Published online Oct 16, 2024. doi: 10.4253/wjge.v16.i10.545
Revised: August 16, 2024
Accepted: September 5, 2024
Published online: October 16, 2024
Processing time: 111 Days and 15.3 Hours
Gastric mesenchymal tumors (GMT) are identified as soft tissue neoplasms that arise from mesenchymal stem cells within the gastrointestinal tract. GMT pri
To assess the safety and effectiveness of ECLR in managing small GMT (sGMT) with a maximum diameter ≤ 20 mm by comparing to ESE.
This retrospective analysis involved patients who were hospitalized in our institution between November 2021 and March 2023, underwent endoscopic resection, and received a pathological diagnosis of GMT. Cases with a tumor diameter ≤ 20 mm were chosen and categorized into two cohorts: Study and control groups. The study group was composed of patients treated with ECLR, whereas the control group was composed of those treated with ESE. Data on general clinical characteristics (gender, age, tumor diameter, tumor growth direction, tumor pathological type, and risk grade), surgery-related information (complete tumor resection rate, operation duration, hospitalization duration, hospitalization cost, and surgical complications), and postoperative follow-up were collected for both groups. The aforementioned data were subsequently analyzed and compared.
Five hundred and eighty-nine individuals were included, with 297 cases in the control group and 292 in the study group. After propensity score matching, the final analysis incorporated 260 subjects in each cohort. The findings indicated that the study group exhibited shorter operation duration and lowered medical expenses relative to the control group. Furthermore, the study group reported less postoperative abdominal pain and had a lower inci
ECLR is a viable and effective approach for managing sGMT.
Core Tip: Geometric mesenchymal tumors (GMT) have a certain malignant tendency. Endoscopic submucosal excavation is commonly used to treat this tumor clinically. We designed a new endoscopic technique [endoscopic “calabash” ligation and resection (ECLR)] to treat the tumor. Studies have shown that ECLR is an effective method for the treatment of GMT.
- Citation: Lin XM, Peng YM, Zeng HT, Yang JX, Xu ZL. Endoscopic “calabash” ligation and resection for small gastric mesenchymal tumors. World J Gastrointest Endosc 2024; 16(10): 545-556
- URL: https://www.wjgnet.com/1948-5190/full/v16/i10/545.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i10.545
Gastrointestinal mesenchymal tumors (GIMT) are identified as soft tissue neoplasms that arise from mesenchymal stem cells within the gastrointestinal tract, exhibiting non-epithelial and non-lymphatic characteristics[1], which are predominantly located in the stomach. GIMT distributed in the stomach are called gastric mesenchymal tumors (GMT). Initially, all digestive tract tumors thought to originate from smooth muscle were classified as gastrointestinal stromal tumors (GIST)[2]. However, advancements in immunohistochemical techniques have enabled precise diagnosis and classification of these tumors. Clinically, GMT primarily encompass gastric stromal tumors (GST), gastric leiomyomas (GL), and gastric schwannomas (GS).
GIST represent the most prevalent subtype of GIMT, constituting 1%-2% of all gastrointestinal tumors[3]. Research indicates that the global incidence rate of GIST is approximately 10%-15% per million annually[4]. GIST, which arise from the interstitial cells of Cajal or their precursor stem cells, are predominantly found in the stomach, accounting for approximately 60%-70% of cases[5]. Histologically, GST under a light microscope exhibit similarities to smooth muscle and Schwann cells, with pathological specimens primarily composed of spindle cells, occasionally accompanied by epithelioid cells. GST’s symptoms are nonspecific and are contingent on the lesion’s size and location. Common clinical manifestations include abdominal distension and pain, while severe cases may present with hematemesis or melena. Studies suggest that benign GIST are more frequently found in the stomach, whereas malignant GIST are more commonly identified in the colon[6]. Among them, GST have a better prognosis than small intestinal or rectal stromal tumors[7]. Nonetheless, GST still possess the potential for malignant transformation. GST are categorized as low-risk or high-risk malignant tumors depending on tumor size and mitotic count[8]. As per the National Institutes of Health 2008 guidelines, the invasive risk of GST can be categorized into four levels: Very low, low, intermediate, or high[9]. Consequently, surgical resection remains the preferred therapeutic approach for GST.
GL are a prevalent type of GIMT, representing 60%-70% of benign gastric tumors[10]. They originate from the submucosa, muscularis mucosa, and muscularis propria, primarily occurring in the cardia and frequently involving the gastroesophageal junction, with a few cases found in the fundus[11]. The occurrence of GL shows no correlation with gender or age[12], and most patients exhibit no significant clinical symptoms. Therefore, distinguishing GL from GST using traditional white light endoscopy and endoscopic ultrasonography (EUS) is challenging. Seo et al[13] developed a scoring system to differentiate tumors based on endoscopic appearance and EUS findings. A score of 0 to 1 indicates a high likelihood of GL, with a sensitivity and specificity of 75.9% and 99.5%, respectively, whereas a score of 2 to 3 suggests a higher probability of GST, with a sensitivity and specificity of 75.8% and 85.4%, respectively. Pathological examination remains the definitive gold standard for diagnosis. Historically, GL were considered to have minimal malignant potential. However, a study by Yamamoto et al[14] revealed that GL can undergo malignant transformation into leiomyosarcomas. Thus, early surgical intervention remains the primary treatment for GL.
GS, another variant of GIMT, originate from Schwann cells within the gastrointestinal neural plexus[15,16]. GS are comparatively rare, constituting merely 0.2% of all gastric tumors[1]. The age of onset for GS varies widely, with a higher incidence in females[17]. GS predominantly appear in the gastric body[11], followed by the antrum and fundus[16]. Patients generally do not display significant clinical symptoms, though some may experience abdominal discomfort. Larger tumors may lead to gastrointestinal bleeding[15]. Physical examination might reveal a palpable abdominal mass. GS are typically benign but hold the potential for malignant transformation[16]. Nevertheless, the prognosis post-resection is favorable[5]. Therefore, prompt surgical removal is recommended upon GS diagnosis.
In summary, early detection and intervention are crucial factors affecting the prognosis of GMT, with surgical treatment being the preferred approach. Historically, surgical resection was the standard method for treating GMT. Recently, advancements in endoscopic techniques have shown that the resection rate of endoscopic procedures is comparable to that of traditional open surgery, particularly for small GMT (sGMT, diameter ≤ 2 cm). Furthermore, endoscopic minimally invasive surgery provides advantages such as reduced trauma, faster recovery, shorter operation time, fewer complications, improved quality of life, and lower hospitalization costs, making it the preferred treatment for sGMT. Current endoscopic techniques include endoscopic submucosal dissection (ESD), endoscopic submucosal excavation (ESE), endoscopic full-thickness resection (EFTR), and submucosal tunneling endoscopic resection[18]. ESE is both effective and safe for GMT treatment, preserving the normal physiological structure of the digestive tract while excising the tumor, and is widely used in clinical practice. A novel endoscopic surgical method for GMT, termed en
A cohort of 520 patients diagnosed with GMT via biopsy following endoscopic treatment at our hospital between November 2021 and March 2023 was selected for this retrospective analysis. This study received approval from the hospital’s ethics committee, and all individuals, along with their families, provided informed consent.
The inclusion criteria were: (1) Age ≥ 18 years; (2) Preoperative endoscopic ultrasound indicating a single lesion, without excluding GMT; (3) Preoperative computed tomography (CT) or magnetic resonance imaging (MRI) showing no evidence of metastatic spread to lymph nodes or distant sites; (4) Tumor diameter ≤ 20 mm; (5) Postoperative pathological confirmation of the resected lesion as GST, GL, or GS; and (6) Treatment completion through ESE or ECLR. In addition, all cases included in this investigation did not change the endoscopic treatment.
The exclusion criteria included: (1) Individuals with severe primary diseases affecting the heart, liver, kidney, or hematopoietic system; (2) Detection of tumor metastasis by preoperative imaging such as CT or MRI; (3) Patients with other conditions significantly increasing treatment risk and hospital stay duration; (4) Postoperative pathology of the resected lesion not confirming GST, GL, or GS, or incomplete immunohistochemical examination; and (5) Fragmented patient files. Individuals were allocated to either a control group (GMT cases managed with ESE) or a study group (GMT individuals receiving ECLR therapy).
All endoscopists involved in this study held positions as either chief physician or associate chief physician, with a cumulative experience of more than 500 cases in advanced endoscopic procedures, including ESD, ESE, EFTR, and ECLR. They possessed substantial expertise in ESE and ECLR techniques and had completed standardized training in surgical protocols and the use of instruments.
The instruments employed in this research consisted of gastroscope (GIF-Q260J; Olympus Corporation, Tokyo, Japan), endoscopic ultrasound system (SU-9000; Hitachi High-Tech Group, Tokyo, Japan), argon plasma coagulation system (VIO-200s; ERBE Elektromedizin GmbH, Tübingen, Germany), IT-Knife2 (KD-611 L; Olympus Corporation), DualKnife (KD-650 L; Olympus Corporation), disposable multifunctional mucosal cutting knives (Anrei, Zhejiang Province, China), disposable injection needles (NM-200 L-0423; Olympus Corporation), disposable electrocircular ligators (MTNPFS01-02423180; Nanjing Nanwei Medical Technology Co., Ltd.), hot biopsy forceps (HBF-16/1800; Nanjing Nanwei Medical Technology Co., Ltd.), titanium clips (ROCC-D-26-195; Nanjing Nanwei Medical Technology Co., Ltd., China), nylon ligation rings (Olympus Corporation), and transparent ligation caps (Olympus Corporation). The submucosal injection solution comprised 250 mL of normal saline, 0.5 mg of methylene blue, and 1 mg of epinephrine. Midazolam, pethidine, or propofol was utilized for the administration of intravenous anesthesia.
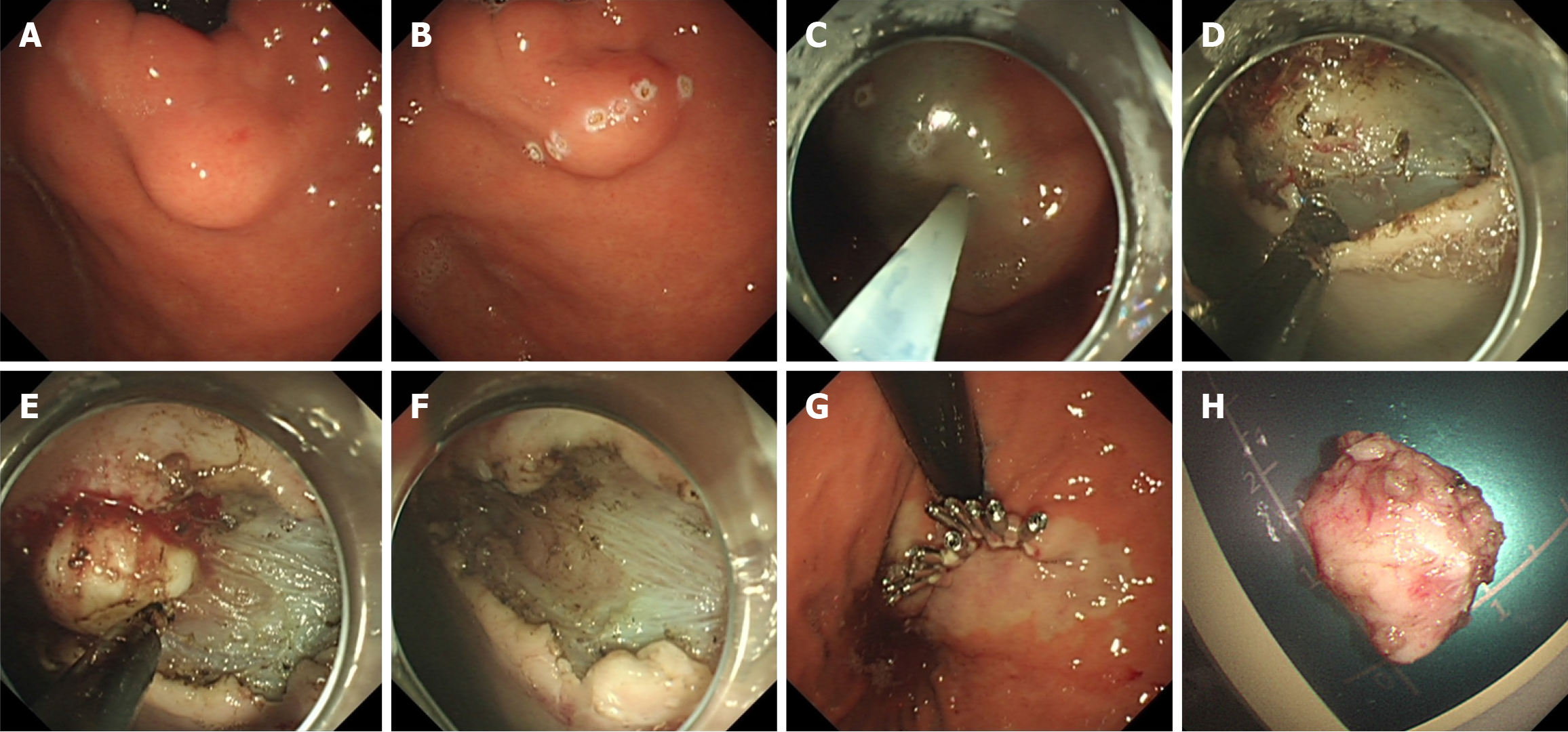
ESE: The subject was positioned in the left-side lying posture, with a cardiac monitor for monitoring vital signs (Figure 1). Intravenous anesthesia was administered. Initially, the surface mucosa of the sGMT was marked with electrocoagulation using a mucosal incision knife (MIK) and several injections were delivered into the submucosal layer around the lesion. Following this, a MIK was employed to cut through the mucosa and submucosa at the apex of the sGMT, revealing the tumor surface, dissecting the tumor layer by layer, and resecting the sGMT along the tumor base. Finally, the wound was closed with titanium clips and/or nylon loops. Residual blood in the gastric cavity was rinsed out. Once it was confirmed that no active bleeding was present at the treatment site, the endoscope was withdrawn. A gastric tube linked to a ne
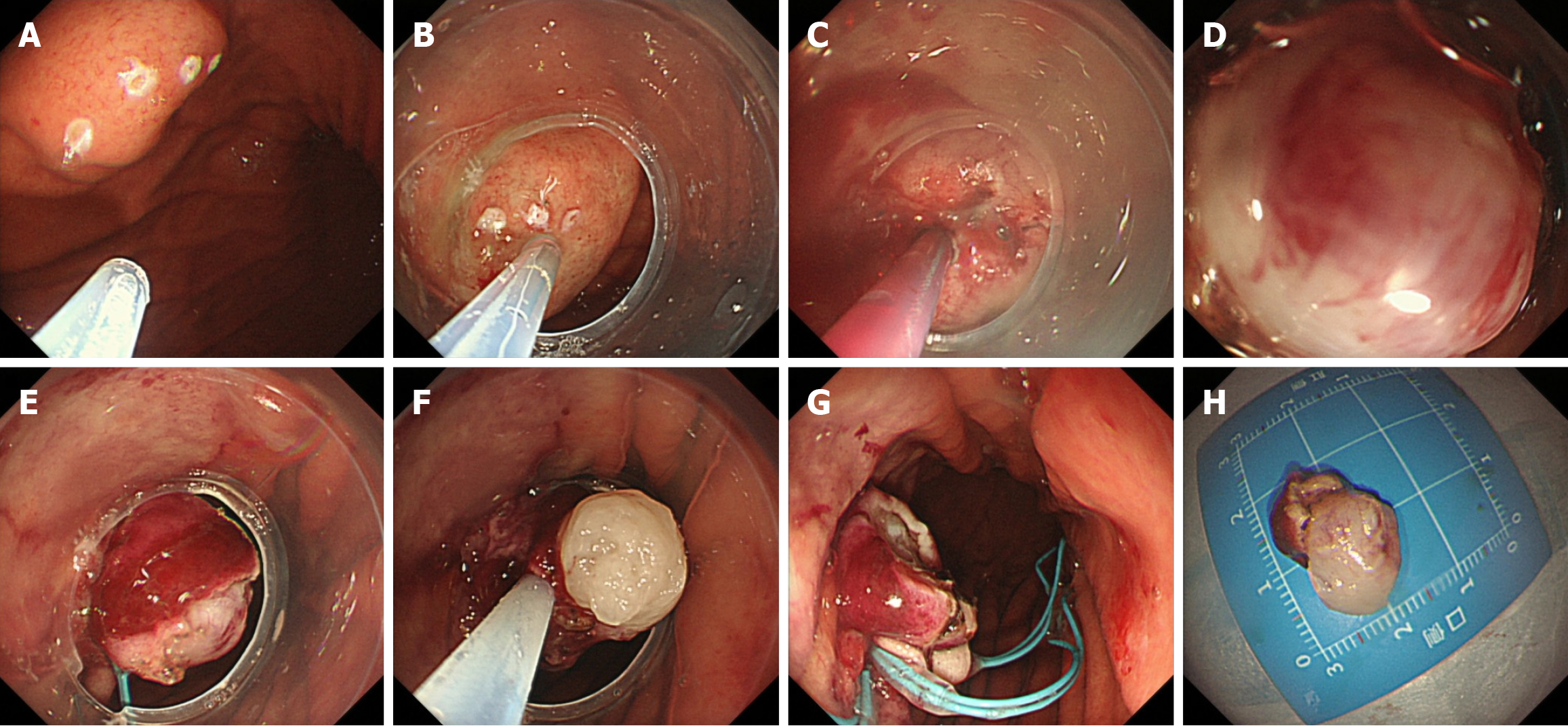
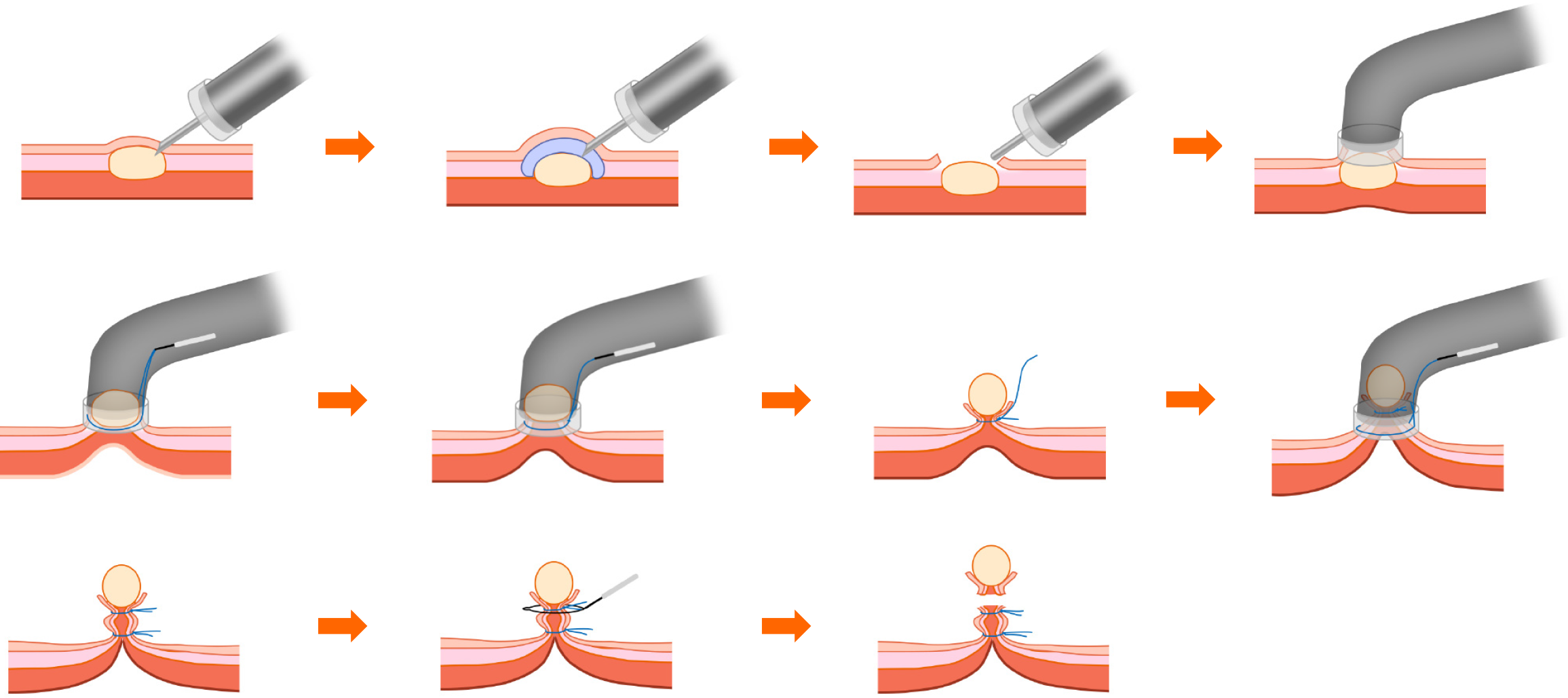
ECLR: The subject was positioned in the left-side lying posture, with a cardiac monitor for monitoring vital signs (Figures 2 and 3). Intravenous anesthesia was administered. Initially, electrocoagulation markers were placed on the surface of the sGMT utilizing a MIK or an electrocircular snare. Subsequently, several injections were made into the submucosal layer bordering the sGMT, utilizing an injection needle. Following this, an incision was made on the surface of the tumor with the tip of the MIK or electrocircular snare, ensuring that it was large enough to cover the tumor’s diameter and deep enough to reach the mucosal and submucosal layers, thereby exposing the tumor. Using negative pressure suction, the tumor was aspirated into the transparent ligation cap at the endoscope’s tip. If the tumor could not be aspirated, further dissection was performed. The initial nylon loop was situated at the foundation of the tumor, while the subsequent loop was strategically placed inferior to the first, ensuring that it could tighten the full-thickness gastric wall. The suction device was reactivated to fully aspirate the tumor into the ligation cap. At this point, the maximum negative pressure was applied to aspirate the complete tumor into the ligation cap while the assistant gradually tightened the nylon loop. Consequently, the nylon loop and tumor in the surgical area resembled a “calabash”. The surgeon then used the MIK or electrocircular snare to excise the tumor above the first nylon loop, effectively removing the upper part of the “calabash”. The inferior portion remained intact to prevent perforation or bleeding. In the case of intraoperative perforation, a nylon loop could be re-used for full-thickness ligation, or titanium clips combined with a nylon loop could be used for closure.
The potential complications during treatment include the following: (1) Resection failure: The inability to remove the GMT endoscopically or the necessity to convert to surgical intervention midway due to various reasons; (2) Intraoperative massive bleeding: Defined as intraoperative blood loss exceeding 100 mL or bleeding that is uncontrollable under endoscopy; (3) Intraoperative perforation: The occurrence of full-thickness gastric defects during the procedure; (4) Postoperative delayed bleeding: Characterized by the presence of bright red drainage fluid from the gastric tube post-procedure, the patient experiencing hematemesis, or the discovery of active bleeding on follow-up endoscopy; (5) Postoperative delayed perforation: Identification of full-thickness gastric defects after the procedure; (6) Abdominal infection: Defined as the development of fever in the patient, accompanied by increased white blood cell count, elevated neutrophil percentage, elevated procalcitonin and C-reactive protein levels, or (and) CT examination indicating ascites or (and) abscess formation; (7) Postoperative infections in locations beyond the abdominal region, including but not limited to respiratory tract infections; (8) Postoperative electrocoagulation syndrome: The onset of fever, localized abdominal tenderness and rebound tenderness, and increased white blood cell count (≥ 10.8 × 109/L) within 2 d following the endoscopic operation, but excluding gastric perforation as indicated by abdominal X-ray or CT examination; and (9) Postoperative abdominal pain: The occurrence of abdominal pain post-procedure, which can be assessed according to pain intensity using a pain rating scale.
Following the operation, a gastric tube was placed and linked to a negative pressure drainage box to monitor for delayed bleeding. All patients were administered proton pump inhibitors to suppress gastric acid for 8 wk postoperatively. If the patient’s condition remained stable without delayed bleeding, perforation, infection, or other complications, a full liquid diet was initiated 24 h after the procedure. For individuals with large surgical incisions, marked postoperative abdominal pain, or postoperative bleeding complications, the fasting period needed to be extended. Patients with postoperative infections were treated with antibiotics. In cases of delayed perforation after the procedure, gastrointestinal decom
The criteria for discharge are as follows: (1) Absence of complications, including delayed bleeding, perforation, or infection; (2) The patient reported no discomfort after 1 d of a liquid diet; (3) Pain score ≤ 2 points; and (4) Patients po
One week after discharge, patients must attend a follow-up appointment at the outpatient clinic to assess postoperative recovery and receive dietary and activity guidance. Telephone follow-ups were conducted at 2 and 4 weeks after discharge to assess for any latent adverse events, including bleeding, perforation, or infection. Additionally, all individuals were advised to schedule their first follow-up endoscopy within 3 to 6 months post-discharge to evaluate wound healing and check for tumor recurrence. Subsequent endoscopies should be performed annually. If follow-up examinations revealed the presence of residual or recurrent tumors, a second endoscopic or surgical intervention should be considered.
Data analyses were carried out utilizing Statistical Product and Service Solutions 25.0 software (International Business Machines Corporation, Armonk, NY, United States). Propensity score matching (PSM) was employed to mitigate retrospective bias, with a caliper set at 0.02. Comparative analysis was performed among the study group and the control group, taking into account general clinical data (gender, age, tumor length, tumor growth direction, tumor pathological type, and risk grade), surgical-related information (complete tumor resection rate, operation time, length of hospital stay, hospitalization expenses, and surgical complications), and postoperative follow-up outcomes. Measurement data are denoted as the mean ± SD, whereas count data are represented by frequencies or percentages. Independent sample t tests were utilized to compare measurement data between the two groups, and χ2 tests were utilized for count data comparisons. Non-parametric Wilcoxon tests were employed for data comparisons involving non-normally distributed data or unequal variances. Two-tailed P < 0.050 was deemed statistically significant.
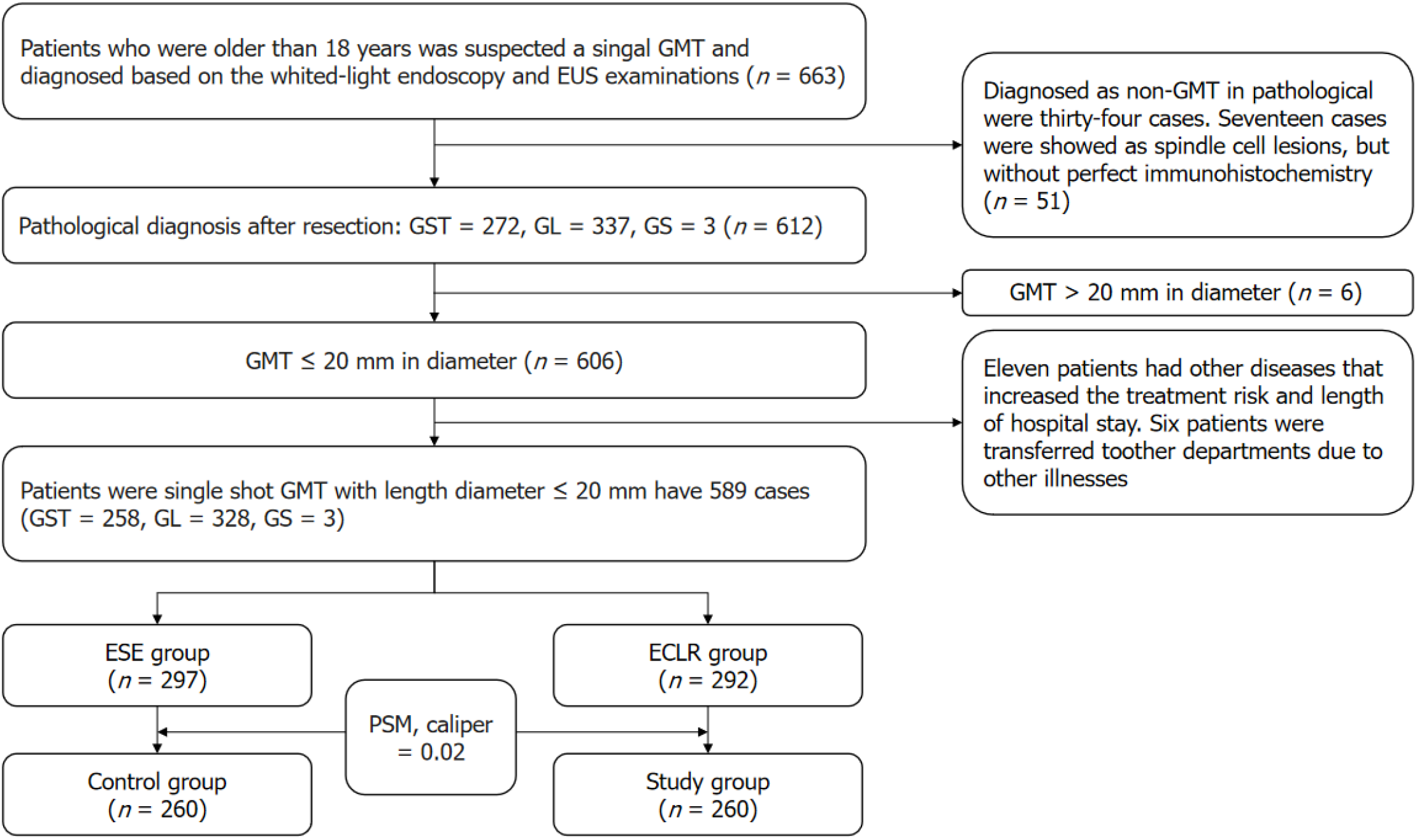
The selection process for study subjects is depicted in Figure 4. From November 2021 to March 2023, 663 patients were suspected of having a single GMT based on white light endoscopy and EUS examination. Upon acquiring patient authorization, these patients received endoscopic surgical treatment. Postoperative pathology reported 272 cases of GST, 337 cases of GL, and 3 cases of GS. Based on the preoperative EUS examination results, 606 cases of GMT with a long diameter ≤ 20 mm were identified. Among them, 11 patients presented with comorbidities that elevated the risks as
Comparison of baseline characteristics: The control group consisted of 95 males and 165 females, with an average age of 53.01 years ± 10.37 years (range: 26 years to 78 years) (Table 1). The average maximum diameter of the GMT was 6.72 mm ± 2.22 mm (range: 2.4 mm to 15.9 mm). GMT were situated within the gastric fundus in 143 cases and in the gastric body in 117. Most GMT (253 cases, 97.30%) exhibited endophytic growth, while a minority (7 cases, 2.70%) showed partial exophytic growth. There were no completely exophytic GMT observed. In terms of pathological types, there were 99 cases of GST and 161 cases of GL, with no cases of GS. Among the GST cases, 98 were classified as very low risk, and only one case was categorized as low risk. There were no intermediate or high-risk cases determined.
| Characteristic | Control group (n = 260) | Study group (n = 260) | P value | |
| Gender | Male/female | 95/165 | 99/161 | 0.717 |
| Age (years) | 53.01 ± 10.37 | 52.23 ± 11.92 | 0.428 | |
| Tumor location | Gastric fundus/gastric body | 143/117 | 146/114 | 0.791 |
| Tumor long diameter | 6.72 ± 2.22 | 6.53 ± 2.16 | 0.320 | |
| Tumor growth pattern | Endoluminal/partially exophytic | 253/7 | 253/7 | > 0.999 |
| Tumor pathological type | Leiomyoma | 161 | 139 | 0.062 |
| Schwannoma | 0 | 1 | > 0.999 | |
| Gastrointestinal stromal tumor | 99 | 120 | 0.076 | |
| Gastrointestinal stromal tumor risk stratification | Very low risk | 98 | 120 | 0.452 |
| Low risk | 1 | 0 | ||
| Intermediate risk | 0 | 0 | ||
| High risk | 0 | 0 | ||
The research cohort comprised 99 males and 161 females, with an average age of 52.23 years ± 11.92 years (range: 20 years to 87 years). The average diameter of the GMT was 6.53 mm ± 2.16 mm (range: 2.5 mm to 14.7 mm). GMT were primarily situated within the gastric fundus in 146 cases and in the gastric body in 114. A total of 253 GMT (97.30%) exhibited endophytic growth, while 7 (2.70%) showed partial exophytic growth. There were no completely exophytic GMT observed. The pathological types included 120 GST, 139 GL, and 1 GS case. All GST patients (120 cases) were categorized as very low-risk, with no low-risk, intermediate-risk, or high-risk cases. There were no statistically significant differences between the two groups regarding gender (P = 0.717), age (P = 0.428), GMT distribution (P = 0.791), average GMT diameter (P = 0.320), GMT growth direction (P > 0.999), GMT pathological type (P > 0.999), or GST risk stratification (P = 0.452).
Comparison of efficacy of endoscopic treatments: Postoperative pathological biopsy confirmed that all cases had intact tumor capsules and total excision of the GMT (Table 2).
| Control group (n = 260) | Study group (n = 260) | P value | ||
| Endoscopic treatment outcomes | Complete resection (cases) | 260 | 260 | > 0.999 |
| Incomplete resection (cases) | 0 | 0 | > 0.999 | |
| Intraoperative complications | Intraoperative bleeding (cases) | 0 | 0 | > 0.999 |
| Intraoperative perforation (cases) | 28 (3.08) | 3 (1.15) | < 0.001 | |
| Intraoperative endoscopic examination failure and conversion to open surgery (cases) | 0 | 0 | > 0.999 | |
| Postoperative complications | Postoperative abdominal pain score (points) | 0.25 ± 0.56 | 0.16 ± 0.43 | 0.029 |
| Postoperative delayed bleeding (cases) | 1 (0.38) | 0 (0) | > 0.999 | |
| Average volume of red blood cell suspension transfused (U) | 2 | 0 | 0.318 | |
| Postoperative delayed perforation (cases) | 0 | 0 (0) | > 0.999 | |
| Postoperative abdominal infection (cases) | 1 (0.38) | 0 (0) | > 0.999 | |
| Postoperative respiratory tract infection (cases) | 3 (1.15) | 1 (0.38) | 0.624 | |
| Postoperative electrocoagulation syndrome (cases) | 8 (3.08) | 1 (0.38) | 0.037 |
Comparison of intraoperative complications: There were no cases of massive intraoperative bleeding in either group. Intraoperative perforation occurred in 28 cases (3.08%) in the control group and 3 cases (1.15%) in the study group. All perforations were endoscopically closed without the need for surgical intervention. Neither group required conversion to surgical treatment owing to endoscopic treatment failure (Table 2).
Comparison of postoperative complications: Postoperative abdominal pain scores were 0.25 points ± 0.56 points (range: 0 points to 3 points) in the control group and 0.16 points ± 0.43 points (range: 0 points to 3 points) in the study group (Table 2). The pain score was markedly reduced in the study group relative to the control group (P = 0.029). Despite the statistical significance, pain levels were low in both groups, indicating no substantial clinical difference. One individual in the control group experienced delayed bleeding postoperatively (incidence rate: 0.38%), occurring on the third day after discharge due to excessive food intake. This patient returned to the hospital for treatment and received a transfusion of two units of leukocyte-reduced red blood cell suspension, subsequently recovering and being discharged. There were no cases of postoperative delayed bleeding occurring in the study group, and the difference between the groups was not statistically significant (P > 0.999). There were no cases of postoperative delayed perforation observed in either group, with no statistically significant difference (P > 0.999). One case of postoperative intraabdominal infection was discovered in the control group (incidence rate: 0.38%), with no statistically significant difference between the groups (P > 0.999). Respiratory tract infections occurred postoperatively in three cases in the control group and one case in the study group (incidence rates: 1.15% and 0.38%, respectively), with no statistically significant difference (P = 0.624). The incidence rate of postoperative electrocoagulation syndrome in the study group was 0.38% (1/260), significantly lower than the 3.08% (8/260) in the control group (P = 0.037).
Comparison of endoscopic surgery duration: The duration of endoscopic treatment was 54.00 min ± 9.47 min (range: 27 min to 69 min) in the control group and 33.49 min ± 8.46 min (range: 20 min to 49 min) in the study group. Treatment time was significantly shorter in the study group relative to the control group (P < 0.001) (Table 3).
| Control group (n = 260) | Study group (n = 260) | P value | |
| Operative time (min) | 54.00 ± 9.47 | 33.49 ± 8.46 | < 0.001 |
| Length of hospital stay (d) | 5.04 ± 1.46 | 5.24 ± 1.45 | 0.117 |
| Hospitalization expenses (RMB) | 16111.40 ± 3709.71 | 14435.25 ± 3458.43 | < 0.001 |
| Materials expenses (RMB) | 4115.81 ± 1486.70 | 3571.96 ± 1507.32 | < 0.001 |
Comparison of hospitalization duration: The length of hospital stay was 5.04 d ± 1.46 d (range: 2 d to 13 d) in the control group and 5.24 d ± 1.45 d (range: 3 d to 11 d) in the study group, with no statistically significant difference between the two groups (P = 0.117) (Table 3).
Comparison of medical costs: Medical costs were RMB 16111.40 ± 3709.71 (range: RMB 9601.95 to 35910.45) in the control group and RMB 14435.25 ± 3458.43 (range: 7590.25 to 30412.48) in the study group. In addition, during tumor resection, the two groups had the same operation charge code, which means that operation cost was same. However, material costs of the two groups were different. Material costs were RMB 4115.81 ± 1486.70 (range: RMB 91242.13 to 10804.39) in the control group and RMB 3571.96 ± 1507.32 (range: RMB 718.27 to 8943.39) in the study group. Costs in the study group were significantly reduced relative to the control group (P < 0.001) (Table 3).
Comparison of follow-up results: There was no statistically significant difference in follow-up duration between the two cohorts. During the follow-up period, no tumor recurrence, metastasis, or death was discovered in either group (Table 4).
| Control group (n = 260) | Study group (n = 260) | P value | |
| Mean postoperative follow-up time (mo) | 3.51 ± 4.26 | 3.23 ± 4.07 | 0.457 |
| Postoperative recurrence (cases) | 0 | 0 | > 0.999 |
| Postoperative metastasis (cases) | 0 | 0 | > 0.999 |
| Postoperative death (cases) | 0 | 0 | > 0.999 |
GMT comprising GST, GL, and GS are frequently observed as submucosal lesions during endoscopy. EUS identifies GMT as hypoechoic masses within the submucosal or muscularis propria layer[20]. Malignant GST typically present as abnormal echoic masses with irregular borders[21]. EUS can provide preliminary assessments of the nature and origin of lesions, and its extended technique, EUS-guided fine-needle aspiration (EUS-FNA), markedly enhances the diagnostic capability of EUS, reducing the need for surgical diagnostic interventions. The sensitivity, specificity, and accuracy of EUS-FNA in diagnosing upper GIMT are reported to be 82.9%, 73.3%, and 80%, respectively[22]. However, EUS-FNA is a non-sterilized procedure performed in the non-sterile environment of the digestive tract, which may lead to postoperative infection. For malignant tumors, EUS-FNA carries the risk of tumor rupture and dissemination of cancer cells. Conse
The primary goal of surgical treatment is to achieve complete tumor resection. During ESE for GMT resection, the MIK is employed to incise the mucosa and submucosa, separate the tumor capsule, expose the tumor, and finally dissect along the tumor base, ensuring the integrity of the capsule and complete tumor removal. GMT located in the fundus are more challenging to resect than tumors in other locations. ESE employs a “U”-shaped reverse position to fully expose the tumor base[19] and achieve complete resection. In contrast, ECLR utilizes negative pressure suction to draw the entire tumor into the ligation cap, performing ligation and resection at the tumor base. Compared with ESE, ECLR does not require external traction[19] and can still achieve complete tumor resection with a simpler operation. In this study, postoperative pathology of all tumors indicated negative resection margins, demonstrating that both ECLR and ESE can achieve complete resection for GMT with a long diameter of ≤ 20 mm.
Intraoperative bleeding is a significant complication frequently associated with GMT originating from the muscularis propria. This is due to the relatively abundant blood supply in the muscularis propria of the gastric submucosa. Addi
In the control group, one patient (0.38%) experienced delayed bleeding postoperatively, with no instances of pos
The average postoperative abdominal pain score was 0.25 ± 0.56 in the control group and 0.16 ± 0.43 in the study group. The postoperative abdominal pain score in the study group was significantly lower relative to that of the control group (P = 0.029). The postoperative abdominal pain in both groups did not affect patients’ daily life.
One case of postoperative intra-abdominal infection was discovered in the control group, caused by the leakage of intraoperative lavage fluid into the abdominal cavity. The patient recovered following antibiotic treatment. Therefore, excessive lavage should be avoided during operations for patients with suspected perforation.
In the control group, three patients (1.15%) developed respiratory tract infections post-treatment, while only one individual (0.38%) in the study group experienced this complication. These infections were attributed to accidental as
Postoperatively, patients in both groups developed electrocoagulation syndrome, with 8 cases (3.08%) in the control group and 1 (0.38%) in the study group. The prevalence rate in the study group was significantly lower relative to that of the control group (P = 0.037). Electrocoagulation syndrome is often caused by heat-induced transmural injury during intraoperative electrocoagulation, characterized by fever and local pain without perforation[25]. This is related to the electrocoagulation method and the duration of the operation. In ESE, frequent electrocoagulation is necessary for he
Compared with ESE, the operation method of ECLR is simpler, and the average operation time in the study group was significantly shorter relative to that of the control group (P < 0.001). There was no statistically significant difference in the length of hospital stay between the two groups. ECLR requires fewer and cheaper instruments than ESE, and the hospitalization cost in the study group was significantly lower relative to that of the control group (P < 0.001). In the follow-up period, no individuals in either group were found to have tumor recurrence or metastasis.
This study demonstrated that for GMT with a long diameter ≤ 20 mm, complete resection was accomplished in all subjects in both the ESE group (control group) and the ECLR group (study group). However, ECLR presents several advantages over ESE, including a simpler procedure, lower treatment costs, and reduced complications such as electrocoagulation syndrome. Consequently, ECLR can be considered an effective endoscopic surgical technique for treating sGMT. However, this study still has limitations. First of all, this investigation is a single-center retrospective analysis with a restricted sample size. There is some information bias and sample selection bias, which may affect the results of this study. Second, different endoscopists may have certain differences in their understanding of different endoscopic techniques. Moreover, the time span for selecting cases in the study is long. As time goes on, the operational skills and proficiency of endoscopists have improved compared to before. It may have a certain impact on the results. Finally, the follow-up period of this study is short. Long-term efficacy of ECLR in the treatment of sGMT is still insufficient. Future research should involve a multi-center prospective study to provide a more robust theoretical basis for the use of ECLR in the treatment of GMT.
First of all, my sincere and hearty thanks and appreciations to my supervisor, Dr. Xu, who has kindly provided me assistance in the course of preparing this paper. In addition, many thanks go to my family, friends, and classmates for their unwavering support. Finally, I am really grateful to all those who devoted much time to reading this paper and gave me much advice, which will benefit me in my later study.
| 1. | Lin CS, Hsu HS, Tsai CH, Li WY, Huang MH. Gastric schwannoma. J Chin Med Assoc. 2004;67:583-586. [PubMed] |
| 2. | Rudolph P, Chiaravalli AM, Pauser U, Oschlies I, Hillemanns M, Gobbo M, Marichal M, Eusebi V, Höfler H, Capella C, Klöppel G. Gastrointestinal mesenchymal tumors - immunophenotypic classification and survival analysis. Virchows Arch. 2002;441:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Colapkulu-Akgul N, Gunel H, Beyazadam D, Ozsoy MS, Alimoglu O. Gastrointestinal Stromal Tumors: Recurrence and Survival Analysis of 49 Patients. Middle East J Dig Dis. 2023;15:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 5. | Saad Abdalla Al-Zawi A, Lahmadi S, Jalilzadeh Afshari S, Kak I, Alowami S. Gastric Schwannoma as an Important and Infrequent Differential Diagnosis of Gastric Mesenchymal Tumours: A Case Report and Review of Literature. Cureus. 2022;14:e32112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Sornmayura P, Howannapakorn J, Karnsombut P. Revision of gastrointestinal mesenchymal tumors. J Med Assoc Thai. 2009;92:87-95. [PubMed] |
| 7. | Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dufresne A, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss SJ, Hall KS, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Gronchi A, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (1)] |
| 8. | Versaci A, Macrì A, Ieni A, Terranova M, Leonello G, Saladino E, Speciale G, Famulari C. [Gastrointestinal stromal tumour: our experience]. Chir Ital. 2009;61:161-169. [PubMed] |
| 9. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 10. | Li HY. [Differential diagnosis and case analysis of two cases of leiomyoma in the stomach]. Zhongguo Yiyao Zhinan. 2015;13:218. [DOI] [Full Text] |
| 11. | Zhong YX, Jin P, Chu JT, Xiong JP, Li Y, Kang WZ, Tian YT. [Diagnosis and treatment analysis of 173 cases of gastric leiomyoma]. Zhongguo Yixue Qianyan Zazhi. 2021;13:49-52. |
| 12. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 13. | Seo SW, Hong SJ, Han JP, Choi MH, Song JY, Kim HK, Lee TH, Ko BM, Cho JY, Lee JS, Lee MS. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013;14:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Yamamoto A, Tateishi Y, Aikou S, Seto Y, Ushiku T. The first case of gastric leiomyosarcoma developed through malignant transformation of leiomyoma. Pathol Int. 2021;71:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Pu C, Zhang K. Gastric schwannoma: a case report and literature review. J Int Med Res. 2020;48:300060520957828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Li B, Liang T, Wei L, Ma M, Huang Y, Xu H, Shi X, Qin C. Endoscopic interventional treatment for gastric schwannoma: a single-center experience. Int J Clin Exp Pathol. 2014;7:6616-6625. [PubMed] |
| 17. | Doyle LA, Hornick JL. Mesenchymal Tumors of the Gastrointestinal Tract Other than GIST. Surg Pathol Clin. 2013;6:425-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Tan XM, Zhao R, Wang Y, Zhu M, Sun YZ, Zhao P, Zhu Y, Yang GX. [Endoscopic submucosal excavation versus endoscopic full-thickness resection in the treatment of gastric stromal tumor]. Zhongguo Linchuang Yanjiu. 2021;34:1025-1032. [DOI] [Full Text] |
| 19. | Peng MS, Zeng HT, Zhang ZL, Chen ZM, Long T, Wang LS, Xu ZL. Efficacy and safety of endoscopic "calabash" ligation and resection for small gastric stromal tumors originating from the muscularis propria. Cancer Med. 2023;12:6825-6841. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Saoud C, Illei PB, Siddiqui MT, Ali SZ. Cytopathology of rare gastric mesenchymal neoplasms: A series of 25 cases and review of literature. Cytopathology. 2023;34:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Zhou XX, Ji F, Xu L, Li L, Chen YP, Lu JJ, Wang CW, Huang W. EUS for choosing best endoscopic treatment of mesenchymal tumors of upper gastrointestinal tract. World J Gastroenterol. 2011;17:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wiersema MJ, Kochman ML, Chak A, Cramer HM, Kesler KA. Real-time endoscopic ultrasound-guided fine-needle aspiration of a mediastinal lymph node. Gastrointest Endosc. 1993;39:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Bao YJ, Ye B, Cao F, Li F. [Progress and current status of surgical research on gastrie gastrointestinal stromal tumor]. Guoji Waikexue Zazhi. 2020;47:768-772. [DOI] [Full Text] |
| 24. | Piccinni G, Marzullo A, Angrisano A, Iacobone D, Nacchiero M. Endoscopic resection of benign very low-risk gastric gastrointestinal stromal tumors. Is it enough? Eur J Gastroenterol Hepatol. 2007;19:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Kimura H, Yabuuchi Y, Notsu A, Yamamoto Y, Yoshida M, Kawata N, Takizawa K, Kishida Y, Imai K, Ito S, Hotta K, Ishiwatari H, Matsubayashi H, Ono H. Features of post-endoscopic submucosal dissection electrocoagulation syndrome for early gastric neoplasm. J Gastroenterol Hepatol. 2021;36:3164-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |