Published online Sep 16, 2023. doi: 10.4253/wjge.v15.i9.545
Peer-review started: March 22, 2023
First decision: April 28, 2023
Revised: May 16, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 16, 2023
Processing time: 174 Days and 9.4 Hours
Antiretroviral treatment (ART) has improved the life expectancy of patients living with human immunodeficiency virus (HIV). As these patients age, they are at increased risk for developing non-acquired immunodeficiency syndrome defining malignancies (NADMs) such as colon cancers.
To determine which factors are associated with the development of precancerous polyps on screening colonoscopy in patients with HIV and to investigate whether HIV disease status, measured by viral load and CD4 count, might influence precancerous polyp development.
A retrospective review of records at two urban academic medical centers was performed for HIV patients who had a screening colonoscopy between 2005-2015. Patients with a history of colorectal cancer or polyps, poor bowel preparation, or inflammatory bowel disease were excluded. Demographic data such as sex, age, race, and body mass index (BMI) as well as information regarding the HIV disease status such as CD4 count, viral load, and medication regimen were collected. Well-controlled patients were defined as those that had viral load < 50 copies, and poorly-controlled patients were those with viral load ≥ 50. Patients were also stratified based on their CD4 count, comparing those with a low CD4 count to those with a high CD4 count. Using colonoscopy reports in the medical record, the size, histology, and number of polyps were recorded for each patient. Precancerous polyps included adenomas and proximal serrated polyps. Data was analyzed using Fisher’s exact tests and logistic regression through SAS 3.8 software.
Two hundred and seven patients met our inclusion criteria. The mean age was 56.13 years, and 58% were males. There were no significant differences in terms of age, race or ethnicity, insurance, and smoking status between patients with CD4 counts above or below 500. BMI was lower in patients with CD4 count < 500 as compared to those with count > 500 (P = 0.0276). In patients with CD4 > 500, 53.85% of patients were female, and 70.87% of patients with CD4 < 500 were male (P = 0.0004). Only 1.92% of patients with CD4 ≥ 500 had precancerous polyps vs 10.68% of patients with CD4 < 500 (P = 0.0102). When controlled for sex, BMI, and ART use, patients with CD4 < 500 were 9.01 times more likely to have precancerous polyps [95% confidence interval (CI): 1.69-47.97; P = 0.0100]. Patients taking non-nucleoside reverse transcriptase inhibitors were also found to be 10.23 times more likely to have precancerous polyps (95%CI: 1.08-97.15; P = 0.0428). There was not a significant difference noted in precancerous polyps between those that had viral loads greater or less than 50 copies.
Patients with low CD4 counts were more likely to have precancerous polyps on their screening colonoscopy although the etiology for this association is unclear. We also found an increased risk of precancerous polyps in patients taking non-nucleoside reverse transcriptase inhibitors, which is contradictory to prior literature showing ART has decreased the risk of development of NADMs. However, there have not been studies looking at colorectal cancer and ART by drug class, to our knowledge. Further prospective studies are needed to determine the effect of HIV control and therapies on polyp development.
Core Tip: Aging human immunodeficiency virus (HIV) patients are at a higher risk for developing non-acquired immunodeficiency syndrome defining malignancies. We investigated the factors associated with the development of precancerous polyps on index colonoscopy and whether HIV disease state might influence precancerous polyps. We divided patients into two groups based on their viral load and CD4 count. We retrieved colonoscopy results, patient demographics, and relevant HIV data from the electronic medical record. We determined that patients with low CD4 counts were more likely to have precancerous polyps on their index colonoscopy. We found an increased risk of precancerous polyps in patients taking non-nucleoside reverse transcriptase inhibitors.
- Citation: Likhtshteyn M, Marzouk E, Arroyo-Mercado FM, Chawla G, Rosengarten S, Lerer R, Ojeda-Martinez H, Thor S. Human immunodeficiency virus patients with low CD4 counts are more likely to have precancerous polyps identified during index colonoscopy. World J Gastrointest Endosc 2023; 15(9): 545-552
- URL: https://www.wjgnet.com/1948-5190/full/v15/i9/545.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i9.545
Antiretroviral therapy has dramatically changed and improved the life expectancy of patients coping with human immunodeficiency virus (HIV). With the introduction of antiretroviral treatment (ART) in 1996, the worldwide life expectancy of HIV-infected people has improved significantly. As the HIV disease state is being better controlled with ART, this patient population is at lower risk for developing acquired immunodeficiency syndrome (AIDS) defining illnesses. However, as these patients live longer, they become vulnerable to developing non-AIDS defining malignancies (NADMs) such as colon cancers[1]. In 1994, Klugman and Schaffner[2] published a case report of a 25-year-old African American man with HIV who was found to have an advanced right sided colonic adenocarcinoma postmortem. At that time, ART therapy had not yet been introduced or widely accepted, and it was thought that the most significant manifestation of HIV in the gastrointestinal tract was Kaposi’s sarcoma[2]. However, even Krugman postulated that HIV might play a role in the development of colon cancer.
While some studies have shown that highly active antiretroviral therapy (HAART) decreases the risk of developing colorectal cancer[3], other studies propose that HIV patients are at higher risk and develop colorectal cancer at younger ages[4,5]. Conversely, other studies have shown that the rates of colorectal cancer are similar between people with and without HIV[6]. According to current guidelines, HIV infection does not change the age at which screening colonoscopies are performed. A consensus regarding HIV infection and colonic neoplasms has not been reached, possibly due to a paucity of data regarding these two diseases. We aimed to identify which factors are associated with the development of precancerous polyps on index (first) screening colonoscopy in patients with HIV and to investigate whether HIV disease status, measured by viral load and CD4 count, may influence precancerous polyp growth.
A retrospective review of medical records at Kings County Hospital and SUNY Downstate Health Sciences University for patients with HIV who had received a screening colonoscopy between 2005 and 2015 was performed. Patient demographics were collected, HIV disease status was documented, and information regarding the colonoscopy was collected. Important factors from the colonoscopy data included the types of polyps, if a polypectomy were performed, if a diagnosis of advanced adenoma was made, or if a diagnosis of adenocarcinoma was made.
Patients with a known history of malignancy, history of colon polyps, inflammatory bowel disease, active gastrointestinal infection, and poor bowel preparation were excluded, as were patients undergoing colonoscopy for surveillance or diagnostic purposes. Data collected for each patient included age, biological sex, ethnicity, age at colonoscopy, body mass index (BMI) at time of colonoscopy, alcohol history use, tobacco use, diabetes history, year of HIV diagnosis, duration in years of HIV diagnosis at time of colonoscopy, CD4 count nadir, CD4 count value closest to colonoscopy date, viral load value closest to colonoscopy date, and ART therapy regimen. Colonoscopy data was collected including the date of colonoscopy, colonoscopy type (screening/diagnostic), proceduralist, family history of polyps, history of polyps or colon cancer, biopsy information (if any), polyp type, polyp size, determination of adenoma, designation of advanced adenoma, diagnosis of adenocarcinoma or anal cancer, quality of preparation, withdrawal time greater than 6 min, and type of anesthesia. Advanced adenomas were categorized as adenomatous polyps being > 1 cm, having greater than 3 adenomas, and/or having a sessile serrated adenoma.
The baseline characteristics and prevalence of polyps in HIV disease groups (based on the viral load and/or CD4 count definitions) were compared using Wilcoxon signed-rank tests and Fisher’s exact tests. For the two groups based on CD4 count, a logistic regression controlling for BMI, sex, and medications while looking at the odds of precancerous polyps was run. All statistics were performed using SAS Studio 3.8 software.
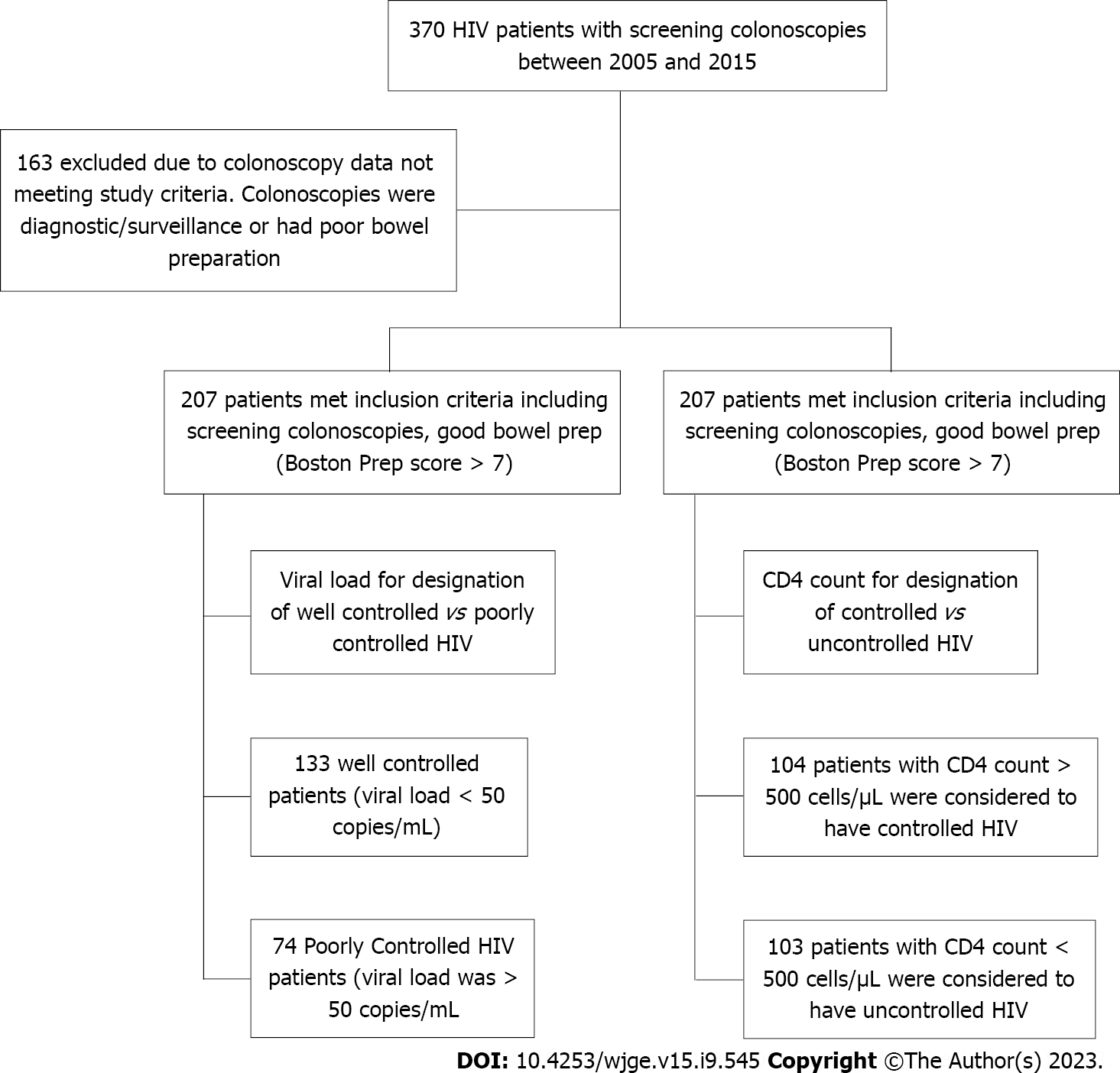
Patients were categorized into two groups based on their HIV disease state. They were determined to be either well-controlled HIV or poorly-controlled HIV patients (using viral load as a designation) and using CD4 count where patients were determined to be controlled or uncontrolled using a CD4 cutoff of 500. HIV patients determined to be well-controlled/controlled were likened to the general population and served as a control group (Figure 1).
A total of 370 records were reviewed. Of these patients, 163 were excluded due to having either a diagnostic colonoscopy, surveillance colonoscopy, or poor preparation. In total, 207 patients were found to have screening colonoscopies with good or excellent prep (Boston Bowel Prep Score > 7) and met our inclusion criteria. The mean age of our patient population was 56.13 years; 58% of our patients were male. Patients were divided into two separate groups based on their HIV disease state using viral load. Patients were denoted to be well-controlled based on a viral load < 50 copies/mL and poorly-controlled if their viral load was > 50 copies/mL. Based on these criteria, we had a total of 133 well-controlled patients and 74 poorly-controlled HIV patients. Using these two defined groups, baseline characteristics between them were compared.
Baseline characteristics between these two groups including age, sex, race, ethnicity, history of diabetes, smoking status, and insurance were not found to be statistically significant. However, the average BMI in well-controlled patients was found to be higher (27.8 kg/m2) than the average (25.9 kg/m2) in poorly-controlled HIV patients (P = 0.0207). See Table 1 for baseline characteristics.
| Clinical features | Parameter, n = 207 total | Controlled, n = 104 | Uncontrolled, n = 103 | P value |
| Age | Yr | 55.9 (47-71) | 56.3 (39-76) | 0.6369 |
| Sex | Male | 46.15 | 70.87 | 0.0004 |
| Race | Black | 93.27 | 90.29 | 0.8201 |
| Hispanic | 3.85 | 5.83 | ||
| White | 2.88 | 2.91 | ||
| Ethnicity | African American | 26.32 | 39.29 | 0.6478 |
| Afro-Caribbean | 31.58 | 25.00 | ||
| Hispanic | 21.05 | 25.00 | ||
| Other | 21.05 | 10.71 | ||
| BMI in kg/m2 | 28.0 | 26.2 | 0.0276 | |
| Diabetes mellitus | Diabetic | 14.42 | 11.76 | 0.6807 |
| Smoking | Current | 18.27 | 27.18 | 0.2451 |
| Insurance | HIV specific | 8.65 | 14.56 | 0.4638 |
| Medicare | 10.58 | 14.56 | ||
| Medicaid | 60.58 | 53.40 | ||
| Private | 16.35 | 16.50 | ||
| Self-Pay | 1.92 | 0.97 |
At an alpha level of 0.05, the prevalence of polyps in both groups was not significantly different. Among the patients with well-controlled HIV, 13% had polyps, while 8% of patients in the poorly-controlled group had polyps. In the well-controlled group, 7.52% of patients had precancerous polyps, and 4.05% of poorly-controlled patients had precancerous polyps. Advanced and right colon adenomas were also found more often in the well-controlled group (5% and 4% vs 1% and 0%, respectively). However, this was not a significant difference.
There is some debate as to whether HIV viral load or CD4 count is the best stratifier to use for HIV disease progression[7-9]. Thus, we also analyzed the effect of CD4 count on polyp prevalence. To designate controlled and uncontrolled HIV, we used a CD4 count cutoff value of 500 cells/μL. Patients with a CD4 count > 500 cells/μL were considered to have controlled HIV while those with a CD4 count equal to or less than 500 cells/μL were considered to have uncontrolled HIV. Using these cutoff values, 104 patients met the criteria for controlled HIV and 103 met the criteria for uncontrolled HIV. With this definition, there were significant differences in the baseline characteristics of sex as well as BMI. As above, BMI was significantly higher in the controlled group (28 kg/m2vs 26.2 kg/m2). Additionally, there was a significantly greater proportion of males in the uncontrolled group (71%) as opposed to the controlled group (46%).
The uncontrolled group had significantly more polyps and precancerous polyps than the controlled group. Among the patients with uncontrolled HIV, 17% had any polyp on colonoscopy, while only 5% of patients with controlled HIV had any polyp. Precancerous polyps were also more likely to be found in the uncontrolled group (11%) vs the controlled group (2%). There were no significant differences in the prevalence of adenomas or other polyp types between the two groups.
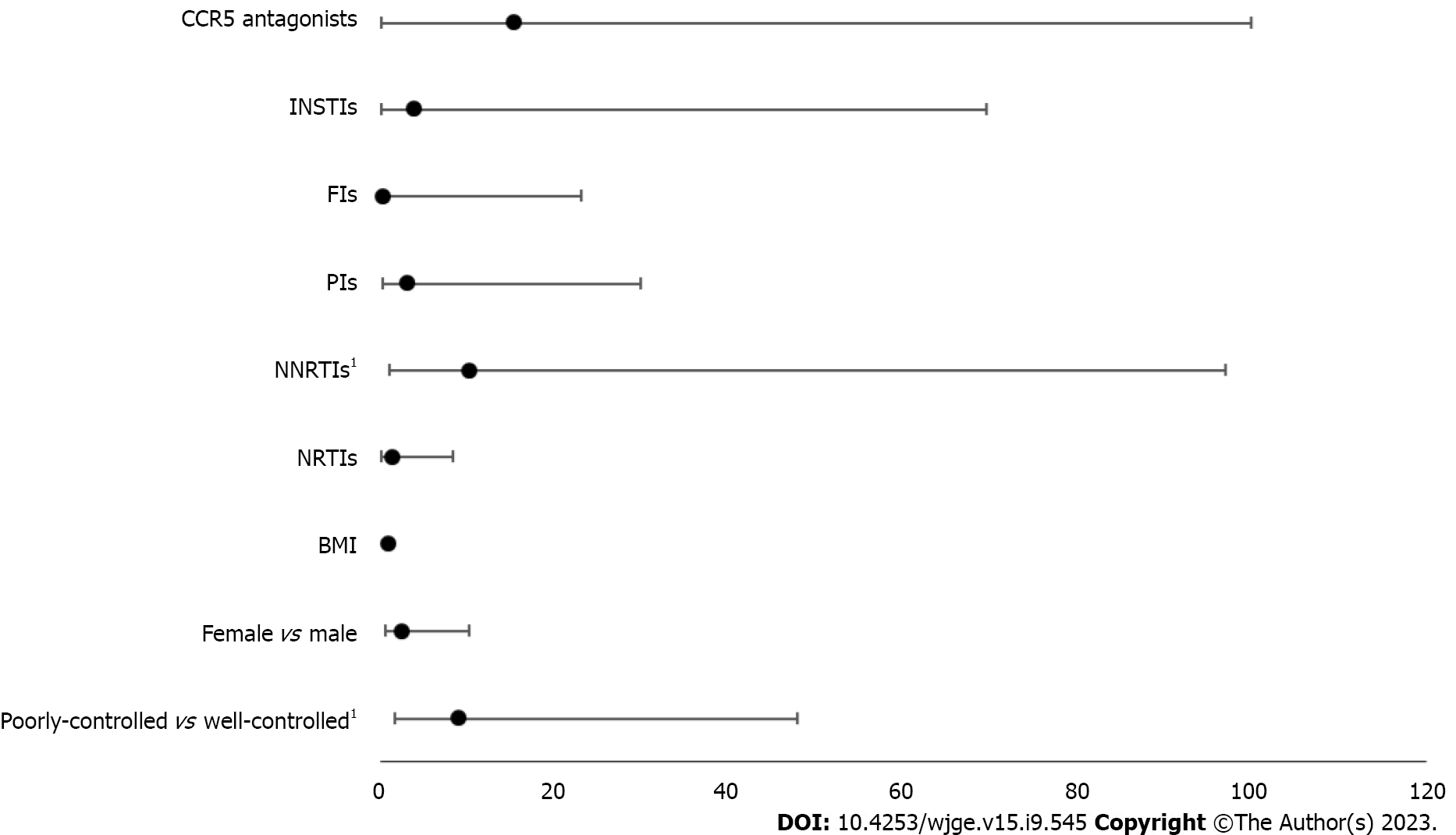
Finally, a logistic regression demonstrated that the odds of precancerous polyps was 9.01 times greater [95% confidence interval (CI): 1.69-47.97] in the uncontrolled group than in the controlled group after adjusting for BMI, sex, and medication types. Of note, non-nucleoside reverse transcriptase inhibitors (NNRTIs) were also associated with increased odds of precancerous polyps (odds ratio: 10.23; 95%CI: 1.08-97.15) (Figure 2).
Using CD4 count > 500 cells/μL as controlled HIV and CD4 count < 500 cells/μL as uncontrolled HIV, there was a significant association between HIV control and precancerous polyp presence. However, when using viral load < 50 copies for the definition of well-controlled vs poorly-controlled, there was not a significant difference in precancerous polyps noted. It was important to investigate these relationships using both viral load and CD4 count as disease status markers because there is debate as to which criteria is superior to demonstrate HIV disease status[9]. Using CD4 count, BMI was again found to be significantly different between the controlled (n = 104) and uncontrolled (n = 103) HIV groups (P = 0.0276).
Interestingly, using CD4 counts to compare groups, 53.85% of controlled patients were females, and 70.87% of uncontrolled patients were males (P = 0.0004). In the controlled group, 1.92% of patients were found to have precancerous polyps, while 10.68% of uncontrolled patients had precancerous polyps, (P = 0.0102) (Table 2). In a logistic regression that was performed to control for and assess the effects of sex, BMI, and antiretroviral use, uncontrolled patients were 9.01 times more likely to have precancerous polyps identified on their colonoscopy (95%CI: 1.69-47.97) (P = 0.0100). Patients taking NNRTIs were also found to be 10.23 times more likely to have precancerous polyps (95%CI: 1.08-97.15) (P = 0.0428) (Table 3). No significant differences were found with other types of HAART medicines. However, it is important to consider that HAART therapy combines multiple medicines.
| Variable | Controlled, n = 104 | Uncontrolled, n = 103 | P value |
| Any polyp | 5 (4.81) | 18 (17.48) | 0.0040 |
| Polyp type | 0.0736 | ||
| Adenoma | 1 (0.96) | 6 (5.83) | |
| Hyperplastic | 3 (2.88) | 4 (3.88) | |
| Serrated | 1 (0.96) | 5 (4.85) | |
| Precancerous | 2 (1.92) | 11 (10.68) | 0.0102 |
| Advanced adenoma | 1 (0.96) | 6 (5.83) | 0.0651 |
| Right colon adenoma | 1 (0.96) | 4 (3.88) | 0.2119 |
| Variable | Odds ratio (95%CI) | P value |
| CD4 < 500 vs CD4 > 500 | 9.01 (1.69-47.97) | 0.0100 |
| Female vs male | 2.58 (0.64-10.34) | 0.1839 |
| BMI | 0.99 (0.89-1.11) | 0.8956 |
| NRTIs | 1.50 (0.27-8.36) | 0.6425 |
| NNRTIs | 10.23 (1.08-97.15) | 0.0428 |
| PIs | 3.23 (0.35-29.98) | 0.3032 |
| FIs | 0.44 (0.01-23.08) | 0.6832 |
| INSTIs | 4.01 (0.23-69.67) | 0.3403 |
| CCR5 antagonists | 15.39 (0.22-999.99) | 0.2073 |
The adenoma detection rate (ADR) for our HIV population was found to be 3.3%, which is seemingly low. However, in similar studies of HIV patients performed in urban academic centers, ADRs ranged between 6.6%-7.8%, and these studies included screening, diagnostic, and surveillance colonoscopies[3,5,6]. It is likely that if other types of colonoscopies such as diagnostic and surveillance were included in our study, our ADR would have been higher. Ultimately, institutions with large HIV patient populations or specialized HIV care may consider further investigating these complex relations, as this would help to increase the study ADR.
Using viral load to determine our two HIV groups, we found no differences in precancerous polyp detection. However, using CD4 count to determine the two groups, we found a significant difference, with uncontrolled patients having more precancerous polyps. Prospective studies involving HIV patients undergoing screening colonoscopy should be performed where CD4 count and HIV viral load are recorded on the day of procedure in order to better classify patients in terms of their disease status as it may relate to their findings on colonoscopy.
Our analysis also suggested an increased risk of precancerous polyps in patients who were taking NNRTIs. Most literature supports the concept that HAART has decreased the risk of HIV patients ever developing NADMs[2,6]; however, there have not been studies analyzing colorectal cancer development and HAART by drug class or drug combination. It is unclear what the mechanism of action may be regarding the use of NNRTIs and polyp growth. A study conducted by Chao et al[10] at Kaiser Permanente suggested that for patients with long-term use of protease inhibitors, there was an associated higher risk of anal cancer[10]. That same study did not show any association between NRRTI use and anal cancer. Similarly, a study by Piketty et al[11] also reported an increased anal cancer risk in HAART users, suggesting that ART therapy does not appear to prevent anal cancer. While anal cancer, advanced polyps, and colon cancer all have different pathogeneses, we highlighted that there is still work to be done to understand the mechanism behind neoplasm development in HIV patients.
Future studies need to be performed to determine if any specific HAART regimen might impact colorectal cancer development. Conversely, some studies have shown that the occurrence of NADMs has increased since the introduction of HAART in 1996. Prior studies show an association between NNRTI use and NADMs[12]. While HAART does not have a direct effect on host DNA, there is substantial evidence that HAART alters gut microbiota[13], which may serve as a theoretical mechanism for the increased ADR in patients on NNRTIs. In addition, it is possible that the use of NNRTIs may increase NADMs by increasing lifespan of HIV patients and the rate of obesity, both of which may contribute to adenomatous polyp development.
In our study, we found there was an increased rate of precancerous polyps in patients who had lower CD4 counts and those taking NNRTIs. While the overall precancerous polyp and ADR was low in this population, further studies are needed to elucidate the possible mechanism of these differences.
Antiretroviral therapies have improved the life expectancy of patients living with human immunodeficiency virus (HIV). As these patients live longer, they can develop non-acquired immunodeficiency syndrome defining malignancies such as colon cancers.
Some studies have shown that highly active anti-retroviral therapy (HAART) decreases the risk of developing colorectal cancer, while other studies propose that HIV patients are at higher risk and develop colorectal cancer at younger ages. There is no recommendation in gastrointestinal guidelines regarding special screening ages for HIV patients.
Our objective was to identify which factors are associated with the development of precancerous polyps on index screening colonoscopy in patients with HIV and to investigate whether HIV disease severity, measured by viral load and CD4 count, might impact adenoma growth.
A retrospective review of electronic medical charts at Kings County Hospital and SUNY Downstate Health Sciences University for patients with HIV who had received a screening colonoscopy between 2005 and 2015 was performed.
We determined there was an increased rate of precancerous polyps in patients who had lower CD4 counts and those taking non-nucleoside reverse transcriptase inhibitors.
We determined there was a relationship between HIV disease status and precancerous polys found on colonoscopy. Further studies need to be done to further explore this relationship in patients with HIV.
Further studies and work need to be done to determine if any specific HAART regimen might impact colorectal cancer development.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bustamante-Lopez LA, Brazil; Lin L, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349-e356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 754] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 2. | Klugman AD, Schaffner J. Colon adenocarcinoma in HIV infection: a case report and review. Am J Gastroenterol. 1994;89:254-256. [PubMed] |
| 3. | Silverberg MJ, Abrams DI. Do antiretrovirals reduce the risk of non-AIDS-defining malignancies? Curr Opin HIV AIDS. 2009;4:42-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Gutkin E, Hussain SA, Mehta P, Kim SH, Pollack S, Rubin M. Prevalence of Adenomas Found on Colonoscopy in Patients With HIV. Gastroenterology Res. 2012;5:52-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Nayudu SK, Balar B. Colorectal cancer screening in human immunodeficiency virus population: Are they at average risk? World J Gastrointest Oncol. 2012;4:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 6. | OʼNeill TJ, Nguemo JD, Tynan AM, Burchell AN, Antoniou T. Risk of Colorectal Cancer and Associated Mortality in HIV: A Systematic Review and Meta-Analysis. J Acquir Immune Defic Syndr. 2017;75:439-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Mahmood E, Lapumnuaypol K, Bandres M, Jain D, Zavala S. Prevalence of Adenoma and Advanced Adenoma in HIV Positive Patients Undergoing Screening Colonoscopy. Am J Gastroenterol. 2018;113 Suppl. [DOI] [Full Text] |
| 8. | Burgi A, Brodine S, Wegner S, Milazzo M, Wallace MR, Spooner K, Blazes DL, Agan BK, Armstrong A, Fraser S, Crum NF. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Chao C, Leyden WA, Xu L, Horberg MA, Klein D, Towner WJ, Quesenberry CP Jr, Abrams DI, Silverberg MJ. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS. 2012;26:2223-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, Costagliola D, Mary-Krause M; FHDH-ANRS CO 4. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, Mandelia S, Møller H, Bower M. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Pinto-Cardoso S, Klatt NR, Reyes-Terán G. Impact of antiretroviral drugs on the microbiome: unknown answers to important questions. Curr Opin HIV AIDS. 2018;13:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |