Published online Feb 16, 2024. doi: 10.4253/wjge.v16.i2.55
- This article has been retracted.
- Retraction in: World J Gastrointest Endosc. Aug 16, 2024; 16(8): 500-501 See also: Errata, Retraction, Duplicate Publication and Comment Policy
Peer-review started: September 22, 2023
First decision: October 20, 2023
Revised: November 16, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: February 16, 2024
Processing time: 130 Days and 18.8 Hours
Colorectal polyps (CPs) are frequently occurring abnormal growths in the co
To investigate the potential association between the TyG index and CPs, as the relation between them has not been documented.
A total of 2537 persons undergoing a routine health physical examination and colonoscopy at The First People's Hospital of Kunshan, Jiangsu Province, China, between January 2020 and December 2022 were included in this retrospective cross-sectional study. After excluding individuals who did not meet the eligibility criteria, descriptive statistics were used to compare characteristics between pa
A nonlinear relation between the TyG index and the prevalence of CPs was identified, and exhibited a curvilinear pattern with a cut-off point of 2.31. A significant association was observed before the turning point, with an odds ratio (95% confidence interval) of 1.70 (1.40, 2.06), P < 0.0001. However, the association between the TyG index and CPs was not significant after the cut-off point, with an odds ratio (95% confidence interval) of 0.57 (0.27, 1.23), P = 0.1521.
Our study revealed a curvilinear association between the TyG index and CPs in Chinese individuals, suggesting its potential utility in developing colonoscopy screening strategies for preventing CRC.
Core Tip: This study represents the first exploration of the association between the triglyceride-glucose (TyG) index and colorectal polyps in a Chinese population. The results showed a curvilinear relation, with a significant association observed before a cut-off point of 2.31. Beyond this cut-off point the association was no longer significant. These results provide valuable insights for future research in this area. Importantly, monitoring the TyG index and managing insulin resistance could potentially aid in identifying individuals at a higher risk of developing colorectal polyps, and implementing timely interventions to prevent their progression to colorectal cancer. This study contributes novel perspectives and avenues for preventing colorectal cancer.
- Citation: Teng YJ, Yang YX, Yang JJ, Lu QY, Shi JY, Xu JH, Bao J, Wang QH. Association between triglyceride-glucose index and colorectal polyps: A retrospective cross-sectional study. World J Gastrointest Endosc 2024; 16(2): 55-63
- URL: https://www.wjgnet.com/1948-5190/full/v16/i2/55.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i2.55
Colorectal polyps (CPs) are common abnormal growths and protrusions on the surface of the colon and rectum[1]. There are various types, including adenomatous polyps and hyperplastic polyps, and they are recognized as the most common precursors of colorectal cancer (CRC)[2]. Adenomatous polyps are the most frequently observed premalignant lesions preceding the development of CRC, and CRC is the most commonly diagnosed cancer and the second most common cause of cancer death after lung cancer[3,4]. The incidence of CPs and CRC has been steadily increasing worldwide in recent decades, making them a significant public health concern[5]. Early detection and removal of CPs are crucial for preventing the development of CRC[6]. Identifying risk factors associated with CPs can help in developing effective screening strategies and implementing preventive measures[7]. Several risk factors have been identified for the development of CPs, including age, family history of CRC, genetic predisposition, dietary factors, and lifestyle choices such as smoking and alcohol consumption[8]. However, there is still a need to explore additional risk factors that may contribute to the development of CPs.
The triglyceride-glucose (TyG) index is a novel marker that has gained significant attention in recent research. It is a composite index that combines the levels of fasting plasma glucose and fasting triglycerides, and provides a comprehensive assessment of metabolic health[9]. Recently, the TyG index has become a popular method for assessing insulin resistance (IR), a forerunner of type 2 diabetes[10]. Studies have demonstrated that an elevation of the TyG index cor
Accumulating evidence has shown that IR may increase the risk of CPs. Qin et al[15] reported a significant correlation between IR and the occurrence of CPs and adenomatous polyps. Furthermore, Keku et al[16] conducted a comprehensive study involving 239 patients with colorectal adenoma and 517 adenoma-free persons, and the results suggested that IR is significantly associated with an elevated risk of developing adenomatous polyps. Additionally, the study revealed a decrease in apoptosis within the normal rectal mucosa among individuals with IR. Similarly, Flood et al[17] found that patients with elevated levels of insulin and glucose have a higher risk of recurrence of adenomatous polyps. Notably, patients with increased glucose levels exhibited an even greater increase in the risk of recurrent advanced adenomatous polyps.
Therefore, the purpose of this study was to investigate the potential association between the TyG index and CPs in a Chinese population. Understanding this association may provide valuable insights into the pathogenesis and early detection of CPs, leading to improved screening strategies and better prevention of CRC.
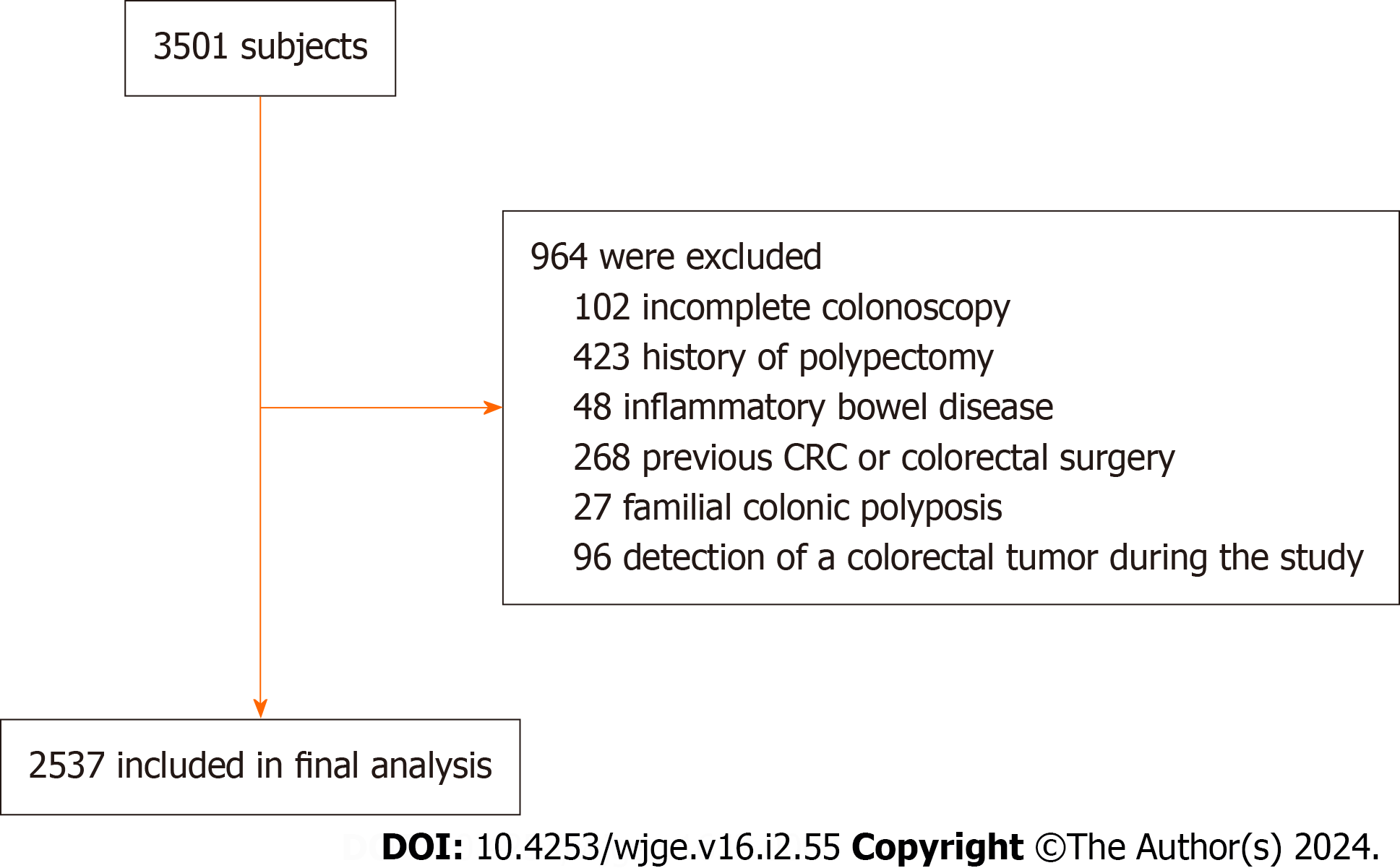
The study included asymptomatic individuals who underwent colonoscopy as part of a comprehensive health screening program at The First People's Hospital of Kunshan, China, between January 2020 and December 2022. The retrospective review included patients between 18 and 79 years of age. Exclusion criteria consisted of incomplete colonoscopy results, history of polypectomy, inflammatory bowel disease, previous CRC or colorectal surgery, familial colonic polyposis, and detection of a colorectal tumor during the examination. The total number of patients included in this study was 2537 (Figure 1). The ethical committee of The First People's Hospital of Kunshan, China, approved the study, and the re
After a 12-h overnight fasting period, blood samples were obtained from each participant. Biochemical analysis of the blood samples was performed using routine enzymatic methods on the VITROS 5600 Integrated System. The blood samples were tested for serum levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), and uric acid (UA). Additionally, the concentrations of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined using previously described methodologies. The TyG index was calculated as Ln [triglycerides (mg/dL) × fasting glucose (mg/dL)/2][18].
Prior to colonoscopy, all patients underwent a bowel preparation involving the use of polyethylene glycol electrolyte powder. The colonoscopies were performed using an ELUXEO 7000 endoscope system (FUJIFILM, Japan) by highly skilled gastroenterologists with a minimum of 5 years of experience in performing colonoscopies. Each endoscopist had performed over 1000 colonoscopies, and performed 6 or more colonoscopy examinations per day. Successful completion of the colonoscopy examination was defined as traversal of the colonoscope to the cecum, which was achieved in 97% of cases.
Pertinent colonoscopy features recorded included the presence or absence of polyps, which were subsequently biopsied or removed. The CPs included adenomatous and non-adenomatous polyps, and patients with polyps diagnosed as malignant were excluded from the study. The histological assessment of the polyps followed the established criteria outlined by the World Health Organization, and was conducted by experienced pathologists. Based on the combined colonoscopy and pathological findings, the patients were categorized into 2 distinct groups: A polyp-free group and a colorectal polyps group (one or more polyps). A subgroup analysis was also performed on the group with adenomatous polyps.
All statistical analyses were performed with R (version 3.5.3) software. Demographic data and risk factors associated with CPs were reported as mean ± SD, or count (percentage). The Kruskal-Wallis test was used to compare continuous variables, while Fisher's exact test was used to compare categorical variables. Multivariate logistic regression was conducted to identify risk factors associated with both adenomatous and non-adenomatous polyps. The consistency of relation was inspected through the use of linear trend tests. Generalized Additive Models (GAMs) and smooth curve fittings were used to examine potential non-linear associations. From the smoothing curve, the turning point was cal
The general characteristics of the study population in relation to colonoscopic findings are summarized in Table 1. In comparison to the polyp-free group, patients with CPs were older and predominantly male. The polyp-free group had lower levels of AST, and higher levels of TGs, TC, LDL-C, HDL-C, UA, and FPG, and a higher TyG index (Table 1).
| Polyp-free | Colorectal polyps | P value vs polyp-free | Adenomatous polyps | P value vs polyp-free | |
| n (%) | 1349 (53.17) | 1188 (46.83) | 276 | ||
| Male | 680 (50.41) | 772 (64.98) | < 0.001 | 170 (14.31) | < 0.001 |
| Age (yr) | 52.92 ± 15.49 | 55.87 ± 11.91 | < 0.001 | 53.62 ± 12.70 | < 0.449 |
| WBC (× 109/L) | 5.74 ± 2.00 | 5.74 ± 1.53 | 0.940 | 5.68 ± 1.43 | 0.235 |
| ALT (U/L) | 27.37 ± 50.07 | 24.96 ± 20.08 | 0.122 | 27.27 ± 20.16 | < 0.001 |
| AST (U/L) | 25.12 ± 36.33 | 22.62 ± 11.14 | 0.023 | 23.24 ± 9.16 | 0.008 |
| TG (mmol/L) | 1.44 ± 1.25 | 1.64 ± 1.12 | < 0.001 | 1.71 ± 1.37 | 0.001 |
| TC (mmol/L) | 4.29 ± 1.07 | 4.67 ± 0.96 | < 0.001 | 4.59 ± 1.01 | < 0.001 |
| LDL-C (mmol/L) | 2.62 ± 0.82 | 2.88 ± 0.77 | < 0.001 | 2.82 ± 0.81 | < 0.001 |
| HDL-C (mmol/L) | 1.33 ± 0.36 | 1.40 ± 0.31 | < 0.001 | 1.39 ± 0.31 | 0.009 |
| UA (mmol/L) | 306.95 ± 90.30 | 332.52 ± 89.48 | < 0.001 | 330.76 ± 88.24 | < 0.001 |
| GLU (mmol/L) | 5.35 ± 1.32 | 5.46 ± 1.14 | < 0.001 | 5.47 ± 1.06 | 0.002 |
| TyG index | 1.15 ± 0.62 | 1.32 ± 0.61 | < 0.001 | 1.31 ± 0.69 | < 0.001 |
A positive correlation between the TyG index and the risk of CPs was identified (Table 2). The association remained significant after adjusting for different variables. In Model I, no covariates were adjusted; in Model II, age and sex were adjusted; and in Model III adjustment was made for age, sex, TC, LDL-C, and HDL-C. The positive association persisted in Model III [odds ratio (OR) = 1.56; 95% confidence interval (CI): 1.03–1.86; P < 0.0001]. These results indicate that each unit increase in the TyG index was associated with a 56% higher risk of CPs. Stratifying the data by age or sex revealed consistent positive associations similar to those observed without stratification (Table 2).
| Variable | Model I | Model II | Model III | |||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Colorectal polyps | ||||||
| TyG index | 1.57 (1.37, 1.78) | < 0.0001 | 1.43 (1.25, 1.64) | < 0.0001 | 1.56 (1.30, 1.86) | < 0.0001 |
| Sex | ||||||
| Male | 1.52 (1.29, 1.80) | < 0.0001 | 1.53 (1.29, 1.81) | < 0.0001 | 1.80 (1.42, 2.28) | < 0.0001 |
| Female | 1.45 (1.18, 1.78) | 0.0005 | 1.36 (1.10, 1.68) | 0.0050 | 1.32 (1.01, 1.76) | 0.0457 |
| Age (yr) | ||||||
| < 50 | 1.81 (1.45, 2.27) | < 0.0001 | 1.62 (1.29, 2.04) | < 0.0001 | 1.66 (1.19, 2.30) | 0.0027 |
| > 50 | 1.37 (1.17, 1.61) | 0.0001 | 1.33 (1.13, 1.57) | 0.0006 | 1.37 (1.11, 1.70) | 0.0037 |
| Adenomous polyps | ||||||
| TyG index | 1.48 (1.21, 1.80) | < 0.0001 | 1.42 (1.16, 1.74) | 0.0006 | 1.84 (1.34, 2.52) | 0.0002 |
| Sex | ||||||
| Male | 1.49 (1.16, 1.91) | 0.0016 | 1.50 (1.17, 1.92) | 0.0015 | 2.04 (1.42, 2.92) | 0.0001 |
| Female | 1.32 (0.94, 1.84) | 0.1097 | 1.33 (0.94, 1.89) | 0.1028 | 1.33 (0.84, 2.11) | 0.2258 |
| Age (yr) | ||||||
| < 50 | 1.94 (1.40, 2.70) | < 0.0001 | 1.85 (1.31, 2.60) | 0.0004 | 2.26 (1.40, 3.64) | 0.0008 |
| > 50 | 1.24 (0.96, 1.60) | 0.0963 | 1.22 (0.95, 1.58) | 0.1197 | 1.40 (0.99, 1.97) | 0.0578 |
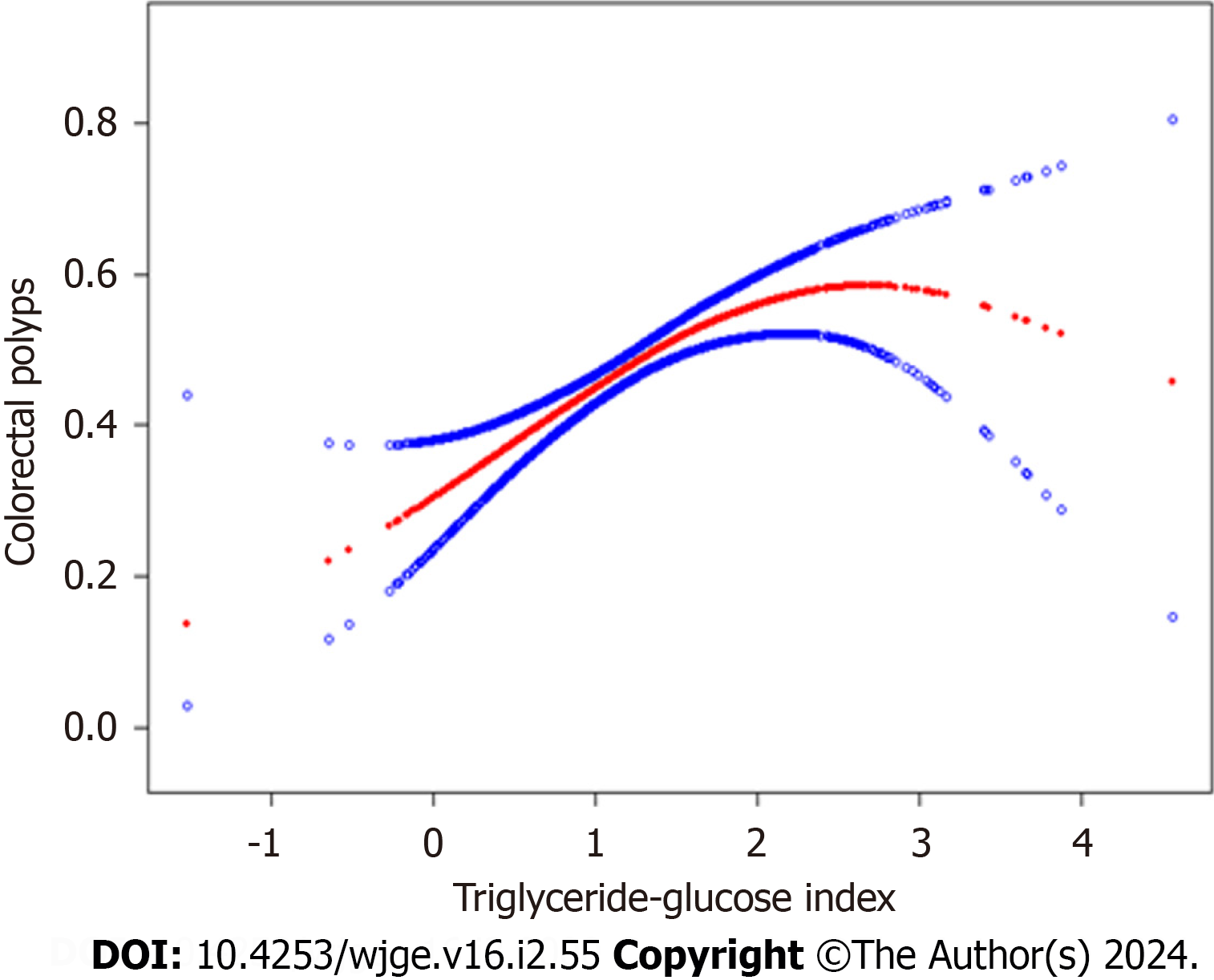
The relation between the TyG index and the prevalence of CPs was non-linear, as evidenced by the GAM and smoo
| Colorectal polyps | Odds ratio (95%CI) | P value |
| TyG index | ||
| Fitting by standard linear model | 1.55 (1.30, 1.86) | < 0.0001 |
| Fitting by two-piecewise linear model | ||
| Inflection point | 2.31 | |
| < 2.31 | 1.7 (1.40, 2.06) | < 0.0001 |
| > 2.31 | 0.57 (0.27, 1.23) | 0.1521 |
| Log-likelihood ratio | 0.009 | |
In this cross-sectional study conducted within a Chinese population, we examined the correlation between the TyG index and CPs. We observed a persistent positive association, even after adjusting for variables such as sex, age, TC, HDL-C, and LDL-C. The relation was non-linear, with a significant turning point at 2.31. Notably, prior to the turning point each unit increase in the TyG index was associated with a 56% higher risk of CPs. This study is the first to investigate the association between the TyG index and CPs in a Chinese population. It is also the first study to uncover a non-linear association between the TyG index and CPs, and subsequently conduct a threshold effects analysis to further examine the non-linear relation.
Our results showed a critical threshold at 2.31 in the non-linear relation between the TyG index and CPs. Specifically, our results suggest that increasing TyG index values are significantly associated with an increased risk of developing CPs up to the turning point of 2.31. Beyond this threshold the relation was no longer statistically significant. It is well esta
Previous research has established a significant association between the TyG index and the development of CRC. It has also suggested that the TyG index should be considered an important factor in determining the need for screening co
The exact mechanisms underlying the relation between the TyG index and CRC development are not fully understood. However, there is substantial evidence linking IR to CRC, and the TyG index is a validated surrogate marker for IR, comparable to the commonly used Homeostatic Model Assessment of IR index[22]. Thus, it is biologically plausible to consider an elevated TyG index as a risk factor for CRC.
Previous studies have shown that IR is a significant risk factor for the development of CRC. Insulin and insulin-like growth factor (IGF) may contribute to CRC development through their anti-apoptotic and mitogenic effects[23]. In a Mendelian randomization analysis conducted by Murphy et al[24] that used blood samples from nearly 400000 persons in the United Kingdom Biobank, an association between circulating levels of IGF1 and CRC was demonstrated. Using genetic data from over 52000 patients with CRC and 46000 persons without CRC, they observed that genetically de
Previous studies have also shed light on the role of insulin/IGF-1 in CRC. It has been shown that insulin/IGF-1 activate signaling pathways such as PI3K/Akt/mTORC and Raf/MAPK, thereby promoting cancer progression. In addition, insulin/IGF-1 activation leads to the activation of glucose transporters like GLUT1 and key glycolytic enzymes including LDHA, LDH5, HK II, and PFKFB3. Moreover, abnormal expression of oncogenes such as MYC and KRAS, as well as overexpression of signaling proteins like HIF-1, TGF-β1, PI3K, ERK, Akt, and mTOR, have been observed in CRC[25-29].
Our study has several limitations which we would like to acknowledge. First, while missing data of covariates were assumed to be missing randomly and the sample size was adequately large to draw a conclusion, we did not use multiple imputation to account for the missing data, which may potentially affect the accuracy of the results. Second, since this was a cross-sectional study, causality between the TyG index and the risk of CPs cannot be determined. Third, this study was conducted at a single center, and therefore multi-center studies should be carried out to verify our findings.
In summary, the TyG index, as a surrogate measure of IR, may potentially mediate the association between insulin-related factors, such as IGF1, and the risk of CRC. Our results clearly demonstrate an association between the TyG index and the development of CPs, providing new evidence in support of colonoscopy screening. Despite the limitations of our study, our findings suggest the need for further research to evaluate the potential role of the TyG index in assessing the risk for CPs and identifying patients who would benefit from colonoscopy screening.
Colorectal polyps (CPs) are widely recognized as precursors to colorectal cancer (CRC), posing a significant global health concern. The triglyceride-glucose (TyG) index, an emerging biomarker, has shown associations with metabolic health and insulin resistance, making it a subject of interest in gastrointestinal cancer research.
The increasing incidence of CPs and CRC worldwide underscores the need for effective screening strategies. This study aims to fill the gap in knowledge by exploring the potential link between the TyG index and CPs in a Chinese population. Understanding this relationship could have implications for developing preventative measures and screening strategies.
The primary objective is to investigate the association between the TyG index and CPs, marking a pioneering exploration in a Chinese demographic. The study endeavors to identify a potential turning point in this relationship, offering valuable insights for future research and interventions.
The retrospective cross-sectional study involves 2537 participants undergoing health examinations and colonoscopies. Thoroughly described methods include participant selection criteria, TyG index calculation, and statistical analyses. By employing logistic regression and a comprehensive approach, the study aims to reveal the nuances of the TyG index's association with CPs.
The study unveils a non-linear relationship between the TyG index and CP prevalence, delineating a significant turning point at 2.31. The analysis indicates a heightened risk of CPs before this threshold, while the association diminishes beyond it. The results contribute to the understanding of the TyG index's role in colorectal health, with potential implications for risk assessment and screening strategies.
This study's novel findings confirm a curvilinear association between the TyG index and colorectal polyps, with a critical cut-off point at 2.31. The persistent positive association before this point highlights the potential utility of the TyG index in identifying individuals at risk. This study's conclusion emphasizes the relevance of these findings in shaping colo
The study prompts further investigation into the mechanisms linking the TyG index, insulin resistance, and colorectal health. Advocating for multi-center studies, the research perspectives underscore the importance of validating findings across diverse populations. The TyG index's potential role in informing future screening guidelines and its broader applicability for assessing colorectal polyp risk remain promising avenues for future research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rathnaswami A, India; Toyoshima O, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Xu ZH
| 1. | Meseeha M, Attia M. Colon Polyps. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Cited in This Article: 1] |
| 2. | Zhu Y, Qiao L, Zhou Y, Ma N, Wang C, Zhou J. Long non-coding RNA FOXD2-AS1 contributes to colorectal cancer proliferation through its interaction with microRNA-185-5p. Cancer Sci. 2018;109:2235-2242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Kaźmierczak-Siedlecka K, Dvořák A, Folwarski M, Daca A, Przewłócka K, Makarewicz W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 50630] [Cited by in RCA: 60277] [Article Influence: 15069.3] [Reference Citation Analysis (171)] |
| 5. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 3058] [Cited by in RCA: 3188] [Article Influence: 398.5] [Reference Citation Analysis (2)] |
| 6. | Kawamura T, Takeuchi Y, Asai S, Yokota I, Akamine E, Kato M, Akamatsu T, Tada K, Komeda Y, Iwatate M, Kawakami K, Nishikawa M, Watanabe D, Yamauchi A, Fukata N, Shimatani M, Ooi M, Fujita K, Sano Y, Kashida H, Hirose S, Iwagami H, Uedo N, Teramukai S, Tanaka K. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2018;67:1950-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 112] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Liljegren A, Lindblom A, Rotstein S, Nilsson B, Rubio C, Jaramillo E. Prevalence and incidence of hyperplastic polyps and adenomas in familial colorectal cancer: correlation between the two types of colon polyps. Gut. 2003;52:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology. 2018;155:355-373.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 103] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | da Silva A, Caldas APS, Rocha DMUP, Bressan J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: A systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;14:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 12] [Cited by in RCA: 394] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 12. | Shi YY, Zheng R, Cai JJ, Qian SZ. The association between triglyceride glucose index and depression: data from NHANES 2005-2018. BMC Psychiatry. 2021;21:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 2] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Yilmaz M, Karaaslan M, Tonyali S, Celik M, Toprak T, Odabas O. Triglyceride-Glucose Index (TyG) is associated with erectile dysfunction: A cross-sectional study. Andrology. 2021;9:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Fritz J, Bjørge T, Nagel G, Manjer J, Engeland A, Häggström C, Concin H, Teleka S, Tretli S, Gylling B, Lang A, Stattin P, Stocks T, Ulmer H. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol. 2020;49:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 20] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Qin M, Wang HP, Song B, Sun YL, Wang DY, Chen M, Shi HX, Zhang H, Li ZJ. [Relationship between insulin resistance, serum VCAM-1, FGF19, IGF-1 and colorectal polyps]. Zhonghua Zhong Liu Za Zhi. 2021;43:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Reference Citation Analysis (0)] |
| 16. | Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2076-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Flood A, Mai V, Pfeiffer R, Kahle L, Remaley AT, Lanza E, Schatzkin A. Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology. 2007;133:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The Triglyceride-Glucose Index, an Insulin Resistance Marker, Was Non-linear Associated With All-Cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med. 2020;7:628109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 24] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 607] [Cited by in RCA: 1059] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 20. | Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Triglyceride-glucose index (TyG index) is a predictor of incident colorectal cancer: a population-based longitudinal study. BMC Endocr Disord. 2020;20:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Liu T, Zhang Q, Wang Y, Ma X, Song M, Cao L, Shi H. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. 2022;22:1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 22. | Low S, Khoo KCJ, Irwan B, Sum CF, Subramaniam T, Lim SC, Wong TKM. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;143:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030-1035. [PubMed] [Cited in This Article: 1] |
| 24. | Murphy N, Carreras-Torres R, Song M, Chan AT, Martin RM, Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Bradbury KE, Besevic J, Rinaldi S, Riboli E, Cross AJ, Travis RC, Agnoli C, Albanes D, Berndt SI, Bézieau S, Bishop DT, Brenner H, Buchanan DD, Onland-Moret NC, Burnett-Hartman A, Campbell PT, Casey G, Castellví-Bel S, Chang-Claude J, Chirlaque MD, de la Chapelle A, English D, Figueiredo JC, Gallinger SJ, Giles GG, Gruber SB, Gsur A, Hampe J, Hampel H, Harrison TA, Hoffmeister M, Hsu L, Huang WY, Huyghe JR, Jenkins MA, Keku TO, Kühn T, Kweon SS, Le Marchand L, Li CI, Li L, Lindblom A, Martín V, Milne RL, Moreno V, Newcomb PA, Offit K, Ogino S, Ose J, Perduca V, Phipps AI, Platz EA, Potter JD, Qu C, Rennert G, Sakoda LC, Schafmayer C, Schoen RE, Slattery ML, Tangen CM, Ulrich CM, van Duijnhoven FJB, Van Guelpen B, Visvanathan K, Vodicka P, Vodickova L, Vymetalkova V, Wang H, White E, Wolk A, Woods MO, Wu AH, Zheng W, Peters U, Gunter MJ. Circulating Levels of Insulin-like Growth Factor 1 and Insulin-like Growth Factor Binding Protein 3 Associate With Risk of Colorectal Cancer Based on Serologic and Mendelian Randomization Analyses. Gastroenterology. 2020;158:1300-1312.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 25. | Khan KH, Yap TA, Yan L, Cunningham D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer. 2013;32:253-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 126] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Wei H, Dong C, Shen Z. Kallikrein-related peptidase (KLK10) cessation blunts colorectal cancer cell growth and glucose metabolism by regulating the PI3K/Akt/mTOR pathway. Neoplasma. 2020;67:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Liu X, Yu M, Xu M, Xiao Y, Ma W, Huang L, Li X, Ye X. Berberine inhibits proliferation and induces G0/G1 phase arrest in colorectal cancer cells by downregulating IGF2BP3. Life Sci. 2020;260:118413. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Mao L, Chen Q, Gong K, Xu X, Xie Y, Zhang W, Cao H, Hu T, Hong X, Zhan YY. Berberine decelerates glucose metabolism via suppression of mTOR-dependent HIF-1α protein synthesis in colon cancer cells. Oncol Rep. 2018;39:2436-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Zhang XL, Li KJ, Feng JX, Liu GJ, Feng YL. Blocking the IGF2BP1-promoted glucose metabolism of colon cancer cells via direct de-stabilizing mRNA of the LDHA enhances anticancer effects. Mol Ther Nucleic Acids. 2021;23:835-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |