Published online Aug 16, 2023. doi: 10.4253/wjge.v15.i8.528
Peer-review started: March 16, 2023
First decision: April 20, 2023
Revised: June 15, 2023
Accepted: July 24, 2023
Article in press: July 24, 2023
Published online: August 16, 2023
Processing time: 142 Days and 22.4 Hours
Subepithelial lesions (SELs) are gastrointestinal tumors with heterogeneous malignant potential. Endoscopic ultrasonography (EUS) is the leading method for evaluation, but without histopathological analysis, precise differentiation of SEL risk is limited. Artificial intelligence (AI) is a promising aid for the diagnosis of gastrointestinal lesions in the absence of histopathology.
To determine the diagnostic accuracy of AI-assisted EUS in diagnosing SELs, especially lesions originating from the muscularis propria layer.
Electronic databases including PubMed, EMBASE, and Cochrane Library were searched. Patients of any sex and > 18 years, with SELs assessed by EUS AI-assisted, with previous histopathological diagnosis, and presented sufficient data values which were extracted to construct a 2 × 2 table. The reference standard was histopathology. The primary outcome was the accuracy of AI for gastrointestinal stromal tumor (GIST). Secondary outcomes were AI-assisted EUS diagnosis for GIST vs gastrointestinal leiomyoma (GIL), the diagnostic performance of experienced endoscopists for GIST, and GIST vs GIL. Pooled sensitivity, specificity, positive, and negative predictive values were calculated. The corresponding summary receiver operating characteristic curve and post-test probability were also analyzed.
Eight retrospective studies with a total of 2355 patients and 44154 images were included in this meta-analysis. The AI-assisted EUS for GIST diagnosis showed a sensitivity of 92% [95% confidence interval (CI): 0.89-0.95; P < 0.01), specificity of 80% (95%CI: 0.75-0.85; P < 0.01), and area under the curve (AUC) of 0.949. For diagnosis of GIST vs GIL by AI-assisted EUS, specificity was 90% (95%CI: 0.88-0.95; P = 0.02) and AUC of 0.966. The experienced endoscopists’ values were sensitivity of 72% (95%CI: 0.67-0.76; P < 0.01), specificity of 70% (95%CI: 0.64-0.76; P < 0.01), and AUC of 0.777 for GIST. Evaluating GIST vs GIL, the experts achieved a sensitivity of 73% (95%CI: 0.65-0.80; P < 0.01) and an AUC of 0.819.
AI-assisted EUS has high diagnostic accuracy for fourth-layer SELs, especially for GIST, demonstrating superiority compared to experienced endoscopists’ and improving their diagnostic performance in the absence of invasive procedures.
Core Tip: Artificial intelligence (AI) has shown itself as a promising tool in diagnostic endoscopic ultrasound. This systematic review and meta-analysis analyze the diagnostic performance of endoscopy ultrasound with AI for subepithelial lesions and compare it with experienced endoscopists. Based on our meta-analysis, the endoscopy ultrasound assisted for AI has high diagnostic accuracy with superiority over experienced endoscopists.
- Citation: Gomes RSA, de Oliveira GHP, de Moura DTH, Kotinda APST, Matsubayashi CO, Hirsch BS, Veras MO, Ribeiro Jordão Sasso JG, Trasolini RP, Bernardo WM, de Moura EGH. Endoscopic ultrasound artificial intelligence-assisted for prediction of gastrointestinal stromal tumors diagnosis: A systematic review and meta-analysis. World J Gastrointest Endosc 2023; 15(8): 528-539
- URL: https://www.wjgnet.com/1948-5190/full/v15/i8/528.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i8.528
Gastrointestinal subepithelial lesions (SELs) are tumors that originate from the muscular mucosa, submucosa, or muscular propria, with the stomach being the most common location where they are identified[1]. Although most SELs are benign and asymptomatic at presentation, up to 15% present malignant potential and may cause symptoms such as bleeding and abdominal pain[1,2]. The most common histological types are gastrointestinal stromal tumor (GIST) and gastrointestinal leiomyoma (GIL), with GIST having malignant potential[3]. One major diagnostic challenge is differentiating between GIST and leiomyoma considering that both commonly originate from the muscular propria and have overlapping features on imaging evaluation[4]. The differentiation among them is imperative due to the difference in prognosis and therapeutic strategy[5,6]. Surgical resection is recommended after GISTs diagnosis due to the risk of malignancy and requires prior histological confirmation, even in small lesions[1].
Endoscopic ultrasonography (EUS) is a valuable tool for SELs because it can characterize them by size, vascularity, internal structure, location, echogenicity, shape, and the layer of origin[6,7]. However, the gold standard for diagnosis is histopathological evaluation, which is indicated in suspected GIST, size > 20 mm, high-risk malignancy, surgical indication, or oncological treatment[1]. In uncertain cases, auxiliary procedures such as fine needle aspiration or fine needle biopsy can be performed for tissue sampling acquisition and immunohistochemical analysis, leading to more accurate results, especially in lesions > 20 mm[8,9]. For lesions < 20 mm with malignancy risk, further analysis with a contrast-enhanced technique can stratify risk to help determine the need for and safety of biopsy[10,11].
Artificial intelligence (AI) has emerged as a powerful and exciting technology impacting many aspects of health care and promoting changes in daily clinical practice, especially for early, accurate, and real-time diagnosis[12]. Since the 1960s, AI systems have been applied in radiology for the recognition and interpretation of images and subsequently expanded to other areas including ophthalmology, cardiology, and neurology[13,14]. Between 2017 and 2018, the Food and Drug Administration approved more than 20 AI tools for medical use, including the endoscopy field[14]. In 2020, AI helped in the rational management of the coronavirus disease 2019 pandemic in various scenarios and countries, from predicting diagnostic imaging, manufacturing vaccines, and preventing viral spread[15].
The use of AI systems able to recognize specific patterns in EUS began in early 2000 and was initially applied to the evaluation of pancreatic disorders, especially differential diagnoses between chronic pancreatitis and pancreatic neoplasms[16]. Subsequently, the excellent results contributed to the development of studies that explored diagnosis and malignancy prediction for gastrointestinal SELs, particularly for GIST[17,18]. Although EUS AI-assisted has promising results, the real benefits, ethical implications, and clinical relevance need scientific evidence that supports the use in diverse clinical settings[19].
Considering the deficiency of research and the need for quality evidence to support the application of AI assistance in the subepithelial tumors EUS evaluation, this systematic review aims to perform an analysis of endoscopic ultrasound with AI assistance for GIST diagnosis. The main outcome was the diagnostic accuracy of AI-assisted EUS for GIST. Furthermore, we evaluated the AI capability that distinguishes between GIST and GIL and the experienced endoscopists’ performance.
This study was structured according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[20] and recent recommendations for diagnostic test accuracy reviews[21]. This study was registered in the International Prospective Register of Systematic Reviews under the file number CRD42023418987.
A comprehensive literature search was performed in the following databases up to December 2022: EMBASE, Cochrane Library, and MEDLINE. Two reviewers screened titles and abstracts of all the identified articles that evaluated the performance of AI for the diagnosis of SELs using EUS. Divergent opinions were resolved by a third reviewer. The MESH Terms for searches used were: (“endoscopic” OR “endoscopy”) AND (“ultrasound” OR “endosonography” OR “echoendosonography”) AND (“artificial intelligence” OR “neural network” OR “computer neural network” OR “deep learning”) AND (“GIST” OR “subepithelial tumor” OR “subepithelial lesion” OR “stromal tumor” OR “gastrointestinal subepithelial tumor”) present in titles, abstracts or full-text articles.
The studies included were performed in adults patients (> 18 years) with SELs assessed by EUS AI-assisted, with histopathological diagnosis established, and presented true-negative, true-positive, false-negative, and false-positive values which were extracted to construct a 2 × 2 table. Case reports, systematic reviews, reviews, editorials, conference abstracts, articles with algorithms different from convolutional neural network (CNN), and articles with incomplete data were excluded.
Using a standardized form, the relevant data from eligible studies were extracted and organized using the following main data: First author, year of publication, study type, geographical setting, number of patients, gender, number of GIST tumors, number of GIL tumors, number of other SELs, number images, tumor location, AI model, external validation, endoscopists comparison, and histopathologic analysis. All relevant texts, tables and figures were reviewed for data extraction.
The risk of bias and quality assessment were assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) too[22]. The quality of the studies was evaluated by two authors independently, and disagreement was resolved by consensus in consultation with the third author.
The main outcomes evaluated were the pooled accuracy, sensitivity, positive likelihood, negative likelihood, and specificity of AI-assisted EUS for the diagnosis of GIST based on analysis of images obtained by EUS of gastrointestinal SELs. The positive post-test probability and negative post-test probability were calculated based on likelihood ratios and GIST mean prevalence values from each article. The accuracy was defined as the area under the summary receiver operating characteristic (SROC) curve. Secondary outcomes were performed to evaluate the diagnostic performance of AI for GIST vs GIL, the diagnostic performance of experienced endoscopists for GIST, and GIST vs GIL. Experienced endoscopists were those who performed more than 500 EUS examinations or had at least 5 years of experience evaluating gastrointestinal SELs.
The pooled data of sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio, were meta-analyzed with a 95% confidence interval (CI) using the random effect model for the accuracy of EUS AI-assisted and experienced endoscopists. A SROC curve was drawn and the area under the curve (AUC) was calculated to estimate the accuracy.
Forest plots were made to show the point estimates in each study in relation to the summary pooled estimate. The width of the point estimates in the forest plots indicated the assigned weight for that study. For 0 values, 0.5 was added, as described by Cox and Snell[23]. The heterogeneity of likelihood ratios and diagnostic odds ratios were tested using Cochran’s Q test based on inverse variance weights. The heterogeneity of the sensitivities and specificities was tested using the likelihood ratio test. Heterogeneity among studies was also tested using SROC curves. Heterogeneity was assessed and data were analyzed using Meta-DiSc (Clinical Biostatistics HRC, Madrid, Spain)[24]. The Bayes model was used to calculate the post-test probability and elaborate Fagan’s Nomogram[25] using estimated mean prevalence data from each article for GIST.
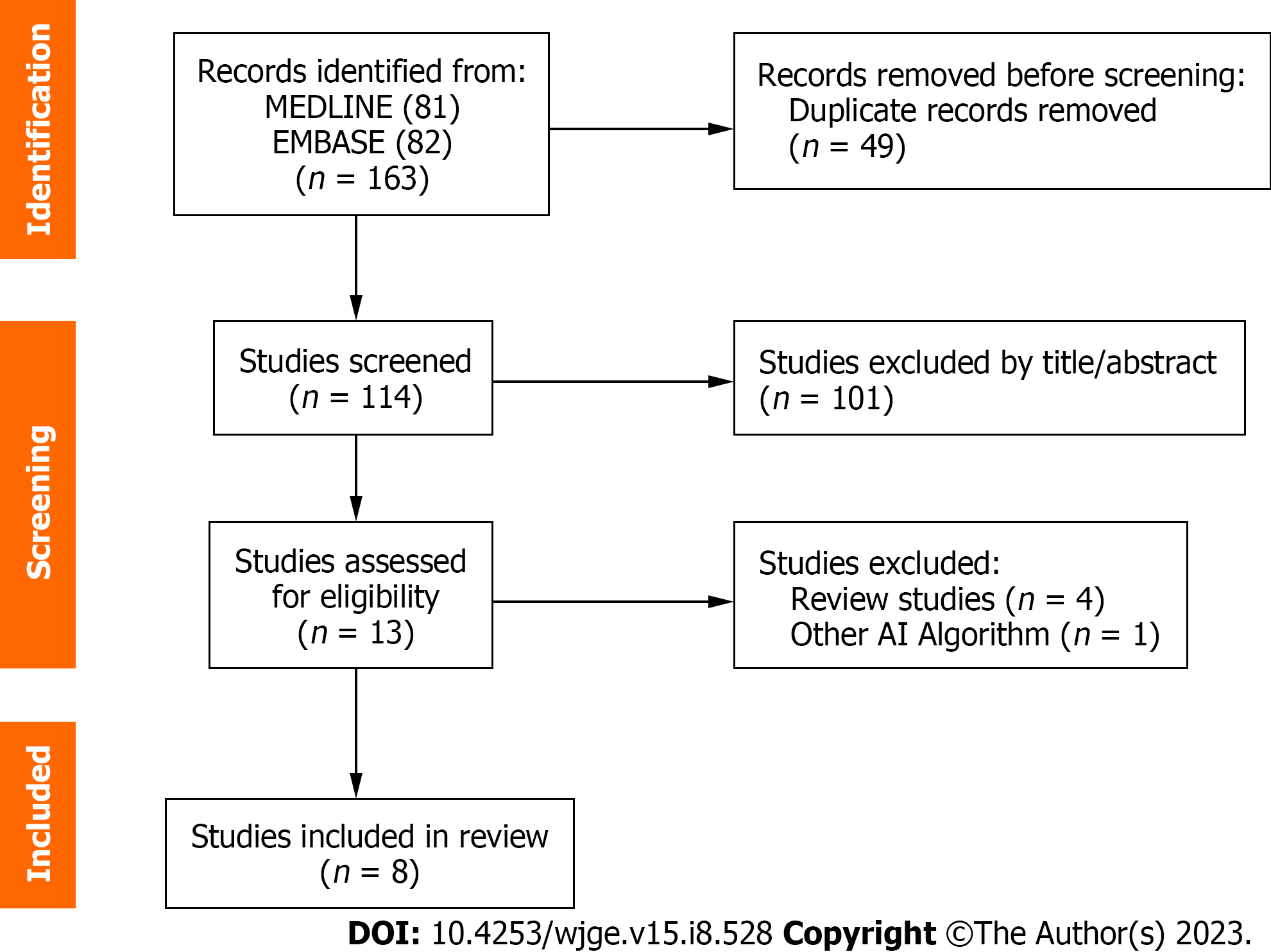
A total of 163 studies were extracted after the search strategy which was shown in Figure 1. After the exclusion of 150 titles, based on the selection criteria, 13 studies were eligible for full-text examination. Of those, 4 were removed for being review articles and one[26] for not using a CNN model. Thus, eight relevant articles were selected for the present meta-analysis (Table 1) with a total of 2355 patients and 44154 images[27-34]. All articles were retrospective studies. The characteristics of the included studies are summarized in Table 1. A total of 1436 patients were diagnosed with GIST, 725 were GIL, and 194 were non-GIST/non-GIL with a GIST prevalence of 68% and leiomyoma being 30% in the present study. The Asian continent has the largest number of publications, a total of 7 articles (4 Japanese, 2 Korean, and 1 Chinese), and Europe has one (Turkey). As for the AI model used, all 8 studies were developed with a CNN algorithm.
| Ref. | Geographical setting | Study type | Patients | Sex (male/female) | GIST | GIL | Other SELs | Images | Tumor location | AI model | External validation | Endoscopists comparison | Histopathology |
| Minoda et al[29], 20221 | Eastern | Retrospective | 52 | 33/19 | 36 | 14 | 2 | 2718 | Esophagus, duodenum, and colon | CNN | Yes | Yes | Yes |
| Hirai et al[33], 2022 | Eastern | Retrospective | 664 | 231/188 | 435 | 97 | 100 | 16110 | Esophagus, stomach, and duodenum | CNN | Yes | Yes | Yes |
| Tanaka et al[34], 2022 | Eastern | Retrospective | 53 | 28/25 | 42 | 11 | - | 10600 | Stomach | CNN | No | Yes | Yes |
| Yang et al[32], 2022 | Eastern | Retrospective | 752 | 337/415 | 348 | 404 | - | 10439 | Esophagus, stomach, duodenum, colon, and rectum | CNN | Yes | Yes | Yes |
| Oh et al[30], 2021 | Eastern | Retrospective | 168 | NI | 125 | 43 | - | 546 | Stomach | CNN | Yes | Yes | Yes |
| Seven et al[31], 2022 | Eastern | Retrospective | 145 | 72/73 | 109 | 36 | - | 1362 | Esophagus, stomach, and duodenum | CNN | Yes | Yes | Yes |
| Kim et al[27], 2020 | Eastern | Retrospective | 248 | 111/137 | 157 | 55 | 35 | 1117 | Stomach | CNN | Yes | Yes | Yes |
| Minoda et al[28], 2020 | Eastern | Retrospective | 273 | 138/135 | 184 | 65 | 24 | 3980 | Stomach | CNN | Yes | Yes | Yes |
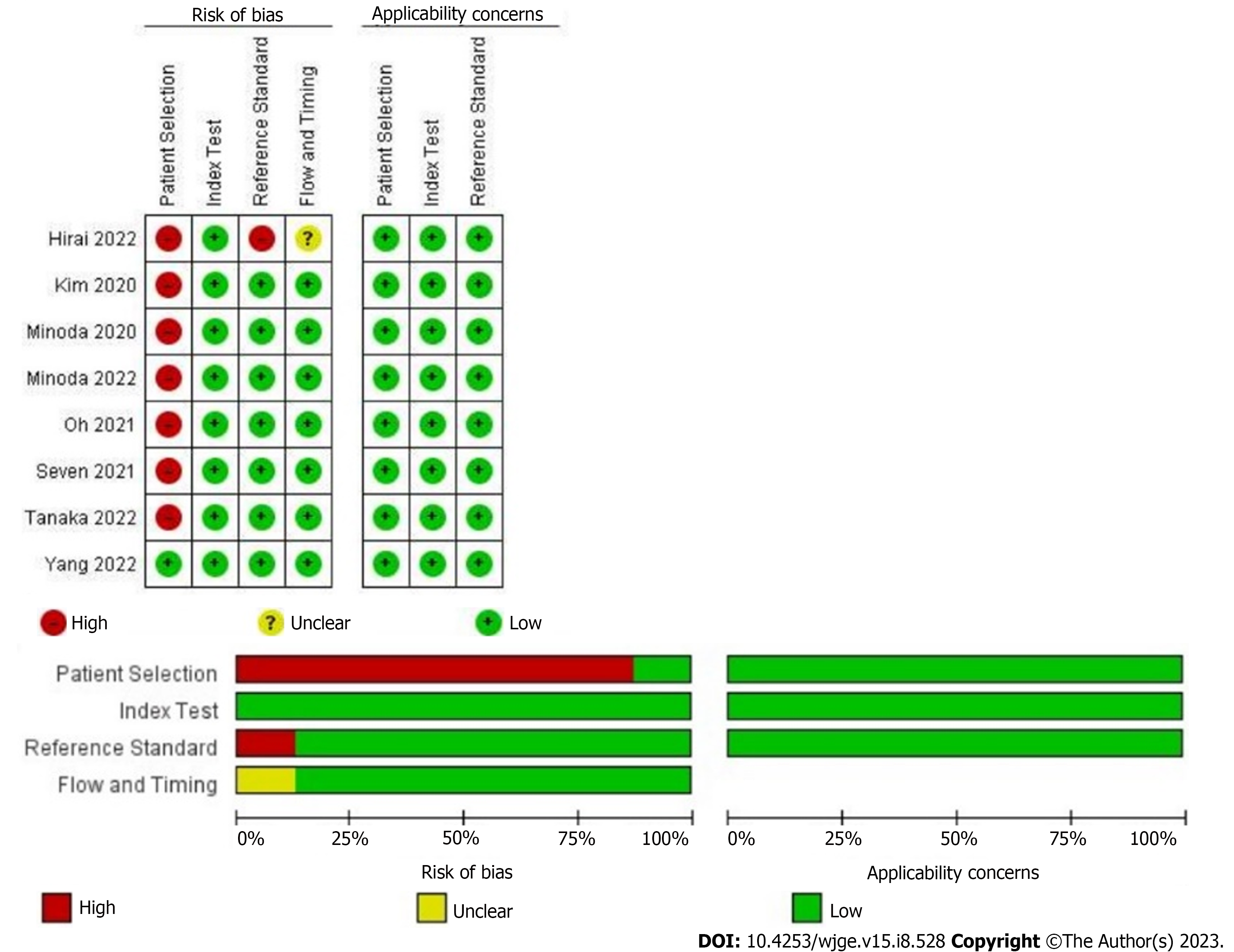
The quality of the included studies was evaluated according to the QUADAS-2 tool. The risk of bias of the 8 studies is shown in Figure 2, where 7 were categorized as high risk or uncertain risk for one or more fundamental elements due to their retrospective designs (Figure 2).
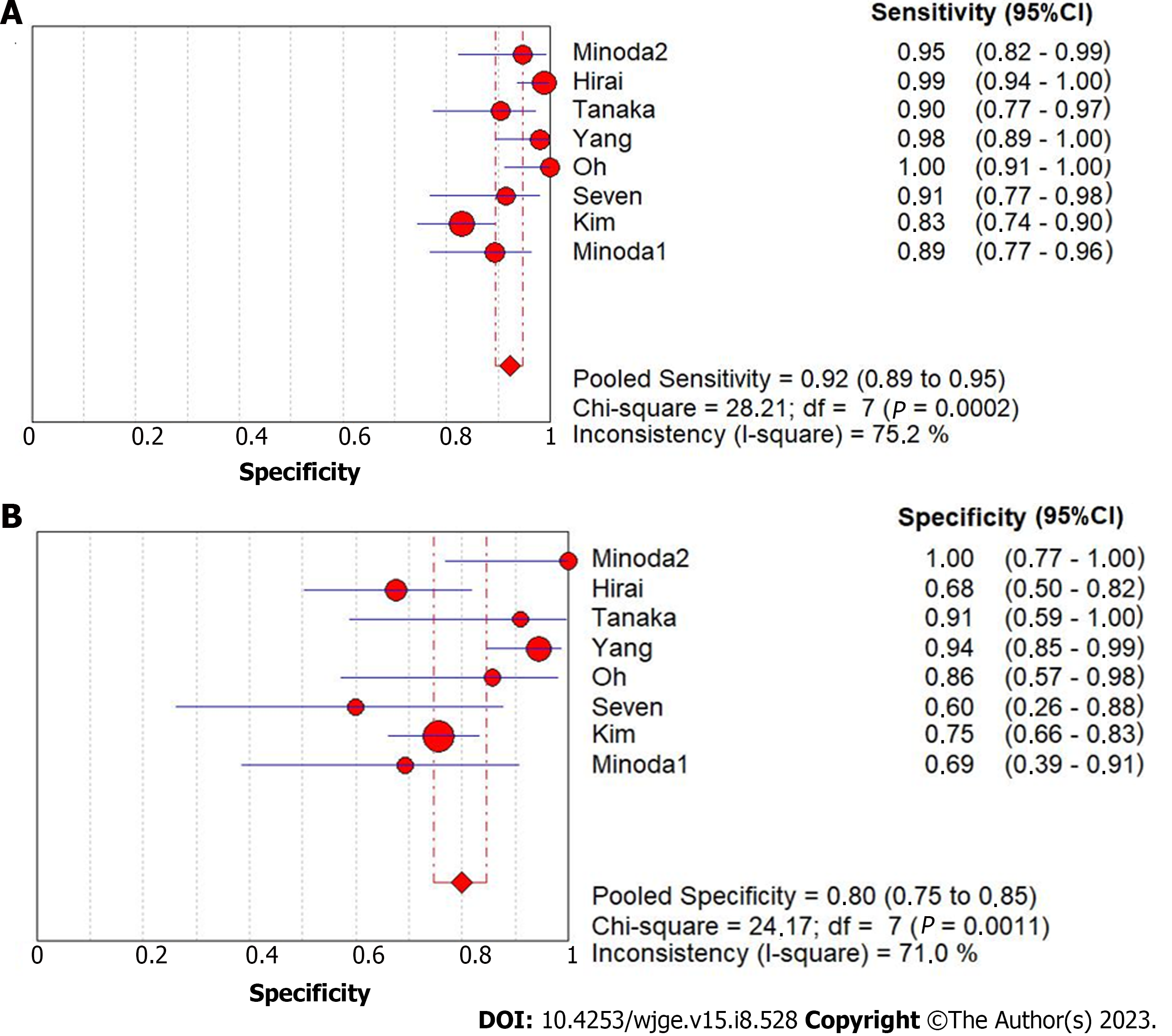
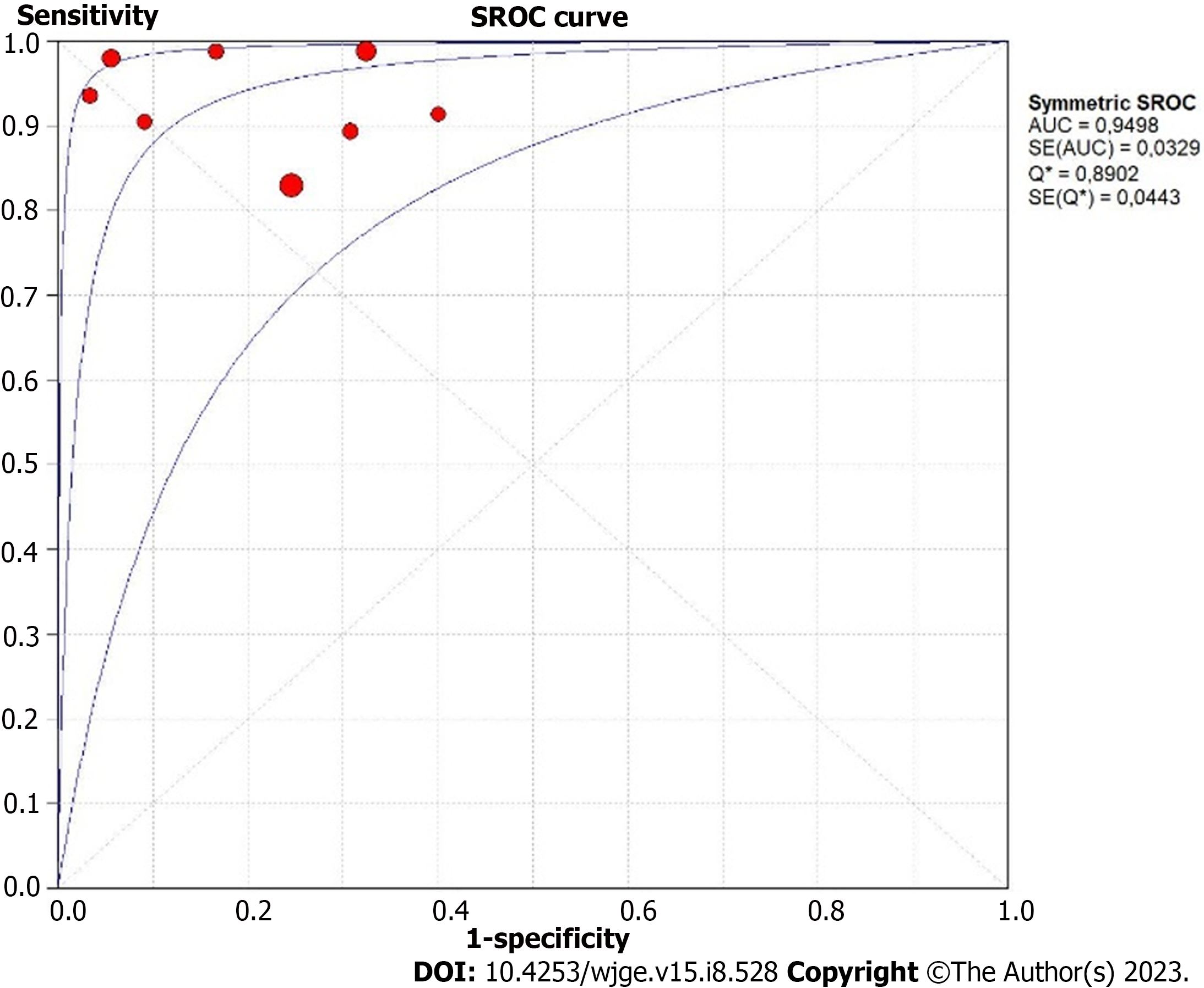
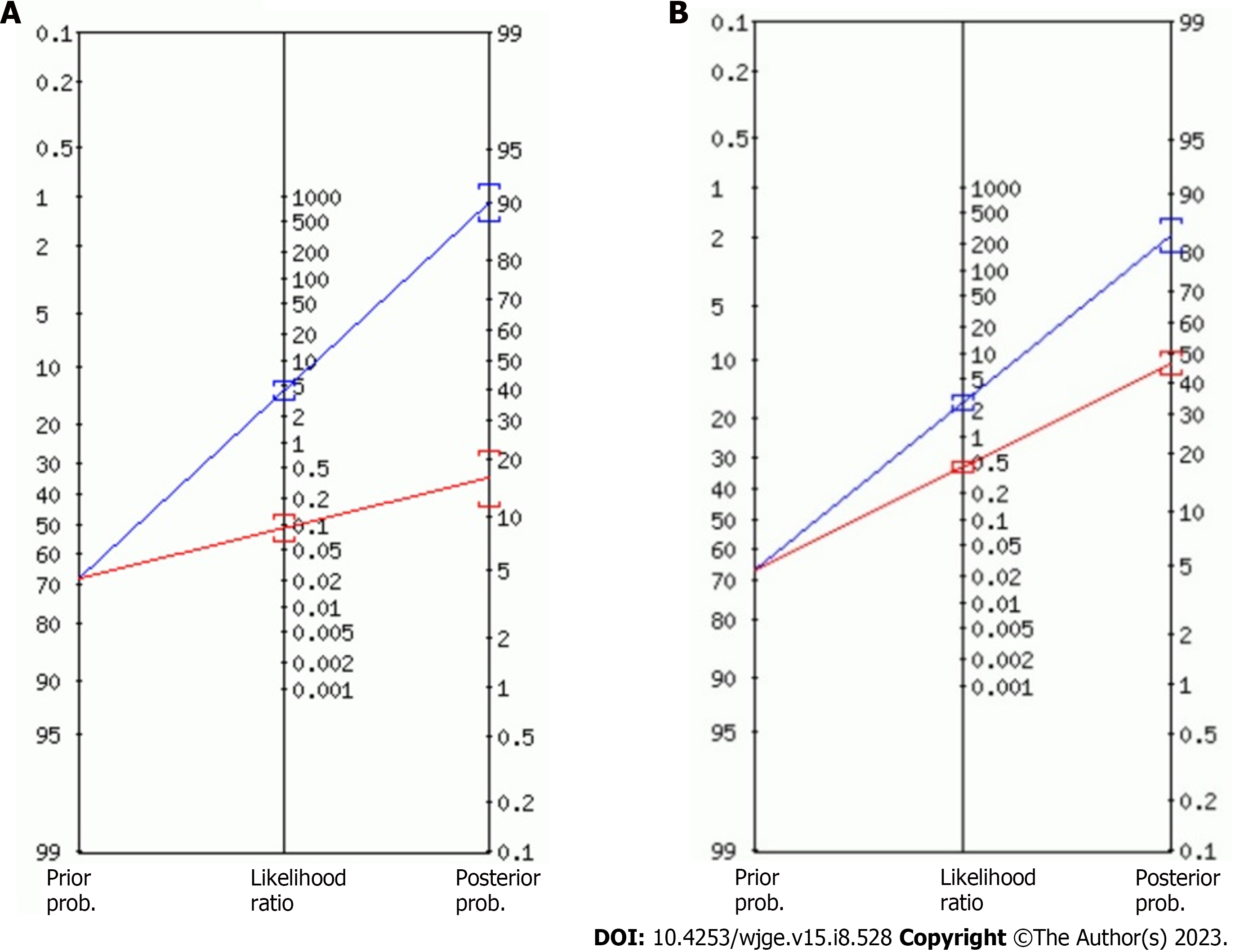
The diagnostic accuracy of GIST for AI-assisted EUS presented summary sensitivity values of 92% (95%CI: 0.89-0.95; P < 0.01), specificity of 80% (95%CI: 0.75-0.75; P < 0.01) (Figure 3), with substantial heterogeneity for both (I² = 75.2% and I² = 71%, respectively). A positive likelihood ratio of 4.26 (95%CI: 2.7-6.7; P = 0.01), negative likelihood ratio of 0.09 (95%CI: 0.04-0.18; P < 0.01), and diagnostic odds ratio of 71.74 (95%CI: 22.43-229.46; P < 0.01) was achieved. Figure 4 shows the SROC curve, with an AUC of 0.949 (P = 0.03) indicating high diagnostic accuracy (Table 2). The positive post-test probability was 90% (95%CI: 0.88-0.92), and the negative post-test probability was 16% (95%CI: 0.11-0.22), as shown in Fagan’s nomogram (Figure 5A).
Diagnostic accuracy of AI-assisted EUS for GIST vs GIL: For differentiation between GIST and GIL, the AI-assisted EUS presented a combined sensitivity of 93% (95%CI: 0.88-0.97; P = 0.08) and combined specificity of 90% (95%CI: 0.88-0.95; P = 0.02), positive likelihood ratio of 6.48 (95%CI: 2.14-19.6; P = 0.01), negative likelihood ratio of 0.06 (95%CI: 0.02-0.21; P = 0.05) and diagnostic odds ratio of 128.18 (95%CI: 18.6-883.25; P = 0.03). The heterogeneity was I2 = 55% for sensibility and I2 = 68.6% for specificity. The area under SROC curve expressed high diagnostic accuracy, with values of 0.966 (AUC).
Diagnostic performance of experts for GIST: Seven studies included in this meta-analysis evaluated the diagnostic performance of experienced echo-endoscopists. The combined general values of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and the area under the summary ROC curve were, respectively, 72% (95%CI: 0.67-0.76; P < 0.01), 70% (95%CI: 0.64-0.76; P < 0.01), 2.51 (95%CI: 1.75-3.61; P = 0.08), 0.42 (95%CI: 0.33-0.52; P = 0.16), 6.88 (95%CI: 3.95-11.99; P = 0.14) and 0.777 (AUC). The heterogeneity was I2 = 77.3% for sensibility and I2 = 73.8% for specificity.
Diagnostic performance of experienced endoscopists’ for GIST vs GIL: Considering only the differentiation of GIST and GIL, the combined sensitivity was 73% (95%CI: 0.65-0.80; P < 0.01), specificity 75% (95%CI: 0.65-0.84; P = 0.91), positive likelihood ratio 2.61 (95%CI: 1.75-3.88; P = 0.70), negative likelihood ratio 0.37 (95%CI: 0.22-0.64; P = 0.02), diagnostic odds ratio of 7.21 (95%CI: 2.95-17.59; P = 0.16) and area under the SROC curve of 0.819 (AUC). The heterogeneity was I2 = 77.3% for sensibility and I2 = 0.0% for specificity. The post- and pre-test probability were, respectively, 84% (95%CI: 0.80-0.86) and 46% (95%CI: 0.42-0.50), as shown in Figure 5B.
AI has the world’s attention for the impacts generated after its implementation in the most diverse fields, especially in diagnostic medicine. The utilization of AI technology in the field of medical imaging enhances the diagnostic process, leading to improved accuracy and the early detection of diseases, thus ensuring enhanced disease management and clinical outcomes[31]. In the present systematic review and meta-analysis, we analyze the application of appropriately trained software on EUS AI-assisted diagnosis of fourth-layer SELs, mainly for GIST, representing the largest pooled data including eight studies with more than 2300 patients and 44154 images. Through the evaluation of AI performance in this review, we achieved a combined sensitivity of 92%, a combined specificity of 80%, a positive post-test probability of 90%, and a negative post-test probability of 16% when distinguishing between GIST and non-GIST SELs based on EUS images.
The imaging modality frequently used to evaluate SELs is the EUS because of its ability to characterize the size, echogenicity, originating layer, shape, vascularity, and location[1,3,35]. Regarding conventional EUS findings for GIST, just stronger echogenicity in comparison with the surrounding muscle echo is an associated independent diagnostic factor[31]. A previous study reported that echogenicity, the presence of hyperechogenic spots and anechoic spaces, tumor shape, and marginal regularity in the EUS were not helpful in differentiating GIST from non-GIST tumors, being homogeneity was the only predictive factor[35]. Thus, the differentiation between GIST from other SELs without histological evaluation is difficult using EUS images only because the interpretation of the features is subjective and dependent on the experience of the endoscopist with a heterogeneous inter-observer agreement[28,31]. Although the gold standard diagnostic is histological evaluation, someone’s SELs can only be monitored with follow-up exams in the absence of risk stigmata and resection indications[9,10].
The implementation of AI technology to improve the EUS diagnostic performance compensates limitations and disagreements discussed previously. Our results for overall GIST diagnosis by AI were superior to the EUS doctors’ performance earlier reported[36], showing the ability to improve differential diagnosis with efficiency, quick evaluation, and reduce unnecessary procedures and surgical interventions. AI can evaluate specific patterns in the pixel-level characteristics of a tumor, making it more accurate than the naked eye in its analysis, and was observed that the size of the lesion increases, diagnostic accuracy increases in parallel, being more expressive from > 20 mm[28,34]. Considering the risks of invasive procedures, the misdiagnosis rate of GIST, the requirement of pathologic specimens for determining malignancy potential, and eligibility for neo-adjuvant therapy, the application of AI has significant improvements in the safety clinical management of SELs[28]. Recently, software developed and trained with gastric GIST images for EUS AI-assisted diagnosis has been used in other gastrointestinal sites with excellent results[29]. Furthermore, EUS assisted by AI developed to diagnose and prediction of the malignancy risk of GIST showed excellent performance[18], assisting in the decision for resection with or without neoadjuvant therapy. Using AI as an auxiliary tool in the diagnosis in endoscopy aims primarily to increase diagnostic accuracy and reduce the number of false negatives and positives.
In the evaluation of SELs originating from the muscular layer, the differentiation between GIST and leiomyoma is one of the most challenging, since they have very similar sonographic characteristics: Both are hypoechoic, usually homoge
The performance of AI was superior to EUS experts, even in cases where the expert was informed of the source layer and the location of the lesion[31], a fact that favors the expert because with some information certain diagnoses become obvious. For example, a soft, hyperechoic lesion of the submucosa, in the antrum, is highly suspicious for lipoma. With a sensitivity of 72% (vs 93% AI-assisted), experts can expect to have a miss rate of approximately 3 in every 10 cases of GIST. The diagnostic accuracy of the experts for GIST, although considered good, was much lower than that of the AI system (AUC 0.819 vs 0.966). A previous study reported an increase in accuracy, specificity, and positive predictive value after joint diagnosis of endoscopists with AI assistance to distinguish GIST from leiomyoma, demonstrating that AI has the potential to help enhance correct diagnosis even for experienced endoscopists. In addition, the rapid development of AI systems capable of performing fast and more specific analyses without increasing operating costs or equipment updates[32] makes it possible and attractively apply in diagnostic centers of lower volume and invariably assists inexperienced endoscopists’ diagnoses.
Although these results are exciting, they should be evaluated with caution due to the dynamic nature of diagnostic examinations. In these studies, experts evaluated images of SEL without being able to perform their usual maneuvers. Moreover, most studies did not provide essential information commonly known in clinical practice, such as the patient’s medical history, color at white light endoscopy, or lesion consistency[37]. Additionally, the expected performance of AI can be influenced by factors such as disease prevalence and severity, the expertise and training of endoscopists, and the interaction between AI and endoscopists[19]. It is crucial to emphasize that the use of machine learning models in the medical field should not be seen as a direct competition to endoscopists. Instead, they should be regarded as auxiliary diagnostic tools and even training aids for less experienced endoscopists.
Despite the number of studies, patients, and reviewed images, this meta-analysis has certain limitations. Firstly, although we included several studies with a large number of patients, all of them were retrospective, thereby reducing the quality of evidence. However, given the scarcity of data, this meta-analysis is crucial in improving our understanding of the current level of evidence for AI-assisted EUS. Secondly, this meta-analysis exhibited significant heterogeneity, likely due to the variability in the populations included in the studies. For instance, two studies evaluated the AI’s performance by categorizing the SELs based on a 20 mm cut-off for size[28,34], which limits the performance evaluation to SELs < 20 mm. Thirdly, the varying quality of EUS devices and images used in the trials limits their applicability in real-world scenarios. Many of these studies only employed internal validation datasets for training the algorithms, which may potentially result in an overestimation of the AI models’ performance. This situation is indicative of overfitting[37], where a machine learning model becomes overly specialized to the training data and performs poorly on new.
In summary, AI-supported EUS demonstrates notable diagnostic precision in retrospective investigations related to the detection of GIST. Furthermore, AI has shown superior accuracy compared to experienced endoscopists, indicating its potential as a significant diagnostic adjunct in this field. The advancement of AI algorithms and EUS devices, along with the increased accessibility of EUS and the availability of high-quality EUS images, creates a favorable environment for robust studies aiming to achieve enhanced diagnostic performance and develop valuable, clinically applicable tools. Consequently, AI technology has the potential to profoundly influence all aspects of healthcare, as indicated by current research findings.
In conclusion, our systematic review and meta-analysis demonstrated the high diagnostic accuracy of EUS AI-assisted for the differentiation of SELs, especially GIST from other fourth-layer subepithelial tumors. AI revealed the potential to become help enhance endoscopists’ diagnostic performance in the EUS evaluation of SELs and avoid unnecessary invasive procedures.
Endoscopic ultrasonography (EUS) with artificial intelligence (AI) has shown high diagnostic accuracy for subepithelial lesions (SELs), particularly gastrointestinal stromal tumors (GISTs). The performance of AI systems has demonstrated superiority over experienced endoscopists and the ability to improve diagnostic power through collaborative diagnosis.
This paper aims to investigate the diagnostic capabilities of AI-assisted EUS for SELs by analyzing images and comparing them with the expertise of experienced endoscopists.
The research aims to assess the accuracy of AI-assisted EUS in diagnosing SELs, particularly those originating from the fourth layer. Additionally, the study analyzes the diagnostic performance of experienced endoscopists and compares it with AI systems.
Retrospective studies were selected of AI-assisted EUS for the diagnosis of SELs, using histopathology as the standard method. The included studies utilized EUS with AI for SELs diagnosis through image analysis. The risk of bias and quality of evidence were assessed, and the analysis was performed using Meta-Disc software.
This meta-analysis included eight retrospective studies with a total of 2355 patients and 44154 images. The AI-assisted EUS for GIST diagnosis showed a sensitivity of 92% [95% confidence interval (CI): 0.89-0.95; P < 0.01], specificity of 80% (95%CI: 0.75-0.85; P < 0.01), and an AUC of 0.949. For the diagnosis of GIST vs gastrointestinal leiomyoma (GIL) by AI-assisted EUS, specificity was 90% (95%CI: 0.88-0.95; P = 0.02) and AUC 0.966. The experienced endoscopists achieved a sensitivity of 72% (95%CI: 0.67-0.76; P < 0.01), specificity of 70% (95%CI: 0.64-0.76; P < 0.01), and an AUC of 0.777 for GIST. Evaluating GIST vs GIL, the experts achieved a sensitivity of 73% (95%CI: 0.65-0.80; P < 0.01) and an AUC of 0.819.
This systematic review and meta-analysis demonstrate the high diagnostic accuracy of AI-assisted EUS in differentiating SELs, particularly GIST, from other fourth-layer subepithelial tumors.
This study demonstrated that by integrating machine learning techniques with EUS images, AI can aid in distinguishing benign from malignant lesions and guiding treatment decisions, with high accuracy. Additionally, through AI assistance image recognition can enhance real-time diagnosis during EUS evaluations, increasing the performance of even experienced endoscopists.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq; Naganuma H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (1)] |
| 2. | Park CH, Kim EH, Jung DH, Chung H, Park JC, Shin SK, Lee YC, Kim H, Lee SK. Impact of periodic endoscopy on incidentally diagnosed gastric gastrointestinal stromal tumors: findings in surgically resected and confirmed lesions. Ann Surg Oncol. 2015;22:2933-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Standards of Practice Committee; Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (9)] |
| 5. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 6. | Park EY, Kim GH. Diagnosis of Gastric Subepithelial Tumors Using Endoscopic Ultrasonography or Abdominopelvic Computed Tomography: Which is Better? Clin Endosc. 2019;52:519-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Alkhatib AA, Faigel DO. Endoscopic ultrasonography-guided diagnosis of subepithelial tumors. Gastrointest Endosc Clin N Am. 2012;22:187-205, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | de Moura DTH, McCarty TR, Jirapinyo P, Ribeiro IB, Flumignan VK, Najdawai F, Ryou M, Lee LS, Thompson CC. EUS-guided fine-needle biopsy sampling versus FNA in the diagnosis of subepithelial lesions: a large multicenter study. Gastrointest Endosc. 2020;92:108-119.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Cheng S, Matuguma SE, de Oliveira GHP, Silva GLR, Cheng H, Sánchez-Luna SA, Minata MK. Colonoscopic Ultrasound-Guided Fine-Needle Aspiration Using a Curvilinear Array Transducer: A Single-Center Retrospective Cohort Study. Dis Colon Rectum. 2022;65:e80-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kamata K, Takenaka M, Kitano M, Omoto S, Miyata T, Minaga K, Yamao K, Imai H, Sakurai T, Watanabe T, Nishida N, Chikugo T, Chiba Y, Imamoto H, Yasuda T, Lisotti A, Fusaroli P, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of submucosal tumors of the upper gastrointestinal tract. J Gastroenterol Hepatol. 2017;32:1686-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Oka A, Ishimura N, Ishihara S. A New Dawn for the Use of Artificial Intelligence in Gastroenterology, Hepatology and Pancreatology. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2838] [Article Influence: 473.0] [Reference Citation Analysis (0)] |
| 14. | Gao J, Jiang Q, Zhou B, Chen D. Convolutional neural networks for computer-aided detection or diagnosis in medical image analysis: An overview. Math Biosci Eng. 2019;16:6536-6561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Mijwil MM, Abttan RA, Alkhazraji A. Artificial intelligence for COVID-19: A Short Article. AJPNMS. 2022;10. [DOI] [Full Text] |
| 16. | Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Chen T, Xu L, Dong X, Li Y, Yu J, Xiong W, Li G. The roles of CT and EUS in the preoperative evaluation of gastric gastrointestinal stromal tumors larger than 2 cm. Eur Radiol. 2019;29:2481-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Seven G, Silahtaroglu G, Kochan K, Ince AT, Arici DS, Senturk H. Use of Artificial Intelligence in the Prediction of Malignant Potential of Gastric Gastrointestinal Stromal Tumors. Dig Dis Sci. 2022;67:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Messmann H, Bisschops R, Antonelli G, Libânio D, Sinonquel P, Abdelrahim M, Ahmad OF, Areia M, Bergman JJGHM, Bhandari P, Boskoski I, Dekker E, Domagk D, Ebigbo A, Eelbode T, Eliakim R, Häfner M, Haidry RJ, Jover R, Kaminski MF, Kuvaev R, Mori Y, Palazzo M, Repici A, Rondonotti E, Rutter MD, Saito Y, Sharma P, Spada C, Spadaccini M, Veitch A, Gralnek IM, Hassan C, Dinis-Ribeiro M. Expected value of artificial intelligence in gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2022;54:1211-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13355] [Article Influence: 834.7] [Reference Citation Analysis (0)] |
| 21. | Leeflang MM, Deeks JJ, Takwoingi Y, Macaskill P. Cochrane diagnostic test accuracy reviews. Syst Rev. 2013;2:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 22. | Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, Deeks JJ, Leeflang MMG; QUADAS-C Group†, Bossuyt PMM, Brazzelli MG, Dinnes J, Gurusamy KS, Jones HE, Lange S, Langendam MW, Macaskill P, McInnes MDF, Reitsma JB, Rutjes AWS, Sinclair A, de Vet HCW, Virgili G, Wade R, Westwood ME. QUADAS-C: A Tool for Assessing Risk of Bias in Comparative Diagnostic Accuracy Studies. Ann Intern Med. 2021;174:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 23. | Cox DR, Snell EJ. The Analysis of Binary Data. In: The Concise Encyclopedia of Statistics. New York: Springer New York, 1989: 4-5. |
| 24. | Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1446] [Cited by in RCA: 1573] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 25. | Fagan TJ. Letter: Nomogram for Bayes theorem. N Engl J Med. 1975;293:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 573] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Nguyen VX, Nguyen CC, Li B, Das A. Digital image analysis is a useful adjunct to endoscopic ultrasonographic diagnosis of subepithelial lesions of the gastrointestinal tract. J Ultrasound Med. 2010;29:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kim YH, Kim GH, Kim KB, Lee MW, Lee BE, Baek DH, Kim DH, Park JC. Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Minoda Y, Ihara E, Komori K, Ogino H, Otsuka Y, Chinen T, Tsuda Y, Ando K, Yamamoto H, Ogawa Y. Efficacy of endoscopic ultrasound with artificial intelligence for the diagnosis of gastrointestinal stromal tumors. J Gastroenterol. 2020;55:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Minoda Y, Ihara E, Fujimori N, Nagatomo S, Esaki M, Hata Y, Bai X, Tanaka Y, Ogino H, Chinen T, Hu Q, Oki E, Yamamoto H, Ogawa Y. Efficacy of ultrasound endoscopy with artificial intelligence for the differential diagnosis of non-gastric gastrointestinal stromal tumors. Sci Rep. 2022;12:16640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Oh CK, Kim T, Cho YK, Cheung DY, Lee BI, Cho YS, Kim JI, Choi MG, Lee HH, Lee S. Convolutional neural network-based object detection model to identify gastrointestinal stromal tumors in endoscopic ultrasound images. J Gastroenterol Hepatol. 2021;36:3387-3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Seven G, Silahtaroglu G, Seven OO, Senturk H. Differentiating Gastrointestinal Stromal Tumors from Leiomyomas Using a Neural Network Trained on Endoscopic Ultrasonography Images. Dig Dis. 2022;40:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Yang X, Wang H, Dong Q, Xu Y, Liu H, Ma X, Yan J, Li Q, Yang C, Li X. An artificial intelligence system for distinguishing between gastrointestinal stromal tumors and leiomyomas using endoscopic ultrasonography. Endoscopy. 2022;54:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Hirai K, Kuwahara T, Furukawa K, Kakushima N, Furune S, Yamamoto H, Marukawa T, Asai H, Matsui K, Sasaki Y, Sakai D, Yamada K, Nishikawa T, Hayashi D, Obayashi T, Komiyama T, Ishikawa E, Sawada T, Maeda K, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Ishigami M, Fujishiro M. Artificial intelligence-based diagnosis of upper gastrointestinal subepithelial lesions on endoscopic ultrasonography images. Gastric Cancer. 2022;25:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Tanaka H, Kamata K, Ishihara R, Handa H, Otsuka Y, Yoshida A, Yoshikawa T, Ishikawa R, Okamoto A, Yamazaki T, Nakai A, Omoto S, Minaga K, Yamao K, Takenaka M, Watanabe T, Nishida N, Kudo M. Value of artificial intelligence with novel tumor tracking technology in the diagnosis of gastric submucosal tumors by contrast-enhanced harmonic endoscopic ultrasonography. J Gastroenterol Hepatol. 2022;37:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Kim SM, Kim EY, Cho JW, Jeon SW, Kim JH, Kim TH, Moon JS, Kim JO; Research Group for Endoscopic Ultrasound of the Korean Society of Gastrointestinal Endoscopy. Predictive Factors for Differentiating Gastrointestinal Stromal Tumors from Leiomyomas Based on Endoscopic Ultrasonography Findings in Patients with Gastric Subepithelial Tumors: A Multicenter Retrospective Study. Clin Endosc. 2021;54:872-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Seo SW, Hong SJ, Han JP, Choi MH, Song JY, Kim HK, Lee TH, Ko BM, Cho JY, Lee JS, Lee MS. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013;14:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Park SH, Han K. Methodologic Guide for Evaluating Clinical Performance and Effect of Artificial Intelligence Technology for Medical Diagnosis and Prediction. Radiology. 2018;286:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 498] [Article Influence: 71.1] [Reference Citation Analysis (0)] |