Published online Nov 16, 2024. doi: 10.4253/wjge.v16.i11.595
Revised: September 20, 2024
Accepted: October 15, 2024
Published online: November 16, 2024
Processing time: 162 Days and 6.5 Hours
The concept of macroscopic on-site evaluation (MOSE) was introduced in 2015 when the endoscopist observed better diagnostic yield when the macroscopically visible core on MOSE was superior to 4 mm. Recent studies suggest that MOSE by the endoscopist may be an excellent alternative to rapid on-site evaluation, and some classifications have been published. Few studies have assessed the adequacy of histologic cores in MOSE during endoscopic ultrasound-guided fine-needle aspiration/biopsy (EUS-FNA/FNB).
To evaluate the performance of MOSE during EUS-FNA/FNB.
This multicentric prospective study was conducted in 16 centers in 3 countries (Egypt, Iraq, and Morocco) and included 1108 patients with pancreatic, biliary, or gastrointestinal pathology who were referred for EUS examination. We prospectively analyzed the MOSE in 1008 patients with available histopathological reports according to 2 classifications to determine the adequacy of the histological core samples. Data management and analysis were performed using a Statistical Package for Social Sciences (SPSS) version 27.
A total of 1074 solid lesions were biopsied in 1008 patients with available cytopathological reports. Mean age was 59 years, and 509 patients (50.5%) were male. The mean lesion size was 38 mm. The most frequently utilized needles were FNB-Franseen (74.5%) and 22 G (93.4%), with a median of 2 passes. According to 2 classifications, 618 non-bloody cores (61.3%) and 964 good samples (95.6%) were adequate for histological evaluation. The overall diagnostic yield of cytopathology was 95.5%. The cytological examination confirmed the diagnosis of malignancy in 861 patients (85.4%), while 45 samples (4.5%) were inconclusive. Post-procedural adverse events occurred in 33 patients (3.3%). Statistical analysis showed a difference between needle types (P = 0.035) with a high sensitivity of FNB (97%). The analysis of the relationship between the MOSE-score and the final diagnosis showed a significant difference between the different scores of the MOSE (P < 0.001).
MOSE is a simple method that allows endoscopists to increase needle passes to improve sample quality. There is significantly higher FNB sensitivity and cytopathology diagnostic yield with good MOSE cores.
Core Tip: This work is a multicentric international prospective study of 1108 patients to evaluate macroscopic on-site evaluation (MOSE) performance in endoscopic ultrasound-guided fine-needle aspiration/biopsy for diagnostic accuracy. MOSE is a simple procedure that allows the endoscopist to increase the number of needle passes to improve sample quality. The current study confirmed the relationship between good cores by MOSE scoring and a high diagnostic yield in cytopathology and showed a higher sensitivity of fine-needle biopsy (97%).
- Citation: Okasha HH, Hussein HA, Ragab KM, Abdallah O, Rouibaa F, Mohamed B, Ghalim F, Farouk M, Lasheen M, Elbasiony MA, Alzamzamy AE, El Deeb A, Atalla H, El-Ansary M, Mohamed S, Elshair M, Khannoussi W, Abu-Amer MZ, Elmekkaoui A, Naguib MS, Ait Errami A, El-Meligui A, El-Habashi AH, Ameen MG, Abdelfatah D, Kaddah M, Delsa H. Role of macroscopic on-site evaluation of endoscopic ultrasound-guided fine-needle aspiration/biopsy: Results of a multicentric prospective study. World J Gastrointest Endosc 2024; 16(11): 595-606
- URL: https://www.wjgnet.com/1948-5190/full/v16/i11/595.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i11.595
Endoscopic ultrasound-guided fine needle aspiration/biopsy (EUS-FNA/FNB) are highly accurate for pathological diagnosis. To increase their diagnostic yield, macroscopic on-site evaluation (MOSE), performed by an endoscopist, was described in 2015 as an alternative to rapid on-site evaluation (ROSE)[1-3]. The MOSE of the biopsy involves a direct assessment of the adequacy of the sample through visual inspection of the core tissue obtained during the puncture. The endoscopist observed a better diagnostic yield when the macroscopically visible core (MVC) on MOSE was greater than 4 mm[3,4]. Various classifications have been published based on the appearance of a MVC in histological specimens[5,6].
Only a few studies have evaluated the adequacy of histology cores in MOSE during EUS-FNA/FNB. However, without robust data, MOSE has not been broadly adopted as a standard technique[4]. Although evidence supports the utility of MOSE, diagnostic yield varies with needle type and number of passes. This prospective multicentre study aimed to evaluate the performance of MOSE using multiple EUS-FNA/FNB needles (19 G, 20 G, and 22 G) and the feasibility of using 2 classifications to determine histological core adequacy.
This study was conducted at 16 centers in 3 countries (Egypt, Iraq, and Morocco). Ethical approval for the study was obtained from the Research Ethics Committee of the University of Cairo (protocol number: MD-319-2022). All patients provided written informed consent.
This prospective multicentric study included 1108 patients with pancreatic, biliary, or gastrointestinal pathology who were referred for EUS examination and underwent EUSFNA/FNB. Patient demographics, EUS findings, lesion characteristics, FNA and FNB methods, MOSE classification, and histological results were collected and analyzed. Patients with unknown cytopathology data were excluded from the present study.
All procedures were performed under anesthesia (deep sedation) using the linear array echoendoscope by an experienced operator in each center. The needle types used for EUS-FNB were Franseen (Acquire, Boston Scientific Corporation, Natick, MA, United States), Microtech (Nanjing, China), Procore (Cook Medical, Winston-Salem, NC, United States), and Medtronic (Medtronic, Minneapolis, MN, United States). In contrast, for EUS-FNA, the needles used were Expect (Boston Scientific Corporation, Natick, MA, United States) and Echotip (Cook Medical, Winston-Salem, NC, United States). The needle sizes used in the procedures were 22 G, 20 G, and 19 G. If insufficient material was obtained, many passes were made. After each pass, the sample was immediately analyzed by the endosonographer to classify the MOSE. The adequacy of the histological core samples was determined using two classifications. For each patient, the highest MOSE score was considered.
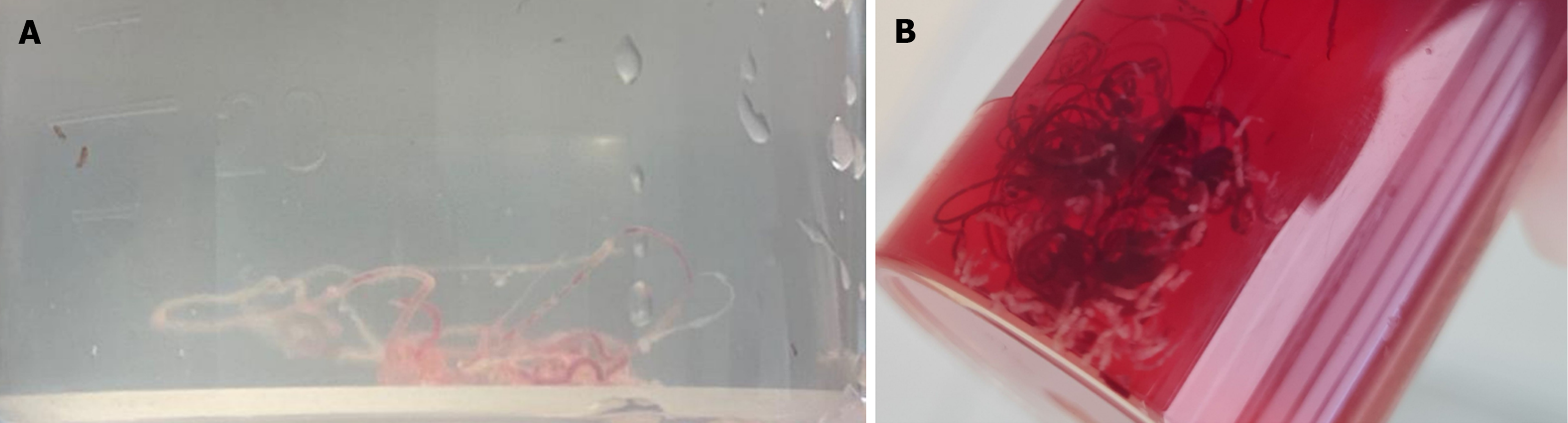
MOSE-1 classification: After each puncture, the material was carefully examined for visible cores. A whitish tissue with apparent bulk was defined as a visible core. Macroscopic evaluation of the core samples was categorized as follows[7]: Score 1: Definitely visible tissue core with scanty blood clots. Score 2: Visible tissue core with moderate blood clots. Score 3: Scanty tissue core with mainly blood clots. Score 1 was considered to be the most optimal sample on MOSE.
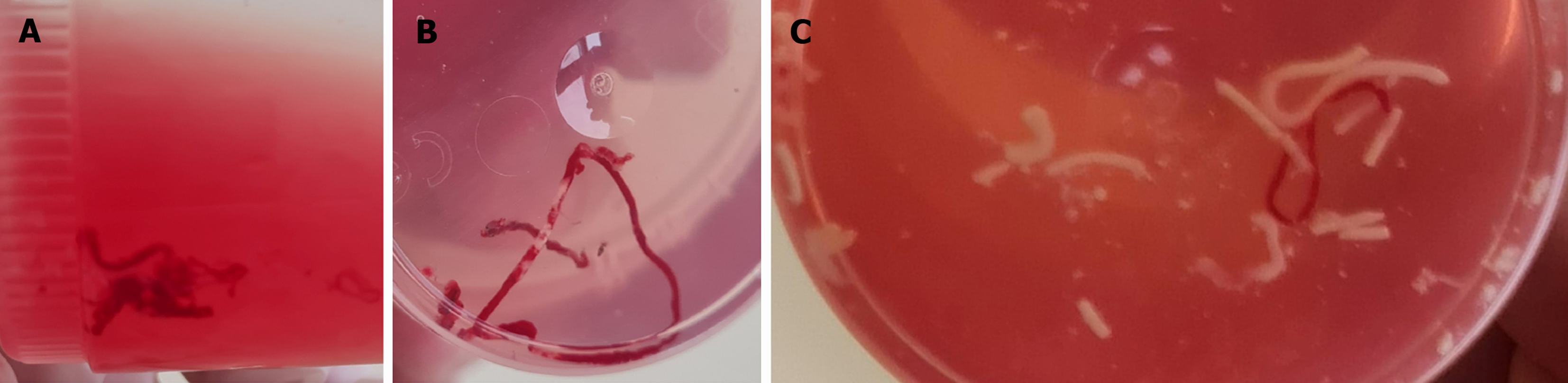
MOSE-2 classification: Furthermore, in the current study, the sample was classified based on the size of the yellowish-white core, as shown in Table 1, using the MOSE classification proposed by Gaia et al[5] in 2022.
After inspection of the core by the operator, all specimens were fixed in 10% formalin solution or 95% absolute alcohol for cytological analysis. An experienced gastrointestinal and pancreaticobiliary pathologist evaluated the specimens.
In the current study, the primary outcome was diagnostic accuracy using MOSE. Sensitivity, specificity, and diagnostic accuracy were calculated to determine the efficacy of EUS-FNA/FNB in diagnosing tumors. The number of passes, needle type and size, and procedural adverse events were assessed. Sample histopathology was categorized as benign, malignant, or inconclusive. Inconclusive samples were defined as hypocellular or acellular smears, which are insufficient to diagnose a malignant or benign disease. MOSE efficacy was determined by calculating the accuracy of MOSE classifications 1 and 2 in obtaining the conclusive sample.
Data management and analysis were performed using Statistical Package for Social Sciences (SPSS) version 27. Numerical data were summarized using means and standard deviations. Categorical data were summarized as numbers and percentages. Estimates of the frequency were performed using the numbers and percentages. Numerical data were explored for normality using the Kolmogrov-Smirnov and Shapiro-Wilk tests. The χ2 or Fisher’s tests were used to compare the independent groups for categorical data, as appropriate. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of the MOSE technique were calculated using a 2 × 2 table against the gold standard method (pathology). All tests were two-tailed; a probability (P value) ≤ 0.05 was considered significant.
Over 1108 patients were screened for eligibility, 1008 patients were finally included, and 100 patients with unavailable cytopathology results were excluded from the study. This multicenter study was conducted in three countries, including 1008 patients: Six hundred thirty-three from Egypt (62.8%), 200 from Iraq (19.8%), and 175 from Morocco (17.4%). The mean age was 59 ± 12 years, with 509 (50.5%) males. The baseline characteristics of enrolled patients are reported in Table 2. A total of 1074 solid lesions underwent biopsy, comprising 664 pancreatic (62%) and 410 extrapancreatic (38%) lesions. The mean lesion size was 38 ± 17 mm.
| Characteristics | |
| Age (years), mean ± SD | 59 ± 12 |
| Sex, n (%) | |
| Female | 499 (49.5) |
| Male | 509 (50.5) |
| Lesion location, n (%) | |
| Pancreas | 664 (62) |
| Stomach | 101 (9.4) |
| Lymph nodes | 96 (8.9) |
| Liver | 46 (4.2) |
| Mediastinum | 38 (3.5) |
| Other | 129 (12) |
| Total | 1074 (100) |
| The mean size of the target lesion on EUS (mm), mean ± SD | 38 ± 17 |
| Approach, n (%) | |
| Transduodenal | 585 (58) |
| Transgastric | 350 (34.7) |
| Transesophageal | 62 (6.2) |
| Transrectal | 11 (1.1) |
| Type of the needle (FNA or FNB), n (%) | |
| FNA | 101 (10) |
| FNA-Expect-Boston | 68 (6.7) |
| FNA-EchoTip-Cook | 33 (3.3) |
| FNB | 907 (90) |
| FNB-Acquire-Boston | 751 (74.5) |
| FNB-Medtronic | 20 (2) |
| FNB-ProCore-Cook | 40 (4) |
| FNB-Trident-Microtech | 96 (9.5) |
| Specimen acquisition method, n (%) | |
| Suction method | 381 (37.8) |
| Capillary method | 290 (28.8) |
| Both | 337 (33.4) |
| Number of needle passes (FNB), n (%) | |
| 1 | 154 (15.3) |
| 2 | 680 (67.5) |
| 3 | 149 (14.8) |
| 4 | 25 (2.5) |
| Final diagnosis (conclusive or inconclusive), n (%) | |
| Conclusive | 963 (95.5) |
| Benign | 102 (10.1) |
| Malignant | 861 (85.4) |
| Inconclusive | 45 (4.5) |
| Postprocedural adverse events, n (%) | |
| No | 975 (96.7) |
| Yes | 33 (3.3) |
| Abdominal pain | 17 (1.7) |
| Small blood collection | 10 (1) |
| Transient fever | 6 (0.6) |
FNB was the predominant procedure, performed in 907 patients (90%), whereas only 101 patients (10%) underwent FNA. The most common needles used were “Acquire” from Boston Scientific in 751 patients (74.5%) for EUS-FNB and “Expect” from Boston Scientific in 68 patients (6.7%) for EUS-FNA. Needle sizes used during the procedures were 22 G (93.4%), 20 G (3.8%), and 19 G (2.9%), with a median number of needle passes of 2 (1-4 passes).
According to MOSE classification 1 (Figure 1), the endosonographer classified 618 non-bloody cores (61.3%), and according to MOSE classification 2 (Figure 2), 964 good specimens of scores 2 and 3 (95.6%) were adequate for histological evaluation. The detailed description of MOSE 1 and MOSE 2 classifications is illustrated in Table 3. A statistically significant correlation existed between MOSE classifications and needle type, number of needle passes, and tissue sampling techniques (Table 4). The 22 G needle demonstrated statistical superiority in generating good cores when classified according to MOSE-1. There was a statistically significant difference of 97.3% for both techniques (capillary and suction) compared to 93.4% for suction alone in the MOSE-2 classification. Out of the 1008 samples examined by experienced cytopathologists, 45 (4.5%) were inconclusive; however, the overall diagnostic yield of cytopathology was 95.5%. The cytological examination confirmed the diagnosis of malignancy in 861 patients (85.4%) and benign lesions in 102 cases (10.1%).
| Classification | |
| MOSE-1 classification, n (%) | |
| Score 1: Definite visible tissue core with scanty blood clots | 618 (61.3) |
| Score 2: Visible tissue core with moderate blood clots | 325 (32.2) |
| Score 3: Scanty tissue core with mainly blood clots | 65 (6.5) |
| MOSE-2 classification, n (%) | |
| Score 0: Punctio sicca/no material | 0 (0) |
| Score 1: Only necrotic or haematic material | 44 (4.4) |
| Score 2: ≥ 1 core tissue, ≤ 2 mm yellowish-white | 194 (19.2) |
| Score 3: ≥ 1 core tissue, > 2 mm yellowish-white | 770 (76.4) |
| MOSE-1 classification | MOSE-2 classification | ||||||
| 1, n (%)1 | 2, n (%)1 | 3, n (%)1 | P value | Scores 0 and 1, n (%)1 | Scores 2 and 3, n (%)1 | P value | |
| Type of the needle | |||||||
| FNA-EchoTip | 13 (39.4) | 15 (45.5) | 5 (15.2) | < 0.001 | 1 (3) | 32 (97) | 0.009 |
| FNA-Expect | 37 (54.4) | 28 (41.2) | 3 (4.4) | 7 (10.3) | 61 (89.7) | ||
| FNB-Acquire | 465 (61.9) | 241 (32.1) | 45 (6) | 33 (4.4) | 718 (95.6) | ||
| FNB-Medtronic | 4 (20) | 10 (50) | 6 (30) | 3 (15) | 17 (85) | ||
| FNB-Pro Core | 12 (30) | 22 (55) | 6 (15) | 0 (0) | 40 (100) | ||
| FNB-Trident-Microtech | 87 (90.6) | 9 (9.4) | 0 (0) | 0 (0) | 96 (100) | ||
| Type of the needle (FNA or FNB) | |||||||
| FNA | 50 (49.5) | 43 (42.6) | 8 (7.9) | 0.036 | 8 (7.9) | 93 (92.1) | 0.073 |
| FNB | 568 (62.6) | 282 (31.1) | 57 (6.3) | 36 (4) | 871 (96) | ||
| Size of the needle | |||||||
| 19 G | 13 (44.8) | 11 (37.9) | 5 (17.2) | 0.034 | 4 (13.8) | 25 (86.2) | 0.249 |
| 20 G | 11 (28.9) | 20 (52.6) | 7 (18.4) | 1 (2.6) | 37 (97.4) | ||
| 22 G | 594 (63.1) | 294 (31.2) | 53 (5.6) | 39 (4.1) | 902 (95.9) | ||
| Specimen acquisition method | |||||||
| Both | 248 (73.6) | 69 (20.5) | 20 (5.9) | < 0.001 | 9 (2.7) | 328 (97.3) | 0.0262 |
| Capillary method | 150 (51.7) | 116 (40) | 24 (8.3) | 10 (3.4) | 280 (96.6) | ||
| Suction method | 220 (57.7) | 140 (36.7) | 21 (5.5) | 25 (6.6) | 356 (93.4) | ||
| Number of needle passes | |||||||
| 1 | 89 (57.8) | 60 (39) | 5 (3.2) | < 0.001 | 4 (2.6) | 150 (97.4) | < 0.001 |
| 2 | 451 (66.3) | 197 (29) | 32 (4.7) | 15 (2.2) | 665 (97.8) | ||
| 3 | 67 (44.9) | 56 (37.6) | 26 (17.5) | 16 (10.7) | 133 (89.3) | ||
| 4 | 11 (44) | 12 (48) | 2 (8) | 9 (36) | 16 (64) | ||
Comparing the two groups with inconclusive and conclusive diagnoses, a statistically significant difference between needle types was observed (P = 0.035), with the greatest disparity seen between FNB-Trident-Microtech (100% conclusive results) and FNA-EchoTip-Cook (87.9%). However, the 2 groups were comparable in needle size and specimen acquisition methods (Table 5). When comparing score 1 with scores 2 and 3 in the MOSE-1 classification, score 1 showed a sensitivity of 95%, specificity of 31%, a PPV of 97%, NPV of 22%, and an overall accuracy of 92% in obtaining conclusive samples, whether benign or malignant. According to the MOSE-2 classification, score 3 has a statistically significant difference in diagnostic accuracy (97.5%) with a P value of 0.002 and in malignancy detection (89.6%) with a P value < 0.001 (Table 6). Further analysis revealed that the MOSE-2 classification with FNA had a sensitivity of 92.7%, a specificity of 20%, a PPV of 96%, an NPV of 12.5%, and an overall accuracy of 89% in providing conclusive, whether benign or malignant, samples. With FNB, the MOSE-2 classification has demonstrated a sensitivity of 97%, specificity of 25%, PPV of 97%, NPV of 28%, and overall accuracy of 94% in obtaining conclusive samples.
| Final diagnosis | |||
| Inconclusive, n (%)1 | Conclusive, n (%)1 | P value | |
| Type of needle | |||
| FNA-EchoTip-Cook | 4 (12.1) | 29 (87.9) | 0.035a |
| FNA-Expect-Boston | 1 (1.5) | 67 (98.5) | |
| FNB-Franseen Acquire-Boston | 39 (5.2) | 712 (94.8) | |
| FNB-Medtronic | 0 (0) | 20 (100) | |
| FNB-ProCore-Cook | 1 (2.5) | 39 (97.5) | |
| FNB-Trident-Microtech | 0 (0) | 96 (100) | |
| Type of needle (FNA or FNB) | |||
| FNA | 5 (5) | 96 (95) | 0.803 |
| FNB | 40 (4.4) | 867 (95.6) | |
| Size of the needle | |||
| 19 G | 3 (10.3) | 26 (89.7) | 0.176 |
| 20 G | 3 (7.9) | 35 (92.1) | |
| 22 G | 39 (4.1) | 902 (95.9) | |
| Specimen acquisition method | |||
| Both | 10 (3) | 327 (97) | 0.079 |
| Capillary method | 11 (3.8) | 279 (96.2) | |
| Suction method | 24 (6.3) | 357 (93.7) | |
| MOSE-1 classification | |||
| Good cores (score 1) | 16 (2.6) | 602 (97.4) | < 0.001a |
| Bloody cores (scores 2 and 3) | 29 (7.4) | 361 (92.6) | |
| MOSE-2 classification | |||
| Score 2 | 14 (7.2) | 180 (92.8) | 0.002a |
| Score 3 | 20 (2.6) | 750 (97.4) | |
| MOSE-2 classification | P value | ||
| Score 2, n (%)1 | Score 3, n (%)1 | ||
| Diagnosis conclusive or inconclusive | |||
| Inconclusive | 14 (7.2) | 20 (2.6) | 0.002a |
| Conclusive | 180 (92.8) | 750 (97.4) | |
| Final diagnosis | |||
| Inconclusive | 14 (7.2) | 20 (2.6) | < 0.001a |
| Benign | 35 (18) | 60 (7.8) | |
| Malignant | 145 (74.7) | 690 (89.6) | |
| Number of needle passes FNB1 | |||
| 1 | 32 (16.5) | 118 (15.3) | < 0.001a |
| 2 | 103 (53.1) | 562 (73) | |
| 3 | 56 (28.9) | 77 (10) | |
| 4 | 3 (1.5) | 13 (1.7) | |
Post-procedural adverse events occurred in only 33 patients (3.3%) and included tolerable abdominal pain in 17 patients (1.7%), self-limiting small blood collection in 10 patients (1%), and transient fever in 6 patients (0.6%). No significant complications or mortality occurred.
Since its description in 2015, MOSE has demonstrated simplicity and ease of use, effectively enhancing the diagnostic yield of biopsies performed under EUS. The recent study was conducted by Sundaram et al[8], published in 2023, and involved 155 patients with solid pancreatic lesions. It compared the efficacy of EUS-FNB in terms of adequacy as assessed by MOSE and smear cytology with adequacy as confirmed by ROSE obtained with the same needle. This study confirmed that MOSE and ROSE effectively assess sampling adequacy, with no discernible difference in overall diagnostic accuracy for solid pancreatic lesions[8]. In addition, in a meta-analysis encompassing 2147 patients, EUS-FNB plus ROSE did not exhibit superiority over EUS-FNB with newer end-cutting needles[9]. Therefore, considering the additional costs and logistics involved, the utility of ROSE should be deliberated[8,9]. Moreover, the Mohan et al’s meta-analysis of 1508 lesions confirmed the efficacy of MOSE with a high pathologic diagnosis[4].
The multicentric prospective study of Mangiavillano, including 504 samples, confirmed a strong correlation between MOSE after the first pass and histologic adequacy, with a high rate of concordance (90%)[10]. Regardless of the first pass MOSE result, this study showed that a second pass is necessary to increase diagnostic accuracy[10]. Visible cores superior to or equal to 4 mm on MOSE may indicate sample adequacy for pathological interpretation, as reported in the first studies on MOSE[3]. However, recent studies have confirmed that the accuracy of EUS-FNB improves as the length of the visible core increases[10]. Visible cores of at least 10 mm are strongly associated with the probability of a correct diagnosis[9,10]. These findings may indicate that the 10 mm white-yellow core adequacy cut-off may be proposed[10,11].
The current study demonstrated that a score of 3 according to the MOSE 2 classification (≥ 1 core tissue > 2 mm yellowish-white) exhibited a statistically significant increase in diagnostic accuracy (97.5%), correlating with an improvement in EUS-FNB accuracy with tissue length. Regarding needle type, previous studies on solid lesions confirmed that EUS-FNB is superior to EUS-FNA in diagnosing solid lesions because it allows more cell blocks to be assessed with a similar number of passes. Sensitivity was identical between EUS-FNA with ROSE, EUS-FNB with ROSE, and EUS-FNB alone[12,13]. The current findings are consistent, showing a significantly higher rate of conclusive diagnoses with the FNB-Trident Microtech (100%) compared to the FNA-EchoTip Cook (87.9%).
Moreover, Mohan et al’s meta-analysis demonstrated excellent pooled sensitivity, specificity, and PPV for EUS-guided MOSE (91.5%, 98.9%, and 98.8%, respectively)[4]. Similarly, the study of 155 solid pancreatic lesions using FNB with MOSE reported 96.12% sensitivity and 100% specificity[8]. In a study of 79 patients undergoing EUS-FNB to diagnose abdominal mass using the MOSE-1 classification, Oh et al[7] found diagnostic accuracy, sensitivity, and specificity to be 94.5%, 94.3%, and 100%, respectively. Furthermore, Gaia et al’s prospective study of 76 consecutive patients undergoing EUS-FNB for pancreatic and extrapancreatic solid lesions reported better accuracy for score 3 of the MOSE-2 classification (78.3%)[5].
A recent prospective randomized study including 96 patients with solid gastro-intestinal, pancreatic, biliary lesions, and enlarged lymph nodes studied the diagnostic performance of MOSE compared with conventional technique of EUS-FNB using Franseen biopsy needles[14]. They concluded that EUS-FNB with MOSE is a simple reliable technique that can achieve a high and comparable diagnostic accuracy with lesser number of passes. Obtaining longer length and greater number of MVC increase the sensitivity to diagnose malignancy with MOSE[14].
In the current study, MOSE-2 classification by FNA was 92.7% sensitive and 20% specific, whereas FNB was 97% sensitive and 25% specific. Regarding MOSE-1 classification, score 1 compared to score 2 and score 3 had 95% sensitivity, 31% specificity, and 92% overall accuracy. Following the development of ROSE and MOSE, further alternatives have now been described. The introduction of stereomicroscope MOSE (S-MOSE) bridge technology aims to identify the optimal cut-off length for visible white cores that indicate pathology. S-MOSE provides stereomicroscopic magnification to differentiate between core tissue and blood clots, allowing endoscopists to perform assessments comfortably in the endoscopy room. Compared to ROSE, this innovative approach can reduce overall procedure time[15-17]. Additionally, sample isolation processing by stereomicroscopy (SIPS) was employed to acquire stereomicroscopically visible white cores (SVWCs). This method enhances sample quality for diagnosis by isolating the tissue core from red components like red blood cells and fibrin during magnified stereomicroscopic examination. Using an SVWC cut-off of ≥ 3.5 mm or ≥ 4 mm with a 22-gauge Franseen FNB needle, SIPS has a high sensitivity of 98.8% for malignancy in upper gastrointestinal subepithelial lesions[16]. Nevertheless, it is a complicated and time-consuming process and seems to be unnecessary for pancreatic cancer[18,19].
More recently, Nakatani et al[20] introduced stereomicroscope on-site evaluation, which resembles S-MOSE but excludes the SIPS procedures. Stereomicroscope on-site evaluation solely confirms whether the SVWC cut-off (≥ 4 mm) is attained, making it simpler than S-MOSE. Recently Iwashita et al’s review analysed the advances in EUS-FNA/FNB techniques and equipment and highlighted the importance of sample handling, including MOSE, for diagnostic accuracy[21]. This emphasizes the need for a comprehensive understanding of the characteristics of each technique to optimize diagnostic efficiency and procedure safety[21].
MOSE is a feasible and safe method that allows the endoscopist to increase the number of needle passes to improve the sample quality on each EUS-FNA/FNB. Good MOSE cores are significantly associated with a high sensitivity of the FNB and a better diagnostic yield in cytopathology. In the current multicenter study, we affirmed the utility of MOSE using two classifications, noting a notably higher rate of conclusive diagnoses with the FNB-Trident Microtech compared to the FNA-EchoTip Cook. FNB exhibited higher sensitivity (97%) in the MOSE-2 classification, while score 1 yielded the best sensitivity (95%) in the MOSE-1 classification, particularly with 22 G needles. Ultimately, each endoscopist should strive to achieve a MOSE-1 score of 1 and a MOSE-2 score of 3 during observation of the cores.
Okasha Hussein and Delsa Hanane designed the study and revised the manuscript. We thank all the authors for their contribution to the data collection. We also thank Dr Awad Abeer for revising the manuscript.
| 1. | So H, Seo DW, Hwang JS, Ko SW, Oh D, Song TJ, Park DH, Lee SK, Kim MH. Macroscopic on-site evaluation after EUS-guided fine needle biopsy may replace rapid on-site evaluation. Endosc Ultrasound. 2021;10:111-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 2. | Delsa H, Bellahammou K, Okasha HH, Ghalim F. Cheesy material on macroscopic on-site evaluation after endoscopic ultrasound-guided fine-needle biopsy: Don't miss the tuberculosis. World J Clin Cases. 2023;11:2181-2188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Iwashita T, Yasuda I, Mukai T, Doi S, Nakashima M, Uemura S, Mabuchi M, Shimizu M, Hatano Y, Hara A, Moriwaki H. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study). Gastrointest Endosc. 2015;81:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Mohan BP, Madhu D, Reddy N, Chara BS, Khan SR, Garg G, Kassab LL, Muthusamy AK, Singh A, Chandan S, Facciorusso A, Mangiavillano B, Repici A, Adler DG. Diagnostic accuracy of EUS-guided fine-needle biopsy sampling by macroscopic on-site evaluation: a systematic review and meta-analysis. Gastrointest Endosc. 2022;96:909-917.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Gaia S, Rizza S, Bruno M, Ribaldone DG, Maletta F, Sacco M, Pacchioni D, Rizzi F, Saracco GM, Fagoonee S, De Angelis CG. Impact of Macroscopic On-Site Evaluation (MOSE) on Accuracy of Endoscopic Ultrasound-Guided Fine-Needle Biopsy (EUS-FNB) of Pancreatic and Extrapancreatic Solid Lesions: A Prospective Study. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kim DH, Kim GH, Cho CM, Park CH, Na SY, Kim TH, Cho YK, Kim JH, Seo DW; Korean EUS Study Group. Feasibility of a 20-gauge ProCore needle in EUS-guided subepithelial tumor sampling: a prospective multicenter study. BMC Gastroenterol. 2018;18:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Oh D, Seo DW, Hong SM, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. The impact of macroscopic on-site evaluation using filter paper in EUS-guided fine-needle biopsy. Endosc Ultrasound. 2019;8:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Sundaram S, Chhanchure U, Patil P, Seth V, Mahajan A, Bal M, Kaushal RK, Ramadwar M, Prabhudesai N, Bhandare M, Shrikhande SV, Mehta S. Rapid on-site evaluation (ROSE) versus macroscopic on-site evaluation (MOSE) for endoscopic ultrasound-guided sampling of solid pancreatic lesions: a paired comparative analysis using newer-generation fine needle biopsy needles. Ann Gastroenterol. 2023;36:340-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Facciorusso A, Gkolfakis P, Tziatzios G, Ramai D, Papanikolaou IS, Triantafyllou K, Lisotti A, Fusaroli P, Mangiavillano B, Chandan S, Mohan BP, Crinò SF. Comparison between EUS-guided fine-needle biopsy with or without rapid on-site evaluation for tissue sampling of solid pancreatic lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:458-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Mangiavillano B, Facciorusso A, Di Matteo FM, Barbera C, Larghi A, Rizzatti G, Carrara S, Lisotti A, Fusaroli P, De Luca L, Di Leo M, Conti Bellocchi MC, Spadaccini M, Dabizzi E, Auriemma F, Stigliano S, Ramai D, Calabrese F, Manfrin E, Paduano D, Hassan C, Repici A, Crinó SF. Establishing the optimal number of passes during EUS-FNB for diagnosis of pancreatic solid lesions: Prospective multicenter study. Endosc Int Open. 2024;12:E467-E473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Kaneko J, Ishiwatari H, Sasaki K, Satoh T, Sato J, Matsubayashi H, Yabuuchi Y, Kishida Y, Yoshida M, Ito S, Kawata N, Imai K, Kakushima N, Takizawa K, Hotta K, Ono H. Macroscopic on-site evaluation of biopsy specimens for accurate pathological diagnosis during EUS-guided fine needle biopsy using 22-G Franseen needle. Endosc Ultrasound. 2020;9:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Moura DTH, McCarty TR, Jirapinyo P, Ribeiro IB, Farias GFA, Madruga-Neto AC, Ryou M, Thompson CC. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy in solid lesions: A multi-center analysis. World J Clin Cases. 2021;9:10507-10517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Khan MA, Grimm IS, Ali B, Nollan R, Tombazzi C, Ismail MK, Baron TH. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363-E375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Sonthalia N, Kumbar V, Tewari A, Roy A, Ghoshal UC, Goenka MK. Endoscopic ultrasound-guided fine needle biopsy using macroscopic on-site evaluation technique reduces the number passes yet maintains a high diagnostic accuracy: A randomized study. J Gastroenterol Hepatol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (2)] |
| 15. | Okuwaki K, Masutani H, Kida M, Yamauchi H, Iwai T, Miyata E, Hasegawa R, Kaneko T, Imaizumi H, Watanabe M, Kurosu T, Tadehara M, Adachi K, Tamaki A, Koizumi W. Diagnostic efficacy of white core cutoff lengths obtained by EUS-guided fine-needle biopsy using a novel 22G franseen biopsy needle and sample isolation processing by stereomicroscopy for subepithelial lesions. Endosc Ultrasound. 2020;9:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Masutani H, Okuwaki K, Kida M, Yoshida T, Imaizumi H, Yamauchi H, Iwai T, Kaneko T, Hasegawa R, Miyata E, Koizumi W. On-site stereomicroscope quality evaluations to estimate white core cutoff lengths using EUS-FNA biopsy sampling with 22-gauge needles. Gastrointest Endosc. 2019;90:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Cho YK. What method can we choose if rapid on-site evaluation is not available for the endoscopic ultrasound-guided tissue acquisition of upper gastrointestinal subepithelial lesions? Clin Endosc. 2024;57:53-55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Okuwaki K, Imaizumi H, Kida M, Masutani H, Iwai T, Watanabe M, Adachi K, Tadehara M, Hasegawa R, Nakatani S, Kurosu T, Tamaki A, Koizumi W. Usefulness of the automated multiband imaging system for EUS-FNA biopsy specimen evaluation in patients with upper gastrointestinal subepithelial lesions. Endosc Ultrasound. 2022;11:283-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Watanabe M, Okuwaki K, Kida M, Imaizumi H, Matsumoto T, Iwai T, Hasegawa R, Masutani H, Kurosu T, Minato N, Tamaki A, Ishizaki J, Kusano C. Multicenter prospective study of the efficacy of stereomicroscopic on-site evaluation in endoscopic ultrasound-guided tissue acquisition in patients with pancreatic cancer. Pancreatology. 2022;22:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Nakatani S, Okuwaki K, Watanabe M, Imaizumi H, Iwai T, Matsumoto T, Hasegawa R, Masutani H, Kurosu T, Tamaki A, Ishizaki J, Ishizaki A, Kida M, Kusano C. Stereomicroscopic on-site evaluation in endoscopic ultrasound-guided tissue acquisition of upper gastrointestinal subepithelial lesions. Clin Endosc. 2024;57:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Iwashita T, Uemura S, Ryuichi T, Senju A, Iwata S, Ohashi Y, Shimizu M. Advances and efficacy in specimen handling for endoscopic ultrasound-guided fine needle aspiration and biopsy: A comprehensive review. DEN Open. 2024;4:e350. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |