Published online Feb 16, 2024. doi: 10.4253/wjge.v16.i2.64
Peer-review started: November 14, 2023
First decision: December 5, 2023
Revised: December 24, 2024
Accepted: January 11, 2024
Article in press: January 11, 2024
Published online: February 16, 2024
Processing time: 77 Days and 13.6 Hours
A reliable test is essential for diagnosing Helicobacter pylori (H. pylori) infection, and crucial for managing H. pylori-related diseases. Serving as an excellent me
To investigate the discordance between histology and other H. pylori tests, the underlying causes, and the impact on clinical management.
Pathology reports of gastric biopsies were retrieved spanning August 2013 and July 2018. Reports were included in the study only if there were other H. pylori tests within seven days of the biopsy. These additional tests include CLO test, SA, and H. pylori culture. Concordance between histopathology and other tests was determined based on the consistency of results. In instances where histology re
Of 1396 pathology reports were identified, each accompanied by one additional H. pylori test. The concordance rates in detecting H. pylori infection between biopsy and other tests did not exhibit significant differences based on the number of biopsy fragments. 117 discrepant cases were identified. Only 20 cases (9 with CLO test and 11 with SA) had negative biopsy but positive results in other tests. Four cases initially stained with Warthin-Starry turned out to be positive for H. pylori with subsequent IHC staining. Among the remaining 16 true discrepant cases, 10 pa
There are rare discrepant cases with negative biopsy but positive in SA or CLO test. Various factors may contribute to this inconsistency. Most patients in such cases had undergone treatment.
Core Tip: The concordance between histopathology and rapid urease test (CLO test) or stool antigen test (SA) for detecting Helicobacter pylori (H. pylori) detection is excellent. The agreement between histology and H. pylori culture is good. Concordance between histopathology and other tests shows no significant differences based on the number of biopsy fragments. Occasionally, there are rare cases where histology is negative for H. pylori, while the CLO test or SA is positive. The causes of such discrepancies may be multifactorial, necessitating a separate analysis for each case with clinical correlation. Most of these cases were subsequently treated for H. pylori infection.
- Citation: Qi X, Kuan K, El Jabbour T, Lo Y, Liu Q, Fang Y. Retrospective analysis of discordant results between histology and other clinical diagnostic tests on helicobacter pylori infection. World J Gastrointest Endosc 2024; 16(2): 64-71
- URL: https://www.wjgnet.com/1948-5190/full/v16/i2/64.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i2.64
Helicobacter pylori (H. pylori) infection is directly associated with chronic gastritis, gastric/duodenal ulcer, MALT lym
Each diagnostic method has its advantages and disadvantages. No single test is universally acknowledged as a gold standard for detecting H. pylori infection[4]. A well accepted approach is combining two or more detection methods[4]. The selection of diagnostic tests depends on various factors, including test availability, sensitivity, specificity, cost, and turnaround time. Although non-invasive tests like UBT offer high sensitivity and specificity for detecting H. pylori, upper gastrointestinal (GI) endoscopy with biopsy remains the preferred method, particularly for individuals over 60 or those with alarming symptoms, as recommended by the American College of Gastroenterology[6]. In our hospital, the initial evaluation for symptomatic patients typically involves gastric biopsy and another method. Commonly employed other tests include SA, CLO test, and H. pylori culture. Clinicians place particular emphasis on the morphological assessment of gastric biopsy, especially when combined with immunohistochemistry (IHC), considering it one of the most accurate methods. While the results of other clinical tests generally align with biopsy findings, occasional rare discrepancies may pose challenges for clinicians, especially in cases where biopsy results are negative but other clinical tests are positive.
This study aims to clarify and characterize the discrepancies between various diagnostic tests and histological inter
The research received approval from the Institutional Review Board of the hospital (2016-6957). Pathology reports of gastric biopsies were extracted from the hospital's in-house database using Clinical Looking Glass (version 4.4.2) span
The inter-test agreement between histology and another diagnostic test (CLO test, H. pylori culture, or stool test) for detecting H. pylori infection was assessed utilizing the kappa statistic. An excellent agreement was defined as a kappa value ≥ 0.75, fair to good agreement as a kappa value between 0.4 and 0.75, and poor agreement as a kappa value < 0.4. Concordance referred to the alignment between histology and another diagnostic test on H. pylori detection, with the concordance rate calculated as the number of concordant cases divided by the total number of cases. Differences in concordance rates among various diagnostic tests with histology in detecting H. pylori infection were assessed using chi-square tests. Statistical analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC, United States), and p-values of 0.05 or less were deemed statistically significant.
A total of 1396 pathology reports were identified. The majority of biopsies (n = 1199) were stained solely with Warthin-Starry (WS) stain. Only a small number of cases were reported with H. pylori IHC stain only (n = 81), both WS and IHC stains (n = 60), or no special stain used (n = 56) (Table 1). Among them, 392 cases tested positive for H. pylori through morphological examination, with WS stain, and/or with IHC. Each biopsy was accompanied by only one additional H. pylori test (CLO test, SA, or H. pylori culture). Both CLO test and H. pylori culture were invasive, conducted on the day of the biopsy. SA was performed within seven days of the biopsy. The summary of additional test results (CLO test, SA, or H. pylori culture) is presented in Table 2.
| Ancillary stains | Total cases | H. pylori positive | H. pylori negative |
| WS only | 1199 | 333 | 866 |
| IHC only | 81 | 20 | 61 |
| WC/IHC | 60 | 13 | 47 |
| H&E only | 56 | 26 | 30 |
| Total | 1396 | 392 | 1004 |
| Other H. pylori test | CLO test | SA test | H. pylori culture |
| Total cases | 528 | 306 | 562 |
| H. pylori positive cases | 104 | 66 | 145 |
| H. pylori negative cases | 424 | 240 | 417 |
| H. pylori positivity rate | 19.8% | 21.5% | 25.8% |
| Concordance cases | 505 | 282 | 492 |
| Discordance cases | 23 | 24 | 70 |
| Concordance rate with histology | 95.6% | 92.1% | 87.5% |
The overall concordance rate between histology and other diagnostic tests was high (n = 1279; 91.6%). CLO test and stool antigen tests demonstrated significantly higher concordance rates with biopsy in detecting H. pylori infection com
| CLO test positive | CLO test negative | Total | |
| Histology positive | 95 | 14 | 109 |
| Histology negative | 9 | 410 | 419 |
| Total | 104 | 424 | 528 |
| SA positive | SA negative | Total | |
| Histology positive | 55 | 13 | 68 |
| Histology negative | 11 | 227 | 238 |
| Total | 66 | 240 | 306 |
| H. pylori Culture positive | H. pylori Culture negative | Total | |
| Histology positive | 145 | 70 | 215 |
| Histology negative | 0 | 347 | 347 |
| Total | 145 | 417 | 562 |
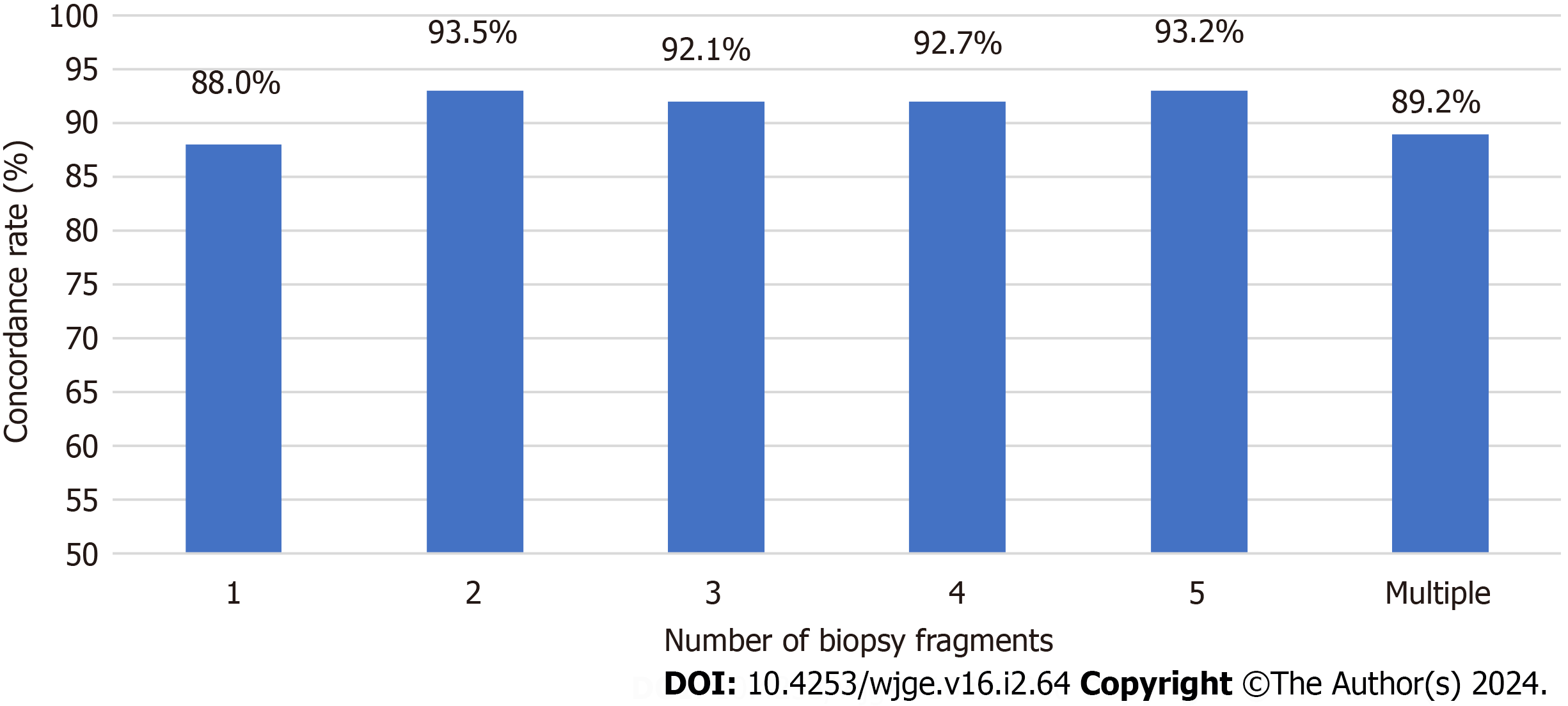
The correlation between concordance rates and the number of biopsy fragments was also examined (Figure 1). The number of biopsy fragments exhibited considerable variation across cases, ranging from 1 to 6 or more: 83 cases (1 frag
The majority of cases exhibiting discordance were those with a positive biopsy but negative results in other H. pylori tests (97 cases). Among these cases, a significant proportion involved bacterial culture (70 cases). Additionally, there were 14 instances of negative CLO tests and 13 cases with negative SA tests, despite a positive histological diagnosis. Con
| Cases # | No. of biopsy specimens | Histology test results | Clinical tests | Treated for H. pylori | ||
| WS/IHC | On PPI/Antibiotics when biopsied | Pos. Clinical Tested | On PPI/Antibiotics when tested | |||
| 1 | 3 | Neg/Neg | NA | CLO | NA | Yes |
| 2 | 2 | Neg/Neg | NA | CLO | NA | Yes |
| 3 | 3 | Neg/Neg | NA | CLO | NA | NA |
| 4 | 2 | Neg/Neg | NA | CLO | NA | NA |
| 5 | 2 | Neg/Pos | No | CLO | No | No |
| 6 | 2 | Neg/Neg | Yes1 | SA | Yes1 | Yes |
| 7 | 1 | Neg/Neg | No | SA | Yes1 | Yes |
| 8 | M | NP/Neg | Yes2 | SA | No | Yes |
| 9 | 3 | Neg/Neg | Yes2 | SA | No | Yes |
| 10 | M | Neg/Pos | Yes1 | SA | Yes1 | Yes |
| 11 | 4 | Neg/Pos | Yes1 | SA | Yes1 | Yes |
| 12 | 3 | NP/Neg | No | SA | No | No |
| 13 | M | Neg/Neg | Yes1 | SA | No | No |
| 14 | 2 | NP/Neg | Yes1 | SA | No | Yes |
| 15 | 1 | NP/Neg | Yes2 | SA | No | Yes |
| 16 | 2 | Neg/Pos | Yes1 | SA | No | Yes |
While histology is commonly regarded as the preferred method for symptomatic patients[6,7], other H. pylori diagnostic tests are equally crucial, often being more convenient, less invasive, and cost-effective, yet maintaining high sensitivity and specificity[4]. Discrepancies between biopsies and the results of other H. pylori diagnostic tests can occasionally arise. No single test is considered the gold standard alone[4]. Nevertheless, clinicians prioritize the morphological assessment of gastric biopsies, especially when augmented with immunohistochemistry, considering it as one of the most accurate approaches. Negative clinical test results may be deemed false negatives when the corresponding biopsy is positive. However, the discrepancy between a negative biopsy and a positive clinical test result can pose a challenge for physi
The CLO test, SA test, and H. pylori bacterial culture were selected for comparison with histology, as they are the most employed tests in conjunction with upper GI endoscopy at our hospital. Both the CLO test and SA test exhibit high sen
The sensitivity and diagnostic accuracy of H. pylori are impacted by the sampling during biopsy. It is recommended by the Sydney protocol[8] to submit four biopsy samples for H. pylori detection: Two from the antrum and two from the body. An additional sample from incisure angularis is advised for gastritis characterization[3,8]. In our study, most cases have less than five biopsy fragments submitted (Figure 1), and there is no available data regarding the origin of these fragments. Interestingly, the number of gastric samples obtained during each endoscopy did not significantly influence the concordance rate between histological diagnosis and other H. pylori tests in our study. However, it is essential to note that concordance does not equate to test accuracy, as concordant results can be either false positives or false negatives. Treatment status at the time of testing is unknown, and both results could be affected by PPI or antibiotics, even if they are concordant.
Most cases have WS stain for histologic diagnosis, as WS stains were automatically ordered from 2013 to 2015 at our hospital. WS stain was preferred over H. pylori IHC stain due to its lower cost and quicker turnaround time. Recent stu
There are 16 discrepant cases, excluding 4 instances where H. pylori was detected by IHC after initially being negative by H&E and WS. Several factors may contribute to the disparity between a negative biopsy and a positive CLO test or SA test. Firstly, the presence of organisms may be reduced or absent due to medications such as PPI and/or antibiotics[12]. Additionally, these medications can alter the appearance of bacteria, making recognition challenging[3,11]. Among the patients, 10 were on PPI before the biopsy procedure and/or other H. pylori tests, with the majority (6 cases) only taking PPI before biopsy, not before other H. pylori tests. For these 6 cases, results from other H. pylori tests are more likely reliable than histology. Secondly, sampling may contribute to the discrepancy, with 12 out of 16 cases having three frag
It is important to note that despite conflicting results presented to clinicians, most patients received treatment without additional testing, except in two cases. This could be attributed to multiple factors, including limited test availability, insurance coverage constraints, challenges in discontinuing medications to minimize testing interference, or delays in treatment. The choice to pursue treatment is influenced by various factors, including clinical symptoms, endoscopic ob
Our findings demonstrated that both CLO test and SA tests exhibit high concordance rates with histological diagnoses. The concordance rate between histology and H. pylori culture is slightly lower, primarily attributed to the lower sensi
Determining Helicobacter pylori (H. pylori) status is essential in the management of H. pylori-related diseases. No single test is universally recognized as the gold standard alone. Typically, symptomatic patients at our hospital undergo upper GI endo
The clinician places particular emphasis on gastric biopsy results, especially when supplemented with immunohistochemistry (IHC), often considering it the most accurate. Rare cases where biopsy results are negative while other clinical tests show positivity can present challenges for clinicians.
The goal of this retrospective study is to examine the discordance between histopathology and alternative H. pylori tests, explore the underlying causes, and assess the implications for clinical management.
Pathology reports of gastric biopsies were retrospectively retrieved from August 2013 to July 2018. Inclusion in the study required the presence of other H. pylori tests within seven days of the biopsy, including rapid urease test (CLO test), stool antigen test (SA), and H. pylori culture. The concordance between histopathology and other tests was evaluated based on result consistency. In cases where histology was negative while other tests showed positivity, the slides underwent reassessment, and the clinical chart was examined.
1396 pathology reports were identified, each accompanied by one additional H. pylori test. The concordance rates between biopsy and other tests did not show significant differences based on the number of biopsy fragments. 117 discrepant cases were identified. Only 20 cases (9 with CLO test and 11 with SA) had negative biopsy but positive results in other tests. Four cases initially stained with Warthin-Starry stain turned out to be positive for H. pylori with subsequent IHC staining. Among the remaining 16 true discrepant cases, 10 patients were on proton pump inhibitors before the biopsy and/or other tests. Most patients underwent treatment, except for two who were untreated, and two patients who were lost to follow-up.
Our findings reveal that both SA and CLO test demonstrate high concordance rates with histological diagnoses. The concordance rate between histology and H. pylori culture is slightly lower, mainly due to the lower sensitivity of the H. pylori culture assay. Importantly, the concordance rate remains consistent regardless of the number of gastric biopsy fragments. Rare instances of discrepancies exist, where H. pylori diagnosis is negative by histology but positive by SA or CLO test. Multiple factors may contribute to the discordance. Despite histological examination showing negative results for H. pylori in these cases with discrepancies, most patients still received treatment. Correlation with clinical history, past laboratory results, and follow-up testing may aid in clinical management.
This retrospective study was conducted at a singular tertiary medical center. It would be intriguing to conduct similar retrospective research in other hospitals to compare discordance rates between histology and other H. pylori tests and variations in clinical management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: College of American Pathologists, 1231720.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li XB, China S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, Wu JY, Kuo CH, Huang YK, Wu DC. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21:11221-11235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 218] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (8)] |
| 2. | Batts KP, Ketover S, Kakar S, Krasinskas AM, Mitchell KA, Wilcox R, Westerhoff M, Rank J, Gibson J, Mattia AR, Cummings OW, Davison JM, Naini BV, Dry SM, Yantiss RK; Rodger C Haggitt Gastrointestinal Pathology Society. Appropriate use of special stains for identifying Helicobacter pylori: Recommendations from the Rodger C. Haggitt Gastrointestinal Pathology Society. Am J Surg Pathol. 2013;37:e12-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015;3:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 4. | Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014;20:12847-12859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (3)] |
| 5. | Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. 2007;21:299-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1016] [Article Influence: 127.0] [Reference Citation Analysis (1)] |
| 7. | Malik GM, Mubarik M, Kadla SA. Helicobacter pylori Infection in Endoscopic Biopsy Specimens of Gastric Antrum: Laboratory Diagnosis and Comparative Efficacy of Three Diagnostic Tests. Diagn Ther Endosc. 1999;6:25-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 9. | Panarelli NC, Ross DS, Bernheim OE, Landzberg ZB, Schuetz AN, Jenkins SG, Landzberg BR, Jessurun J, Yantiss RK. Utility of ancillary stains for Helicobacter pylori in near-normal gastric biopsies. Hum Pathol. 2015;46:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Goldstein NS. Chronic inactive gastritis and coccoid Helicobacter pylori in patients treated for gastroesophageal reflux disease or with H pylori eradication therapy. Am J Clin Pathol. 2002;118:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Chan WY, Hui PK, Leung KM, Chow J, Kwok F, Ng CS. Coccoid forms of Helicobacter pylori in the human stomach. Am J Clin Pathol. 1994;102:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Graham DY, Genta R, Evans DG, Reddy R, Clarridge JE, Olson CA, Edmonds AL, Siepman N. Helicobacter pylori does not migrate from the antrum to the corpus in response to omeprazole. Am J Gastroenterol. 1996;91:2120-2124. [PubMed] |
| 13. | Gisbert JP, Pajares JM. Stool antigen test for the diagnosis of Helicobacter pylori infection: a systematic review. Helicobacter. 2004;9:347-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Kodama M, Murakami K, Okimoto T, Fukuda Y, Shimoyama T, Okuda M, Kato C, Kobayashi I, Fujioka T. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J Gastroenterol. 2012;18:44-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Tepes B. Comparison of two invasive diagnostic tests for Helicobacter pylori after antimicrobial therapy. Scand J Gastroenterol. 2007;42:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015;3:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |