Published online Dec 16, 2024. doi: 10.4253/wjge.v16.i12.668
Revised: September 16, 2024
Accepted: October 14, 2024
Published online: December 16, 2024
Processing time: 140 Days and 20.1 Hours
Thermal damage may lead to inflammation of the peeled mucosal surface during endoscopic submucosal dissection (ESD).
To determine the effect of Joule heat on the onset of post-ESD electrocoagulation syndrome (PECS).
In this prospective study, PECS was characterized by in-hospital fever (white blood cell count: ≥ 10000 μ/L or body temperature ≥ 37.5 °C) and abdominal pain (visual analog scale score ≥ 30 mm during hospitalization or increased by ≥ 20 mm from baseline at admission). High Joule heat was defined as 15390 J. Between April 2020 and April 2024, 209 patients underwent colorectal ESD; those with intraoperative perforation or penetration were excluded. The remaining 202 patients were divided into the PECS and non-PECS groups.
PECS occurred in 30 (14.9%) patients. Multivariate analysis revealed high Joule heat as an independent factor associated with PECS (odds ratio = 7.96; 95% confidence interval: 2.91-21.8, P < 0.01). The procedure time and presence of lesions in the right colon were not associated with PECS.
Accumulated thermal damage on the peeled mucosal surface should be considered during PECS onset. This thermal damage is likely a major component of the mechanism underlying PECS.
Core Tip: This study demonstrated that thermal damage to the peeled mucosal surface during endoscopic submucosal dissection (ESD) is associated with the onset of post-ESD electrocoagulation syndrome (PECS). Analysis of 202 patients with colorectal ESD from April 2020 to April 2024 showed high Joule heat as an independent risk factor for PECS, occurring in 14.9% of the patients. Other factors, such as lesion location in the right colon and procedure time, were not associated with PECS. Accumulated thermal damage on the peeled mucosal surface likely plays an important role in PECS onset.
- Citation: Ochi M, Yamamoto A, Suematsu S, Fukuda K, Morishige K, Oka Y, Ishikawa Y, Ueyama S, Hiroshima Y, Omae Y, Kusano F, Kamoshida T. High Joule heat as a risk factor for post-endoscopic submucosal dissection electrocoagulation syndrome: A multicenter prospective study. World J Gastrointest Endosc 2024; 16(12): 668-677
- URL: https://www.wjgnet.com/1948-5190/full/v16/i12/668.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i12.668
Colorectal endoscopic submucosal dissection (ESD) is performed as a minimally invasive procedure aimed at achieving the optimal en bloc resection for early-stage colorectal tumors[1-4]. However, ESD is linked to significant complications, such as bleeding (0.7%-3.1%) and perforation (2%-14%)[4-10]. Another notable adverse event associated with ESD is the post-ESD electrocoagulation syndrome (PECS). The reported incidence of PECS ranges from 9% to 40%. However, in many cases, there is a tendency for improvement with conservative therapy[11-15]. In some cases, PECS can present as a delayed perforation, highlighting the importance of identifying patients at risk using clinically relevant PECS predictors[12,16].
Currently, two hypotheses have been proposed to elucidate the mechanisms behind PECS. One study suggested that ESD exposes the intestinal mucosa to intestinal bacteria, resulting in inflammation[13,17]. Another study suggested that the thermal damage during ESD may lead to inflammation of the peeled mucosal surface[13,18]. Neither endoscopic clipping closure nor prophylactic antibiotic, aimed at preventing inflammation from intestinal bacterial infection of the exposed mucosa, was effective in reducing the incidence of PECS[19,20]. However, few studies have investigated the thermodynamic impact during ESD on the occurrence of PECS. A recent study reported that transmural thermodynamic impact on the dissected surface during ESD is likely to be the mechanism underlying the occurrence of PECS[21]; however, this was a retrospective trial. Therefore, in this prospective, multicenter, observational study, we aimed to evaluate the thermodynamic impact on the dissected surface on the incidence of PECS in patients receiving treatment with colorectal ESD.
We conducted a prospective, multicenter, observational study involving patients undergoing colorectal ESD as a treatment modality at tertiary Japanese institutions. Patient enrollment was based on the applicability criterion for colorectal ESD outlined in the Japan Gastroenterological Endoscopy Society[22]. In this study, the inclusion criteria were as follows: Carcinomas with shallow T1a invasion (submucosal invasion depth < 1000 μm); lesions that were difficult to remove en bloc using endoscopic mucosal resection, such as large protruding tumors and large depressed tumors; and mucosal lesions accompanied by submucous fibrosis. Patients with T1b invasion (submucosal invasion depth ≥ 1000 μm), multiple lesions per ESD session, and perforation or penetration during colorectal ESD were excluded. Patients underwent outpatient physician’s examinations after 2-3 weeks of colorectal ESD. Physicians evaluated the white blood cell count, C-reactive protein levels, and the intensity of localized abdominal pain using the visual analog scale (VAS) in an outpatient setting.
The study protocol was formulated with Hitachi General Hospital as the main coordinating institution, and was subsequently endorsed by the research ethics committee of joint-research facility. The study was officially registered in the University Hospital Medical Information Network Clinical Trials Registry under the identifier UMIN000041580, and adhered to the guidelines outlined in the 2010 Consolidated Standards of Reporting Trials. Before enrollment, all patients provided written informed consent.
The occurrence of post-PECS was defined by patients meeting both of the following conditions: (1) Localized abdominal pain, evaluated with a VAS score ≥ 30 mm during admission, or an increase of ≥ 20 mm from the baseline VAS score at hospital admission; and (2) Fever, indicated by a white blood cell count ≥ 10000/μL or body temperature ≥ 37.5 °C during admission.
Bowel preparation was conducted by ingesting 2 L of polyethylene glycol before ESD. Subsequently, colorectal ESD procedures were carried out using sedation induced by midazolam (1-5 mg) and/or pentazocine (15-30 mg/kg body weight). Electrocardiographic and respiratory monitoring were performed for each session. ESD was performed by endoscopists comprising experts or trainees. When trainees performed colorectal ESD, they did so under the guidance of experts. Endoscopists who performed > 40 colorectal ESD procedures were defined as experts (four endoscopists), while those who performed < 40 procedures were defined as trainees (13 endoscopists)[23]. A colonoscope (PCF-Q290TI; Olympus, Tokyo, Japan) with a hood (DH-29CR; Fujifilm, Tokyo, Japan) attached to its tip was utilized during colorectal ESD procedures. Marking, mucosal detachment, and mucosal incision were performed using a dual knife (KD-655Q; Olympus). Before performing mucosal detachment, 0.4% sodium hyaluronate diluted in physiological saline was injected directly into the submucosal layer beneath the lesion. We achieved hemostasis using a coagrasper (FD-411QR; Olympus) and an endoscopic clip (HX-610-090; Olympus) for bleeding that occurred during marking, mucosal detachment, and mucosal incision.
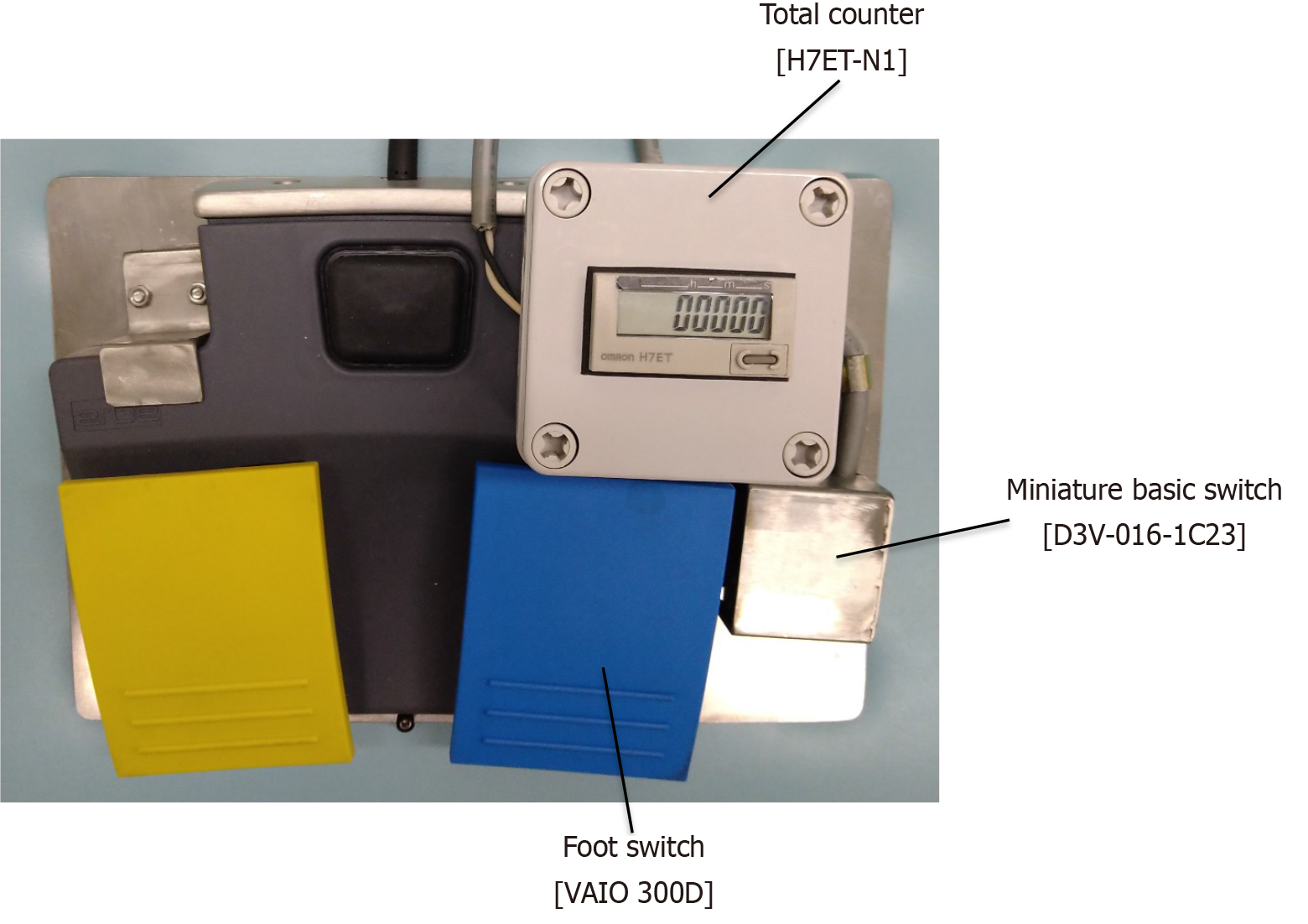
High-frequency was generated using a high-frequency generator (VIO 300D; ERBE Co. Ltd., Tubingen, Germany) equipped with an electrosurgical coagulation unit from the tip of the dual knife for mucosal detachment. We used a device to convert the time measured in the swift coagulation mode into Joule heat by installing a small micro switch [Miniature basic switch (D3V-016-1C23); OMRON Corporation, Kyoto, Japan] adjacent to the foot switch of the electrosurgical coagulation unit. The small micro switch was activated by pressing the foot switch (Figure 1).
We calculated the applied power based on a diagram (Effect 4) depicting the relationship between power (W) and resistance (Ω) using swift coagulation mode[21]. The resistance of the lesion targeted for resection was defined as 635 Ω, considering that the internal resistance of the human body typically falls within the range of 535-735 Ω[24]. The electrosurgical coagulation unit can automatically adjust its power output during lesion detachment, but monitoring this automatic adjustment is challenging. By identifying the intersection of the vertical axis [power (W)] and horizontal axis [resistance (Ω)], the power corresponding to 635 Ω was determined to be 80 W[21]. Based on the relationship that power consumption (J) multiplies power (W) by time (s), we calculated the thermally loaded Joule heat on the mucosal surface. This allowed us to compute the total Joule heat applied to the surface. Additionally, high Joule heat was defined as exceeding 15390 J[21].
In all patients, vital signs were monitored on post-operative day (POD) 1 and blood samples were obtained to assess signs of bleeding. Additionally, patients were assessed for pain using VAS, and chest X-ray examination was performed to verify the presence of free air. The patient was allowed to resume eating on POD 1, beginning with dinner. If bleeding, localized abdominal pain, or fever did not occur following the resumption of eating, the patient was discharged on POD 6.
Clinicopathological factors and outcomes were analyzed using the Mann-Whitney U test (body mass index, age), the χ2 test/Fisher exact test (except body mass index and age) in univariate analysis. The risk assessment of PECS was con
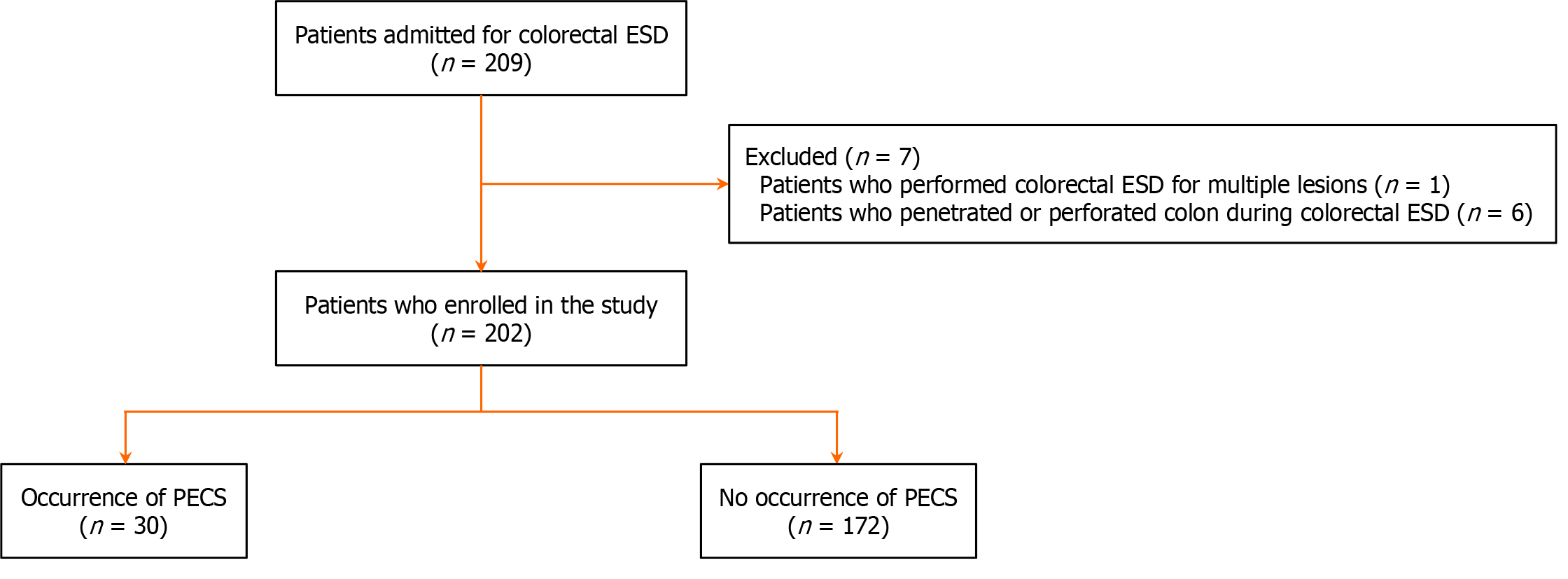
Patients were selected from April 1, 2020 to April 30, 2024. Of the 209 recruited patients, seven were excluded due to multiple lesions (n = 1) and perforation or penetration during ESD (n = 6). Distinguishing between PECS and penetration or perforation was necessary. Additionally, properly evaluating the relationship between PECS and total Joule heat was crucial. Failure to consider these influences could have skewed the results. Ultimately, 202 patients from five tertiary Japanese institutions were enrolled (Figure 2).
The data of 202 patients (mean age: 69.9 years) were statistically analyzed; of these, 72.8% were male. The patients were categorized based on characteristics such as the sample size (≥ 30 mm, < 30 mm), tumor morphology (protruding tumor, non-granular lateral spreading tumors, granular lateral spreading tumors), tumor location (right colon, left colon, rectum), and Eastern Cooperative Oncology Group performance status (0, 1) (Table 1).
| Patient features | |
| Number of patients | 202 |
| Male, n (%) | 147 (72.8) |
| Age (years), mean ± SD | 69.9 ± 10.1 |
| BMI (kg/m2), mean ± SD | 23.7 ± 3.6 |
| Specimen size (mm), mean ± SD | 31.7 ± 12.8 |
| Tumor location, n (%) | |
| Right colon | 81 (40.1) |
| Left colon | 61 (30.2) |
| Rectum | 60 (29.7) |
| Tumor morphology, n (%) | |
| 0-Is/Ip | 88 (43.6) |
| LST-G | 97 (48.0) |
| LST-NG (0-IIc) | 17 (8.4) |
| ECOG performance status after ESD, n (%) | |
| 0 | 194 (96.0) |
| ≥ 1 | 8 (4.0) |
We conducted an evaluation of the factors associated with PECS occurrence, and high Joule heat showed significantly higher results in univariate analysis. Additionally, high Joule heat was identified as a significantly independent factor for PECS occurrence in multivariate analysis (odds ratio = 7.96; 95% confidence interval: 2.91-21.8; P < 0.01) (Table 2).
| PECS group, | Non-PECS group, | P value | OR (95%CI) | ||
| Univariate analysis | Multivariate analysis | ||||
| Sex, n (%) | 0.52 | ||||
| Male | 21 (70.0) | 126 (73.3) | |||
| Female | 9 (30.0) | 46 (26.7) | |||
| Age, n (%) | 0.54 | ||||
| < 75 years | 17 (56.7) | 112 (65.1) | |||
| ≥ 75 years | 13 (43.3) | 60 (34.9) | |||
| Specimen size, n (%) | 0.24 | ||||
| < 30 mm | 11 (36.7) | 83 (48.3) | |||
| ≥ 30 mm | 19 (63.3) | 89 (48.3) | |||
| Tumor location, n (%) | 1.00 | ||||
| Right colon | 12 (40.0) | 69 (40.1) | |||
| Left colon | 10 (33.3) | 51 (29.7) | |||
| Rectum | 8 (26.7) | 52 (30.2) | |||
| Tumor morphology, n (%) | 0.85 | ||||
| 0-Is/Ip | 13 (43.3) | 75 (43.6) | |||
| LST-G | 16 (53.4) | 81 (47.1) | |||
| LST-NG (0-IIc) | 1 (3.3) | 16 (9.3) | |||
| Depth of pathological invasion, n (%) | 0.54 | ||||
| Tis (M) | 28 (93.4) | 152 (88.4) | |||
| T1a (SM < 1000 μm) | 1 (3.3) | 11 (6.4) | |||
| T1b (SM ≥ 1000 μm) | 1 (3.3) | 9 (5.2) | |||
| Endoscopist, n (%) | 0.30 | ||||
| Experts | 12 (40.0) | 54 (31.4) | |||
| Trainees | 18 (60.0) | 118 (68.6) | |||
| Submucosal fibrosis, n (%) | 0.30 | ||||
| Present | 6 (20.0) | 27 (15.7) | |||
| Absent | 24 (80.0) | 145 (84.3) | |||
| ESD procedure time, n (%) | 0.18 | 0.16 | |||
| < 90 minutes | 11 (36.7) | 91 (52.9) | 1 (reference) | ||
| ≥ 90 minutes | 19 (63.3) | 81 (47.1) | 1.76 (0.80-3.86) | ||
| Total Joule heat, n (%) | < 0.01 | < 0.01 | |||
| Low Joule heat | 4 (13.3) | 105 (61.0) | 1 (reference) | ||
| High Joule heat | 26 (86.7) | 67 (39.0) | 7.96 (2.91-21.8) | ||
We compared the long (n = 100) and short (n = 102) procedure time groups, including variables, such as sex, tumor location, ESD procedure performed by trainees, submucosal fibrosis, and PECS. The presence of submucosal fibrosis was significantly higher in the long procedure time group than that in the short procedure time group. However, there was no significant difference in the occurrence of PECS between the two groups (Table 3).
| Long procedure time group (≥ 90 minutes), n = 100 | Short procedure time group (< 90 minutes), n = 102 | P value | |
| Sex, n (%) | 0.75 | ||
| Male | 73 (73.0) | 73 (71.6) | |
| Female | 27 (27.0) | 29 (28.4) | |
| Tumor location, n (%) | 0.67 | ||
| Right colon | 42 (42.0) | 38 (37.3) | |
| Left colon | 26 (26.0) | 35 (34.3) | |
| Rectum | 32 (32.0) | 29 (28.4) | |
| ESD procedure performed by trainees, n (%) | 73 (73.0) | 63 (61.8) | 0.10 |
| Submucosal fibrosis, n (%) | 24 (24.0) | 9 (8.8) | < 0.01 |
| PECS, n (%) | 19 (19.0) | 11 (10.8) | 0.33 |
Perforation or penetration occurred in one patient (one patient with intraprocedural perforation, 3.3%) in the PECS group and in five patients (two patients with intraprocedural perforation and three patients with penetration; total 2.9%) in the non-PECS group. These patients were managed conservatively without surgical treatment (Table 4).
| PECS group | Non-PECS group | |
| Intraprocedural perforation or penetration/number of patients in each group | 1/30 | 5/172 |
| ESD procedure performed by trainees/number of patients in each group | 1/30 | 5/172 |
| Peak CRP (mg/dL), mean ± SD | 4.11 ± not applicable | 1.56 ± 1.42 |
| Duration of hospitalization (days), mean ± SD | 6 ± not applicable | 6.2 ± 2.2 |
To our knowledge, this study is the first to demonstrate the significance of total Joule heat magnitude, calculated using a device developed by us, during colorectal ESD as a risk factor for PECS in a multicenter prospective study. Furthermore, a significant increase in the incidence of PECS occurred when a high Joule heat (≥ 15390 J) was reached.
The mechanism of PECS may involve the occurrence of temporary inflammation due to heat transfer from electrocoagulation to the serous membrane and muscle layer at the resection site[18,27,28]. A previously reported risk factor for PECS is the prolonged procedural time[25,29], resulting in an increased electrical load on the mucosal surface[11]. Furthermore, ischemic changes are caused in the mucosal surface due to excessive electrical load during resection, incision, and hemostasis[30]. These reports suggest that the thermodynamic load imposed on the mucosal surface is connected to the occurrence of PECS. Based on these findings, we demonstrated evidence of an association between the total Joule heat imposed on mucosal surface and the occurrence of PECS[21]. However, this was a retrospective study; therefore, it is necessary to investigate the relationship between the Joule heat of mucosal surface and PECS in a multicenter prospective observational study.
Other reported risk factors for PECS include lesion size[11,13,31], lesion location[11,13,25,26,29], submucosal fibrosis[26,31], and injury to the muscle layer[32]. These risk factors may indirectly result in the need for additional Joule heating to detach the lesions. In previous reports, larger tumor size, fibrosis, and fewer experienced endoscopists were associated with longer procedure times (average: 86 minutes)[22]. This indirectly indicates the burden of thermal damage on the dissection surface. However, in our study, despite longer procedure times (average: 98 minutes), tumor size, fibrosis, and less experienced endoscopists were not identified as risk factors for PECS. The lack of an assessment method for thermal damage is the main reason the risk factors for PECS have not been clarified to date. Risk factors, such as procedure time, lesion size, lesion location, submucosal fibrosis, and injury to the muscle layer, suggest a high Joule heat, and the method of accumulating the coagulation heat emitted from the electrosurgical coagulation unit has provided a rational means to evaluate thermal damage. In our study, high Joule heating emerged as the only independent risk factor for PECS, whe
This study has some potential limitations. Patients who underwent colorectal ESD did not routinely undergo com
We demonstrated that the accumulation of Joule heat on the peeled surface during ESD contributes to the onset of PECS. Thermodynamic damage to this mucosal surface seems to be the central aspect of the mechanism underlying the onset of PECS.
| 1. | Puli SR, Kakugawa Y, Saito Y, Antillon D, Gotoda T, Antillon MR. Successful complete cure en-bloc resection of large nonpedunculated colonic polyps by endoscopic submucosal dissection: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:2147-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 593] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Sugihara K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Wada Y, Kudo SE, Tanaka S, Saito Y, Iishii H, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisabe T, Tsuruta O, Kashida H, Ishikawa H, Sugihara K. Predictive factors for complications in endoscopic resection of large colorectal lesions: a multicenter prospective study. Surg Endosc. 2015;29:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-83; quiz 645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Watabe H, Yamaji Y, Okamoto M, Kondo S, Ohta M, Ikenoue T, Kato J, Togo G, Matsumura M, Yoshida H, Kawabe T, Omata M. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc. 2006;64:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Okamoto K, Watanabe T, Komeda Y, Kono T, Takashima K, Okamoto A, Kono M, Yamada M, Arizumi T, Kamata K, Minaga K, Yamao K, Nagai T, Asakuma Y, Takenaka M, Sakurai T, Matsui S, Nishida N, Chikugo T, Kashida H, Kudo M. Risk Factors for Postoperative Bleeding in Endoscopic Submucosal Dissection of Colorectal Tumors. Oncology. 2017;93 Suppl 1:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Jung D, Youn YH, Jahng J, Kim JH, Park H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy. 2013;45:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Hong MJ, Kim JH, Lee SY, Sung IK, Park HS, Shim CS. Prevalence and clinical features of coagulation syndrome after endoscopic submucosal dissection for colorectal neoplasms. Dig Dis Sci. 2015;60:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Yamashina T, Takeuchi Y, Uedo N, Hamada K, Aoi K, Yamasaki Y, Matsuura N, Kanesaka T, Akasaka T, Yamamoto S, Hanaoka N, Higashino K, Ishihara R, Iishi H. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol. 2016;31:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS, Ki HK. A randomized controlled trial of prophylactic antibiotics in the prevention of electrocoagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2017;86:349-357.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Lee SP, Kim JH, Sung IK, Lee SY, Park HS, Shim CS, Han HS. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol. 2015;30:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Hirasawa K, Sato C, Makazu M, Kaneko H, Kobayashi R, Kokawa A, Maeda S. Coagulation syndrome: Delayed perforation after colorectal endoscopic treatments. World J Gastrointest Endosc. 2015;7:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Mori H, Kobara H, Rafiq K, Nishiyama N, Fujihara S, Oryu M, Masaki T. Effects of gastric irrigation on bacterial counts before endoscopic submucosal dissection: a randomized case control prospective study. PLoS One. 2013;8:e65377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Christie JP, Marrazzo J 3rd. "Mini-perforation" of the colon--not all postpolypectomy perforations require laparotomy. Dis Colon Rectum. 1991;34:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Nomura S, Shimura T, Katano T, Iwai T, Mizuno Y, Yamada T, Ebi M, Hirata Y, Nishie H, Mizushima T, Nojiri Y, Togawa S, Shibata S, Kataoka H. A multicenter, single-blind randomized controlled trial of endoscopic clipping closure for preventing coagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;91:859-867.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Shichijo S, Takeuchi Y, Shimodate Y, Yamashina T, Yamasaki T, Hayashi T, Hirasawa K, Fukunaga S, Yamaguchi S, Asai S, Kawamura T, Fukata N, Yamamoto M, Teramoto A, Kinjo Y, Matsuno K, Kinjo T, Sano Y, Iwatsubo T, Nagaike K, Matsumoto M, Hoki N, Kawamura I, Shimokawa T, Uedo N, Ishikawa H, Tanaka K, Kitano M; Kansai Endoscopic Device Selection Conference in Kansai Research Group. Performance of perioperative antibiotics against post-endoscopic submucosal dissection coagulation syndrome: a multicenter randomized controlled trial. Gastrointest Endosc. 2022;95:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Ochi M, Kawagoe R, Kamoshida T, Hamano Y, Ohkawara H, Ohkawara A, Kakinoki N, Yamaguchi Y, Hirai S, Yanaka A, Tsuchiya K. High total Joule heat increases the risk of post-endoscopic submucosal dissection electrocoagulation syndrome after colorectal endoscopic submucosal dissection. World J Gastroenterol. 2021;27:6442-6452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (3)] |
| 22. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Sugihara KI, Igarashi M, Toyonaga T, Ajioka Y, Kusunoki M, Koike K, Fujimoto K, Tajiri H. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 23. | Hotta K, Oyama T, Shinohara T, Miyata Y, Takahashi A, Kitamura Y, Tomori A. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Bracken TD, Sias GG, Kim C, Senior RS, Patterson RM. Survey of Electrical Utility Worker Body Impedance. IEEE T Power Deliver. 2008;23:1251-1259. [DOI] [Full Text] |
| 25. | Arimoto J, Higurashi T, Kato S, Fuyuki A, Ohkubo H, Nonaka T, Yamaguchi Y, Ashikari K, Chiba H, Goto S, Taguri M, Sakaguchi T, Atsukawa K, Nakajima A. Risk factors for post-colorectal endoscopic submucosal dissection (ESD) coagulation syndrome: a multicenter, prospective, observational study. Endosc Int Open. 2018;6:E342-E349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Ito S, Hotta K, Imai K, Yamaguchi Y, Kishida Y, Takizawa K, Kakushima N, Tanaka M, Kawata N, Yoshida M, Ishiwatari H, Matsubayashi H, Ono H. Risk factors of post-endoscopic submucosal dissection electrocoagulation syndrome for colorectal neoplasm. J Gastroenterol Hepatol. 2018;33:2001-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J Clin Gastroenterol. 1992;15:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 237] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 28. | Cha JM, Lim KS, Lee SH, Joo YE, Hong SP, Kim TI, Kim HG, Park DI, Kim SE, Yang DH, Shin JE. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Yamamoto S, Kinugasa H, Yamasaki Y, Hirai M, Ako S, Takei K, Igawa S, Yasutomi E, Oka S, Ohmori M, Inokuchi T, Harada K, Hiraoka S, Nouso K, Tanaka T, Okada H. Fever and electrocoagulation syndrome after colorectal endoscopic submucosal dissection for patients with immunosuppressants and steroids. DEN Open. 2022;2:e83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 30. | Kobayashi R, Hirasawa K, Sato C, Makazu M, Kaneko H, Ikeda R, Fukuchi T, Sawada A, Ozeki Y, Taguri M, Takebayashi S, Maeda S. Utility of multi-detector computed tomography scans after colorectal endoscopic submucosal dissection: a prospective study. Gastrointest Endosc. 2018;87:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Kim SJ, Kim SY, Lee J. Prognosis and risk factors of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Large cohort study. Surg Endosc. 2022;36:6243-6249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 32. | Omori T, Funasaka K, Horiguchi N, Kamano T, Nagasaka M, Nakagawa Y, Miyahara R, Hashimoto S, Shibata T, Ohmiya N, Hirooka Y. Injury to the muscle layer, increasing the risk of post-colorectal endoscopic submucosal dissection electrocoagulation syndrome. J Gastroenterol Hepatol. 2023;38:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |