosal injection or oral steroid hormone[20-22]. Meanwhile, some novel techniques are being investigated by other scholars[23,24]. However, the effectiveness of these methods requires more clinical evidence and there is no ideal therapy in current clinical practice, so we are also conducting relevant research on this aspect.
By comparing the efficiency and complication rate between ESD, surgery and d-CRT, we summarize the experience of our center: for early EC with infiltration to M1 or M2, no lymph node metastasis, no distant metastasis, and circumferential extent of tumor < 3/4, ESD was the preferred therapy. In particular, for patients with cervical or upper thoracic esophageal carcinoma, ESD is better than surgery. d-CRT should be attempted for patients who are of advanced age, frail, contraindicated to surgery and have an upper and circumferential extent of ≥ 3/4.
In this study, we firstly conducted a retrospective study with a large sample size to compare the efficacy of early EC treated with ESD, surgery and d-CRT, providing some useful suggestions. However, there are some limitations in our study. First, the sample size of our study was still not large enough, especially because the number of patients in the d-CRT group was insufficient. Second, the study was a single-center retrospective study, which has considerable limitations. Last, the data were obtained mainly from the medical records and follow-up, so there was a certain rate of missing visits and missing data. Therefore, multicenter prospective studies with larger sample sizes or randomized controlled studies are needed to supplement the evidence of our study.
This is a first retrospective study to compare OS, RFS and complications of ESD, surgery and d-CRT. In this study, we found that ESD attained better survival benefits and lower hospitalization costs than surgery and d-CRT, and they had similar complications rates. This study provides a more comprehensive analysis of the efficacy and safety of current cT1N0M0 EC treatment patterns and provides new evidence for the use of ESD in cT1N0M0 EC.
For cT1N0M0 esophageal cancer (EC), the current study has mainly focused on surgery and endoscopic submucosal dissection (ESD), while definitive chemoradiotherapy (d-CRT) is a complementary treatment for cT1N0M0 EC. Studies on estimating the therapeutic effect and safety of d-CRT, surgery and ESD are not sufficient, so this study is important.
Early-stage EC is currently increasing year by year, and its treatment methods are also changing rapidly. It is very important to choose the treatment methods with good prognosis and fewer complications, while some patients have the dilemma of treatment choice due to age, cost and other reasons. It is very important to summarize and compare the advantages and disadvantages of the existing treatment methods, which is very important for the health management of patients with EC.
By comparing the efficiency and safety of ESD, surgery and d-CRT for cT1N0M0 EC, to provide a clinical basis for the treatment selection of cT1N0M0 EC and to achieve better prognosis and quality of survival for EC.
We retrospectively analyzed the medical records, pathology, imaging and endoscopic findings, and follow-up results of the cT1N0M0 EC. We met the inclusion criteria and adjusted the effects of confounding factors using the inverse probability of treatment weighting method to conduct survival analysis, Cox proportional risk regression analysis, collected complications and costs, rescue measures after recurrence, and finally evaluated the efficacy and safety of cT1N0M0 EC patients receiving ESD, surgery and d-CRT.
Results showed that ESD had better survival outcomes, lower hospital costs and more acceptable occurrences of complications. This study provides a more comprehensive analysis of the efficacy and safety of current cT1N0M0 EC treatment patterns and provides new evidence for the use of ESD in cT1N0M0 EC. To our knowledge, our study is the first to compare the effects of all three treatments for cT1N0M0 EC. In addition, there are relatively few studies on d-CRT for cT1N0M0 EC patients, and our study can provide relevant evidence of d-CRT for cT1N0M0 EC, so it has a certain new innovation.
This is a first retrospective study to compare overall survival, recurrence-free survival and complication rates of ESD, surgery and d-CRT, and show that ESD attained better survival benefits and lower hospitalization costs than surgery and d-CRT, and they had similar complication rates. This study provides a more comprehensive analysis of the efficacy and safety of current cT1N0M0 EC treatment patterns and provides new evidence for the use of ESD in cT1N0M0 EC.
In the future, we will conduct a subgroup analysis of survival outcomes for the three therapies in cT1N0M0 EC patients, and investigate methods to reduce the occurrence of complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq S-Editor: Liu JH L-Editor: Filipodia P-Editor: Cai YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55641] [Article Influence: 7948.7] [Reference Citation Analysis (131)] |
| 2. | Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev. 2011;12:2461-2466. [PubMed] |
| 3. | Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50-v57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 4. | Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 387] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 5. | Yeh JH, Huang RY, Lee CT, Lin CW, Hsu MH, Wu TC, Hsiao PJ, Wang WL. Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2020;13:1756284820964316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Matsueda K, Ishihara R. Preoperative Diagnosis and Indications for Endoscopic Resection of Superficial Esophageal Squamous Cell Carcinoma. J Clin Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Nagami Y, Ominami M, Shiba M, Minamino H, Fukunaga S, Kameda N, Sugimori S, Machida H, Tanigawa T, Yamagami H, Watanabe T, Tominaga K, Fujiwara Y, Arakawa T. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis. 2017;49:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 729] [Article Influence: 91.1] [Reference Citation Analysis (2)] |
| 9. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1367] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 10. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose vs standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 873] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 11. | Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, Takiuchi H, Komatsu Y, Miyata Y, Fukuda H; Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (JCOG). Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Kato K, Nakajima TE, Ito Y, Katada C, Ishiyama H, Tokunaga SY, Tanaka M, Hironaka S, Hashimoto T, Ura T, Kodaira T, Yoshimura K. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Shinoda M, Ando N, Kato K, Ishikura S, Kato H, Tsubosa Y, Minashi K, Okabe H, Kimura Y, Kawano T, Kosugi S, Toh Y, Nakamura K, Fukuda H; Japan Clinical Oncology Group. Randomized study of low-dose vs standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci. 2015;106:407-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 14. | Kato K, Ito Y, Nozaki I, Daiko H, Kojima T, Yano M, Ueno M, Nakagawa S, Takagi M, Tsunoda S, Abe T, Nakamura T, Okada M, Toh Y, Shibuya Y, Yamamoto S, Katayama H, Nakamura K, Kitagawa Y; Japan Esophageal Oncology Group of the Japan Clinical Oncology Group. Parallel-Group Controlled Trial of Surgery Versus Chemoradiotherapy in Patients With Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology. 2021;161:1878-1886.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Tahara M, Ohtsu A, Hironaka S, Boku N, Ishikura S, Miyata Y, Ogino T, Yoshida S. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol. 2005;35:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Ding H, Chen T, Zhang X, Chen WF, Li Q, Yao L, Korrapati P, Jin XJ, Zhang YX, Xu MD, Zhou PH. Outcomes of Endoscopic Submucosal Dissection vs Esophagectomy for T1 Esophageal Squamous Cell Carcinoma in a Real-World Cohort. Clin Gastroenterol Hepatol. 2019;17:73-81.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, Zhou JM, Chen TY, Zhong YS. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Pih GY, Kim DH, Gong EJ, Na HK, Jung KW, Lee JH, Ahn JY, Choi KD, Song HJ, Lee GH, Jung HY. Preventing esophageal strictures with steroids after endoscopic submucosal dissection in superficial esophageal neoplasm. J Dig Dis. 2019;20:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Zhang S, Ye F, Sun L. Use of stent for prevention of esophageal stricture after circumferential endoscopic submucosal dissection. Dig Endosc. 2019;31 Suppl 1:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EG, Hayee B, Igarashi K, Yoshida A, Ikeda H, Onimaru M, Aoyagi Y, Kudo SE. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Sakaguchi Y, Tsuji Y, Shinozaki T, Ohki D, Mizutani H, Minatsuki C, Niimi K, Yamamichi N, Koike K. Steroid injection and polyglycolic acid shielding to prevent stricture after esophageal endoscopic submucosal dissection: a retrospective comparative analysis (with video). Gastrointest Endosc. 2020;92:1176-1186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Nagami Y, Ominami M, Fujiwara Y. Safe technique of steroid injection utilizing submersion in saline to prevent stricture after esophageal endoscopic submucosal dissection. Dig Endosc. 2020;32:e169-e170. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |