Published online Jul 16, 2023. doi: 10.4253/wjge.v15.i7.496
Peer-review started: February 27, 2023
First decision: May 16, 2023
Revised: May 30, 2023
Accepted: June 14, 2023
Article in press: June 14, 2023
Published online: July 16, 2023
Processing time: 134 Days and 14.3 Hours
Recent advancements in endoscopy equipment have facilitated endoscopists’ detection of neoplasms in the oral cavity and pharyngolaryngeal regions. In particular, image-enhanced endoscopy using narrow band imaging or blue laser imaging play an integral role in the endoscopic diagnosis of oral and pharyngolaryngeal cancers. Despite these advancements, limited studies have focused on benign lesions that can be observed during esophagogastroduodenoscopy in the oral and pharyngolaryngeal regions. Therefore, this mini-review aimed to provide essential information on such benign lesions, along with representative endoscopic images of dental caries, cleft palate, palatal torus, bifid uvula, compression by cervical osteophytes, tonsil hyperplasia, black hairy tongue, oral candidiasis, oral and pharyngolaryngeal ulcers, pharyngeal melanosis, oral tattoos associated with dental alloys, retention cysts, papilloma, radiation-induced changes, skin flaps, vocal cord paresis, and vocal fold leukoplakia. Whilst it is imperative to seek consultation from otolaryngologists or dentists in instances where the diagnosis cannot be definitively ascertained by endoscopists, the merits of attaining foundational expertise pertaining to oral and pharyngolaryngeal lesions are unequivocal. This article will be a valuable resource for endoscopists seeking to enhance their understanding of oral and pharyngolaryngeal lesions.
Core Tip: During esophagogastroduodenoscopy, various lesions other than squamous cell carcinoma can be detected in the oral cavity and pharyngolaryngeal regions. These include dental caries, cleft palate, palatal torus, bifid uvula, compression by cervical osteophytes, tonsil hyperplasia, black hairy tongue, oral candidiasis, oral and pharyngolaryngeal ulcers, pharyngeal melanosis, oral tattoos associated with dental alloys, retention cysts, papilloma, radiation-induced changes, skin flaps, vocal cord paresis, and vocal fold leukoplakia. Endoscopists must possess adequate knowledge about these lesions and promptly identify and diagnose them during an endoscopic examination.
- Citation: Iwamuro M, Hamada K, Kawano S, Kawahara Y, Otsuka M. Review of oral and pharyngolaryngeal benign lesions detected during esophagogastroduodenoscopy. World J Gastrointest Endosc 2023; 15(7): 496-509
- URL: https://www.wjgnet.com/1948-5190/full/v15/i7/496.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i7.496
Recent advances in endoscopy equipment have enabled endoscopists to detect neoplasms in the oral cavity and pharyngolaryngeal region. In particular, image-enhanced endoscopy using narrow band imaging (NBI) or blue laser imaging (BLI) play an integral role in the endoscopic diagnosis of oral and pharyngolaryngeal cancers[1-4]. For instance, early-stage squamous cell carcinoma in the oral and pharyngolaryngeal regions typically exhibits a well-demarcated brownish area with irregular microvasculature on NBI or BLI, which resembles the features of early-stage esophageal cancer. A prospective, controlled cohort study on structured screening of the oropharynx, hypopharynx, and larynx using esophagogastroduodenoscopy revealed significantly increased detection rates of precancerous and early cancerous lesions compared with those without structured examination of the pharyngolaryngeal area[5]. A retrospective observational study revealed that the prevalence of pharyngeal cancer, which was detected during esophagogastroduodenoscopy using NBI, was 0.26% (29/11050)[6]. These results reinforce the growing importance of screening examinations of the laryngopharyngeal area. However, despite the increasing number of articles on the endoscopic features and treatment of squamous cell carcinoma, few articles have focused on benign lesions occurring in the oral and pharyngolaryngeal regions. Herein, we present the endoscopic images of 17 types of lesions detected in the oral and pharyngolaryngeal areas, and review articles associated with these lesions.
Dental caries, also known as dental cavities or tooth decay, are areas of the teeth that have been damaged and weakened by acid-producing bacteria. This damage results in a hole or pit in the tooth, which can cause pain, sensitivity, and other oral health problems[7]. Progressive damage results in significant destruction of the teeth. If the infection is left untreated or becomes severe, the bacteria causing dental caries spread to other parts of the body via the bloodstream, i.e., sepsis, which is a life-threatening condition. Thus, it is important to diagnose dental caries promptly, and maintain good oral hygiene from the standpoint of internists. Furthermore, diabetes is significantly correlates with dental caries[8]. High blood glucose levels make the teeth and gums more susceptible to decay and periodontal diseases. Additionally, patients with diabetes may produce less saliva, leading to dry mouth, another factor that contributes to tooth decay. While visual dental examinations by dental professionals such as hygienists and dentists are crucial, esophagogastroduodenoscopy screening of teeth may be advantageous, particularly in individuals with diabetes. Unfavorable dental health and untreated cavities also lead to periodontal diseases, which are reportedly associated with an increased risk of certain digestive conditions, such as gastroesophageal reflux disease and peptic ulcers[9,10].
Dental caries are visible as discolored or darkened spots, rough or uneven surfaces, visible cavities or holes, or even grossly destroyed areas on teeth. Figure 1 shows representative images of grossly decayed teeth. A 28-year-old man (Case 1) underwent craniotomy for craniopharyngioma, and was treated for panhypopituitarism, diabetes insipidus, and diabetes mellitus. The intraoral view revealed multiple residual roots with carious lesions (Figure 1A). In another 69-year-old man with diabetes mellitus (Case 2), esophagogastroduodenoscopy revealed a severely damaged tooth (Figure 1B).
Cleft palate is a congenital anomaly characterized by a split or opening in the palate, which serves as a demarcation between the oral and nasal cavities. This condition arises from inadequate fusion of the tissues that form the palate during fetal development, and may manifest in varying degrees of severity, size, and location, affecting the soft palate (posterior part of the mouth), hard palate (anterior part of the mouth), or both[11-13]. In severe cases, fissures may extend into the nasal cavity. The global incidence of cleft lip and/or palate is approximately 1 in 700 live births, signifying its substantial occurrence as a congenital anomaly[14]. Surgical intervention is the primary treatment modality for cleft palate, although additional therapeutic measures, such as speech therapy and dental management, may be warranted.
A hole on the palate was observed in a 67-year-old man (Case 3) during esophagogastroduodenoscopy (Figure 2). The patient had been previously diagnosed with cleft palate and mild dysarthria.
Palatal torus, or torus palatinus, is a bony lump that develops in the hard palate. Protrusions are benign, non-neoplastic lesions caused by overgrowth of osseous tissue[15-17]. The palatal torus is typically round or lobed and varies in size. Treatment is not required because it is generally asymptomatic.
A 62-year-old man with maxillary cancer (Case 4) underwent esophagogastroduodenoscopy for cancer screening. A protruding lesion was identified in the roof of the mouth (Figure 3A and B). Computed tomography (CT) images showed a bony structure in the hard palate (Figure 3C), confirming the diagnosis of palatal torus. In this patient, although the CT scan was performed for maxillary cancer, the diagnosis of palatal torus was easily and definitively established through palpation of the protrusion with the index finger, thereby confirming its bony solidity.
Bifid uvula is a congenital anomaly in which the uvula is split into two lobes or appears notched[18,19]. The bifurcation of the uvula is considered a minor variation in the normal anatomy, and is usually not associated with any health problems. The prevalence of bifid uvula is estimated to be 0.4%–3.3% among the general population[20].
A uvula with a bisecting tip was unexpectedly identified in a 75-year-old woman (Case 5) (Figure 4). As the patient did not present with either cleft palate or any discernible subjective symptoms, no intervention was deemed necessary for the bifid uvula.
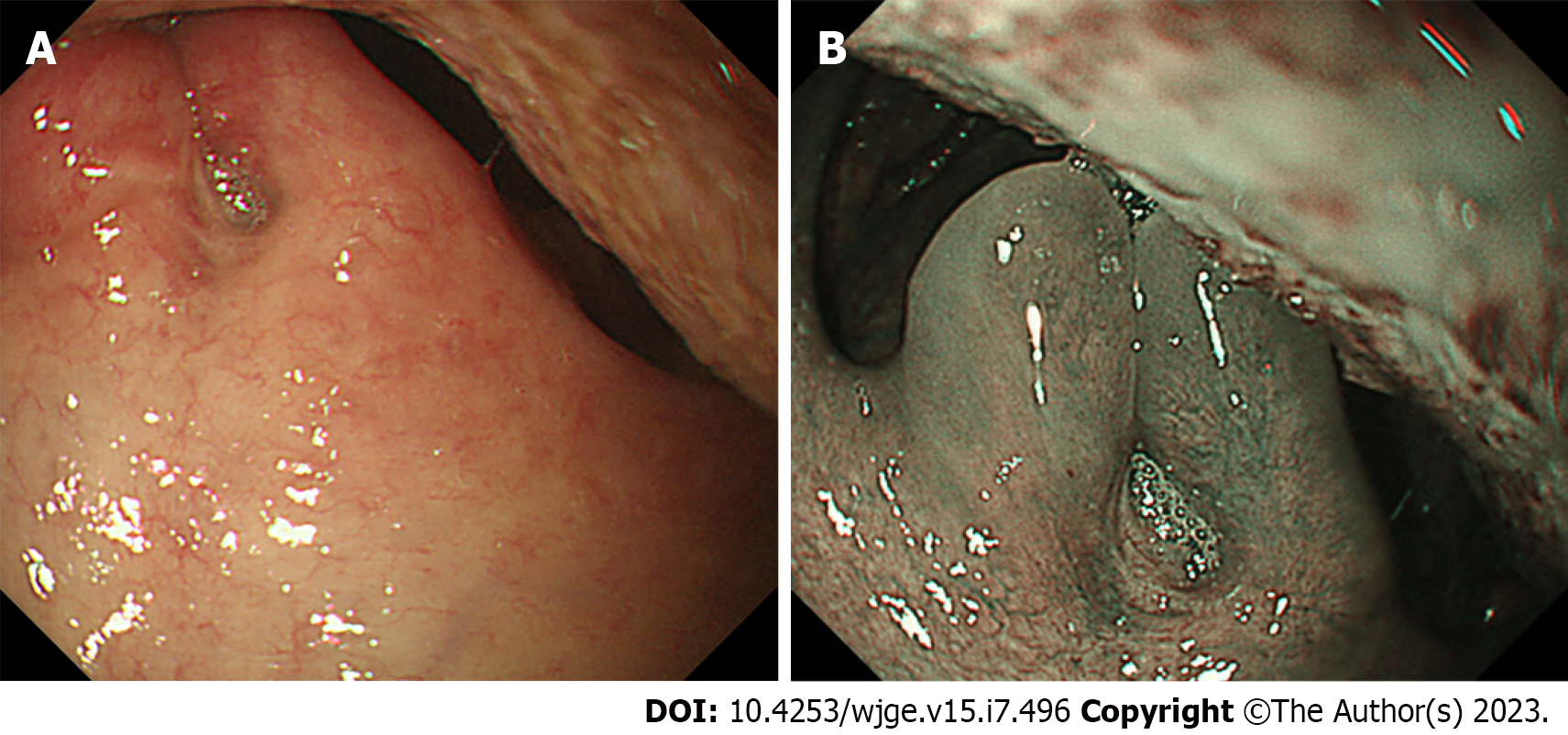
Compression by cervical osteophytes, which are bony outgrowths on the cervical vertebrae, can cause deformities in the oropharynx, hypopharynx, and larynx due to their proximity and pressure on these structures. As osteophytes grow and compress, they can lead to narrowing or obstruction of adjacent tissues[21-23]. The presence of a deformity in the pharyngolaryngeal region, specifically in proximity to the pyriform sinus, may pose a challenge during endoscope insertion. The diagnosis of cervical osteophytes is made based on CT imaging. Previous reports have suggested that a similar deformity may manifest with medialization of the common carotid artery[5].
Esophagogastroduodenoscopy revealed a submucosal bulge on the dorsal side of the oropharynx in an 87-year-old man (Case 6) (Figure 5A). CT images showed a bone protrusion on the anterior side of the cervical vertebra (Figure 5B). Another patient (69-year-old man, Case 7) exhibited deformation of the right dorsal side of the hypopharynx (Figure 5C). CT images revealed bone outgrowth on the right anterior side of the cervical vertebra (Figure 5D).
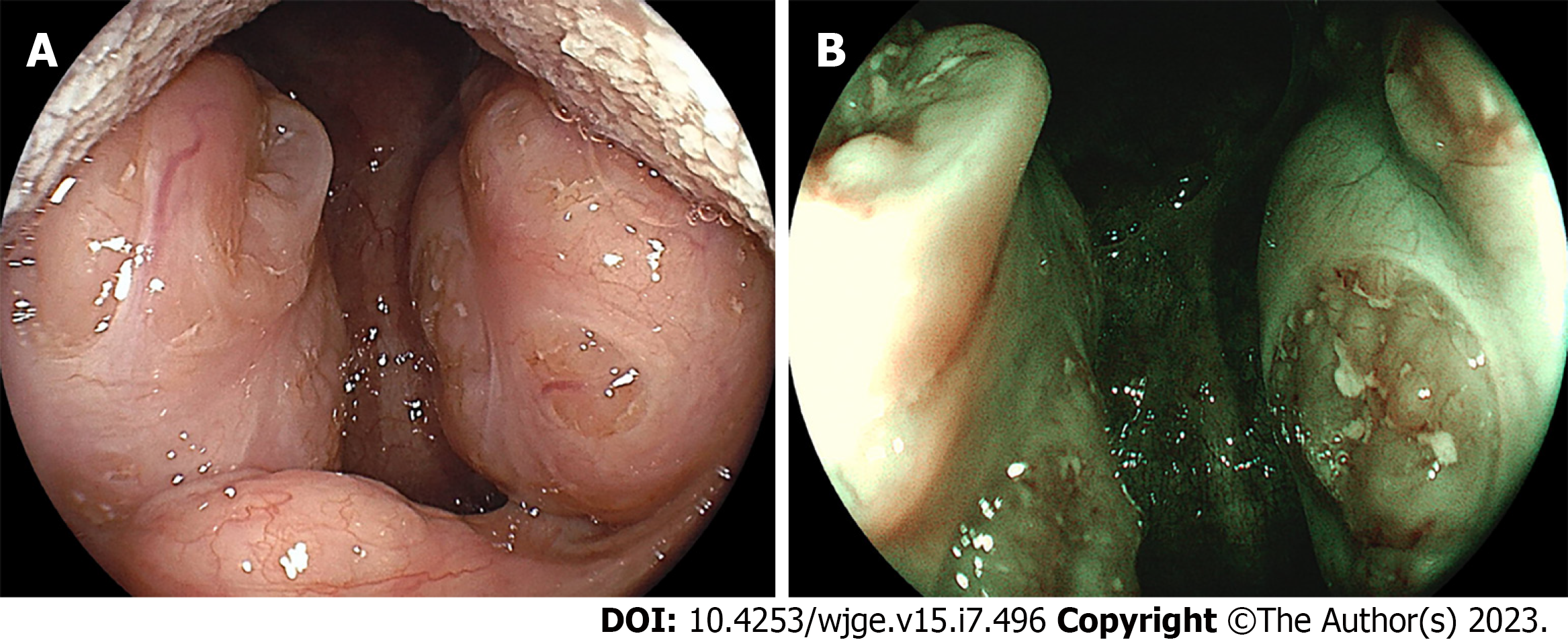
Tonsil hypertrophy can be caused by diverse etiological factors such as recurrent infections, allergies, and genetic factors. Tonsil hypertrophy can lead to several related issues depending on the severity and extent of enlargement[24]. The associated symptoms include difficulty in swallowing, breathing difficulties such as snoring or sleep apnea[25], chronic infections, speech problems, and changes in facial structure called “adenoid face”. Evaluation of the tonsils is generally important before endoscopic examination using sedatives because significant tonsil hypertrophy potentially causes breathing difficulties. If a patient has enlarged tonsils, a healthcare provider may choose to take additional precautions, such as adjusting the dosage of sedative medication, or monitoring the patient's breathing more closely during the sedation procedure. In addition, hypertrophic tonsils prevent endotracheal intubation[26].
A 23-year-old woman (Case 8) underwent screening via esophagogastroduodenoscopy, and hypertrophic tonsils were unexpectedly identified (Figure 6). The patient was asymptomatic, therefore no therapeutic intervention was required for the tonsil hypertrophy.
Black hairy tongue manifests as a superficial, dark, and furry carpet-like growth on the tongue. The precise etiology of this lesion remains uncertain, although it is deemed to be a transient and benign condition that is linked to inadequate oral hygiene, tobacco use, and specific medications such as antibiotics[27-29]. Medical intervention is typically not necessary for black hairy tongue, despite its unattractive appearance. It can be resolved by maintaining better oral hygiene and eliminating causative agents, such as abstaining from smoking and refraining from the use of specific medications.
A 65-year-old man (Case 9) diagnosed with schizophrenia and advanced colon cancer, who had become incapacitated due to mental impairments, was admitted to our hospital. Esophagogastroduodenoscopy revealed a black hairy tongue (Figure 7A). A black hairy tongue was also observed in an 86-year-old woman (Case 10) diagnosed with polymyalgia rheumatica after administration of steroids (10 mg prednisolone) (Figure 7B).
Candida sp. are commensal fungi that reside in the oral mucosa. However, in individuals with compromised immune systems, they can become pathogenic and instigate opportunistic infections. Oral candidiasis can be caused by different species of Candida, among which Candida albicans is the most common pathogen. Other species that can cause oral candidiasis include C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei. Oral candidiasis, also known as oral thrush, is characterized by white or creamy plaques within the mouth[30-32]. Antifungal medication and proper oral hygiene practices are effective for the treatment of oral candidiasis.
Physical examination revealed white adhesions in the mouth of a 63-year-old woman with hypertension and chronic thyroiditis (Case 11). Esophagogastroduodenoscopy revealed multiple white plaques in the oral cavity, pharynx, larynx, and esophagus (Figure 8). A biopsy of the white lesions showed fungi, confirming the diagnosis of oral, phary
Corrosive injury in the oral and pharyngolaryngeal regions occurs following exposure to corrosive agents, including acids, alkalis (bases), or other potent chemicals[33]. The ingestion of corrosive substances primarily occurs as acts of deliberate self-harm in adults and accidentally in children. Corrosive substances can induce profound harm upon contact with the mouth, throat, and larynx. According to a study investigating individuals who ingested ammonia, 69.8% of patients (30 out of 43) displayed oropharyngeal lesions[34]. The severity of the injury is contingent upon multiple factors, including the concentration, volume, and duration of exposure to the corrosive substance, as well as the nature of the chemical involved. Reports indicate that early endoscopy within 12–24 h after ingestion enables careful assessment of anatomical disruptions in the oropharyngolaryngeal areas[35] and the esophagus, stomach, and duodenum.
The manifestation of oral and pharyngolaryngeal ulcers stems from their diverse etiologies. Differential diagnosis depends on the patient's history, physical examination findings, and any accompanying symptoms. Common differential diagnoses include bacterial (e.g., Streptococcus sp.) and viral infections (e.g., herpes simplex virus, Epstein-Barr virus, cytomegalovirus, and Coxsackie virus), autoimmune disorders (e.g., Behcet's disease, systemic lupus erythematosus, and Crohn's disease), skin diseases (e.g., pemphigus vulgaris and mucous membrane pemphigoid), trauma (e.g., biting and scratching), allergic reactions, chemical irritation, and neoplasms[36,37]. Thus, physicians must uncover the underlying cause of the disease in patients with chronic relapsing or intractable ulcers in the oral and pharyngolaryngeal regions.
A 64-year-old woman (Case 12) was referred to our hospital for evaluation of oral ulceration and fever. During her hospitalization, the patient developed macrocytic anemia and neutropenia. Subsequent bone marrow examination led to a diagnosis of myelodysplastic syndrome with trisomy 8. Colonoscopy revealed multiple ulcers involving Bauhin's valve, and esophagogastroduodenoscopy revealed ulcers in the oral cavity (Figure 9). Behçet’s disease-like symptoms have been reported to arise in conjunction with myelodysplastic syndrome involving trisomy 8[38].
Melanosis is characterized by the presence of dark pigmentation in the epithelium. Melanosis in the oral and pharyngolaryngeal regions is also known as smoker's melanosis, as it typically manifests in up to 30% of chronic smokers and gradually regresses following smoking cessation[39]. One study demonstrated a robust correlation between the presence of melanosis and elevated susceptibility to squamous cell carcinoma in the oral cavity, pharynx, larynx, or esophagus[40]. These results suggest that screening for squamous cell carcinoma is important in patients with melanosis.
In a 58-year-old man (Case 13) with advanced esophageal cancer (Figure 10A), melanosis was identified in the pharynx (Figure 10B). The patient had a history of smoking 20 cigarettes daily for 39 years.
Oral tattoos, also known as amalgam tattoos or intraoral tattoos, are characterized by pigmentation or staining of oral tissues, most commonly affecting the gingiva and mucosa of the oral cavity[41-43]. Discoloration arises from exposure to dental restorative materials containing metals including amalgam, gold, and various alloys. Although oral tattoos are generally considered benign and do not cause any symptoms, they can be a source of cosmetic concerns. Additionally, in some instances, they can be misdiagnosed as oral melanoma or other pigmented lesions, warranting an appropriate diagnosis and follow-up by a dental professional or otolaryngologist.
Multiple points of black pigmentation were identified bilaterally on the buccal mucosa of a 68-year-old man (Case 14) (Figure 11)[42]. We performed an endoscopic biopsy of the lesion to exclude melanoma, which revealed no neoplastic cells. Since the pigmented lesions were adjacent to the metal crowns of gold-silver-palladium alloys, we diagnosed oral tattoos associated with dental restorative materials.
Retention cysts are the most prevalent benign lesions of the pharyngeal and laryngeal mucosa. These cysts are lined with epithelial tissue and are characterized by the presence of serous or mucous fluid[44,45]. The pathogenesis of these cysts is believed to involve dilation and obstruction of the mucous gland ducts within the lamina propria or deeper layers of the pharyngolaryngeal region due to the retention of secretions and/or chronic inflammatory processes. Most small and asymptomatic cysts do not require treatment. However, larger cysts or those causing significant symptoms, such as dysphagia, dysphonia, or respiratory distress, may require intervention.
Figure 12A and B show a retention cyst incidentally observed on the ventral side of the epiglottis in a 60-year-old man (Case 15) during esophagogastroduodenoscopy (Figure 12A and B). In an 80-year-old woman (Case 16), a retention cyst was identified on the left side of the epiglottis (Figure 12C). Neither of the patients exhibited any symptoms associated with the presence of epiglottic cysts.
Papillomas in the oral and pharyngolaryngeal regions are benign tumors that generally present exophytic growth with wart-like projections[46,47]. The microscopic features of pharyngeal papillomas typically include papillary architecture, hyperkeratosis, koilocytosis, and fibrovascular cores. Considering the benign nature of papillomas, treatment is reserved only for symptomatic cases.
Reddish wart-like projections were observed on the uvula of a 67-year-old man (Case 17) during esophagogastroduodenoscopy (Figure 13A). Magnifying NBI revealed dilated microvasculature, arranged in an orderly manner, and demarcated into clusters (Figure 13B). Histopathological examination of the endoscopic biopsy specimen confirmed a diagnosis of papilloma.
Radiation therapy is used to treat malignant lesions in the oral cavity, pharynx, and larynx[48,49]. This therapeutic modality induces several mucosal alterations, including mucosal erythema, friability, erosion, and angiectasia. Caution is required during endoscopic observation of irradiated regions, as differentiating between neoplastic lesions (i.e., recurrence of cancer) and radiation-induced mucosal alterations may be challenging.
A 58-year-old man who had undergone radiotherapy for laryngeal cancer (Case 18) showed patchy redness in the pharyngolaryngeal region (Figure 14A). Another 70-year-old man with a history of laryngeal cancer treated with radiotherapy (Case 19) showed vascular dilatation in the larynx (Figure 14B), which was observed more strongly on BLI (Figure 14C).
In reconstructive surgery for oral and pharyngeal defects, skin flaps or skin grafts are sometimes used for both functional and cosmetic restoration. Differentiation between these techniques is defined by vascularization, as a skin graft relies on the vascular bed of the recipient site for blood supply, whereas a skin flap retains its intrinsic blood supply from the donor site[50,51].
We performed a screening esophagogastroduodenoscopy in a 68-year-old man (Case 20). The patient underwent surgery for laryngeal cancer and reconstruction with a pectoralis major myocutaneous flap. Esophagogastroduodenoscopy revealed a clearly demarcated, yellowish-white skin flap through the pharynx, larynx, and esophagus (Figure 15A and B). In a 71-year-old man who underwent reconstructive surgery for laryngeal cancer (Case 21), a pectoralis major myocutaneous flap was observed during esophagogastroduodenoscopy (Figure 15C).
Paresis of the vocal cords denotes a condition in which one or both the vocal cords suffer an impairment in their motility or function[52,53]. The possible etiologies of vocal cord paresis include congenital factors, infectious agents, neoplasms, traumatic incidents, endocrine diseases (e.g., thyroid disorders), and systemic neurological disorders. Hoarseness, breathiness, dysphonia, dysphagia, and/or dyspnea may occur because of vocal cord paresis.
A 65-year-old man (Case 22) underwent surgery for advanced esophageal cancer. His right recurrent laryngeal nerve was injured during surgery, resulting in unilateral vocal cord paresis. Subsequent esophagogastroduodenoscopy revealed that the right vocal cord displayed no movement during respiration (Figure 16A) and phonation (Figure 16B) owing to paresis.
Vocal fold leukoplakia, also known as laryngeal leukoplakia, refers to the manifestation of white patches or plaques on the mucosa of the vocal cords[54,55]. Smoking or chewing tobacco is a primary risk factor for the development of vocal fold leukoplakia. In addition, this condition is commonly linked to excessive alcohol consumption, viral infections such as human papillomavirus, chronic laryngopharyngeal reflux, and voice misuse[56]. It is important to note that the term “leukoplakia” does not indicate a specific histological diagnosis, as it encompasses several histological features, including benign, premalignant, and malignant lesions[57]. Although excisional surgery is the primary modality for treating vocal fold leukoplakia, a definitive threshold for surgical intervention remains elusive owing to the need for judicious therapeutic decision-making that optimizes both vocal function and oncologic safety[58]. Consequently, referral to an otolaryngologist is a crucial step when vocal fold leukoplakia is identified during esophagogastroduodenoscopy.
Figure 17 shows vocal fold leukoplakia observed in a 68-year-old man (Case 23). A whitish nodular lesion was observed mainly on the right side of the vocal fold (Figure 17A), which was emphasized on NBI (Figure 17B). The patient had undergone a biopsy of the leukoplakia lesion at 64 years of age. A pathological diagnosis of dysplasia was made, and the vocal fold lesion was under active surveillance by otolaryngologists.
We presented representative endoscopic images of dental caries, cleft palate, palatal torus, bifid uvula, compression by cervical osteophytes, tonsil hyperplasia, black hairy tongue, oral candidiasis, oral and pharyngolaryngeal ulcers, pharyngeal melanosis, oral tattoos associated with dental alloys, epiglottic cysts, papilloma, radiation-induced changes, skin flap, vocal cord paresis, and vocal fold leukoplakia. The images show various lesions observed in the oral and pharyngolaryngeal regions during esophagogastroduodenoscopy. While it is essential to consult otolaryngologists or dentists when the diagnosis cannot be established by endoscopists, the benefits of acquiring foundational knowledge concerning oral and pharyngolaryngeal lesions and identifying them for the welfare of patients cannot be denied. We believe that this mini-review will be valuable to endoscopists seeking to enhance their understanding of oral and pharyngolaryngeal lesions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Rathi PM, India; Vissink A, Netherlands; Yu H, China; Zharikov YO, Russia S-Editor: Lin C L-Editor: A P-Editor: Xu ZH
| 1. | Hamada K, Ishihara R, Yamasaki Y, Akasaka T, Arao M, Iwatsubo T, Shichijo S, Matsuura N, Nakahira H, Kanesaka T, Yamamoto S, Takeuchi Y, Higashino K, Uedo N, Kawahara Y, Okada H. Transoral endoscopic examination of head and neck region. Dig Endosc. 2018;30:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Okamoto N, Morimoto H, Yamamoto Y, Kanda K, Nankinzan R, Kasamatsu S, Yoshimura S, Kan M, Nakano A, Hosaka S, Watanabe Y, Arahata K, Toyama Y, Okamura A, Yamaguchi T, Yano T. Skill-up study of systemic endoscopic examination technique using narrow band imaging of the head and neck region of patients with esophageal squamous cell carcinoma: Prospective multicenter study. Dig Endosc. 2019;31:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, Katada N, Masaki T, Nakayama M, Okamoto M, Muto M. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, Inoue H, Ishikawa H, Ochiai A, Shimoda T, Watanabe H, Tajiri H, Saito D. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 525] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 5. | Huelsen A, St John AT, Pandey R, Vokes DE, McMaster JJ, Walmsley RS, Holtmann GJ. Structured oropharynx, hypopharynx and larynx assessment during routine esophagogastroduodenoscopy improves detection of pre- and early cancerous lesions: a multicenter, comparative study. Endosc Int Open. 2021;9:E154-E162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Nakanishi H, Doyama H, Takemura K, Yoshida N, Tsuji K, Takeda Y, Asahina Y, Kito Y, Ito R, Hayashi T, Hirano K, Goto Y, Tominaga K, Inagaki S, Waseda Y, Tsuji S, Miwa K, Kaneko Y, Yamada S, Kurumaya H, Sakumoto M, Okada T. Detection of pharyngeal cancer in the overall population undergoing upper GI endoscopy by using narrow-band imaging: a single-center experience, 2009-2012. Gastrointest Endosc. 2014;79:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44 Suppl 18:S94-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 563] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 8. | Tanweer T, Rana NF, Saleem I, Shafique I, Alshahrani SM, Almukhlifi HA, Alotaibi AS, Alshareef SA, Menaa F. Dental Composites with Magnesium Doped Zinc Oxide Nanoparticles Prevent Secondary Caries in the Alloxan-Induced Diabetic Model. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Kuze LS, Fornari F, Collares K, Della Bona A. Association between masticatory dysfunction and gastroesophageal reflux disease: A population-based study in the elderly. J Oral Rehabil. 2023;50:150-156. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Lechien JR, Chiesa-Estomba CM, Calvo Henriquez C, Mouawad F, Ristagno C, Barillari MR, Schindler A, Nacci A, Bouland C, Laino L, Saussez S. Laryngopharyngeal reflux, gastroesophageal reflux and dental disorders: A systematic review. PLoS One. 2020;15:e0237581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Paradowska-Stolarz A, Mikulewicz M, Duś-Ilnicka I. Current Concepts and Challenges in the Treatment of Cleft Lip and Palate Patients-A Comprehensive Review. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Frederick R, Hogan AC, Seabolt N, Stocks RMS. An Ideal Multidisciplinary Cleft Lip and Cleft Palate Care Team. Oral Dis. 2022;28:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, Liang KY, Wu T, Murray T, Fallin MD, Redett RA, Raymond G, Schwender H, Jin SC, Cooper ME, Dunnwald M, Mansilla MA, Leslie E, Bullard S, Lidral AC, Moreno LM, Menezes R, Vieira AR, Petrin A, Wilcox AJ, Lie RT, Jabs EW, Wu-Chou YH, Chen PK, Wang H, Ye X, Huang S, Yeow V, Chong SS, Jee SH, Shi B, Christensen K, Melbye M, Doheny KF, Pugh EW, Ling H, Castilla EE, Czeizel AE, Ma L, Field LL, Brody L, Pangilinan F, Mills JL, Molloy AM, Kirke PN, Scott JM, Arcos-Burgos M, Scott AF. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010;42:525-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 14. | Yılmaz HN, Özbilen EÖ, Üstün T. The Prevalence of Cleft Lip and Palate Patients: A Single-Center Experience for 17 Years. Turk J Orthod. 2019;32:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | García-García AS, Martínez-González JM, Gómez-Font R, Soto-Rivadeneira A, Oviedo-Roldán L. Current status of the torus palatinus and torus mandibularis. Med Oral Patol Oral Cir Bucal. 2010;15:e353-e360. [PubMed] |
| 16. | Bezamat M, Zhou Y, Park T, Vieira AR. Genome-wide family-based study in torus palatinus affected individuals. Arch Oral Biol. 2021;130:105221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | El Sergani AM, Anderton J, Brandebura S, Obniski M, Ginart MT, Padilla C, Butali A, Adeyemo WL, Long RE Jr, Moreno LM, Marazita ML, Weinberg SM. Prevalence of Torus Palatinus and association with dental arch shape in a multi-ethnic cohort. Homo. 2020;71:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Feka P, Banon J, Leuchter I, La Scala GC. Prevalence of bifid uvula in primary school children. Int J Pediatr Otorhinolaryngol. 2019;116:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Vilacosta I, Cañadas Godoy V. Images in clinical medicine. Bifid uvula and aortic aneurysm. N Engl J Med. 2008;359:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Sales SAG, Santos ML, Machado RA, Dias VO, Nascimento JE, Swerts MSO, Júnior HM, Martelli DRB. Incidence of bifid uvula and its relationship to submucous cleft palate and a family history of oral cleft in the Brazilian population. Braz J Otorhinolaryngol. 2018;84:687-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Srivastava SK, Bhosale SK, Lohiya TA, Aggarwal RA. Giant Cervical Osteophyte: An Unusual Cause of Dysphagia. J Clin Diagn Res. 2016;10:MD01-MD02. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Mallepally AR, Tandon V, Chhabra HS. Dysphagia in a Young Adult: Rare Case of Giant Cervical Osteophyte. Asian J Neurosurg. 2020;15:218-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Hwang JS, Chough CK, Joo WI. Giant anterior cervical osteophyte leading to Dysphagia. Korean J Spine. 2013;10:200-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Mashat GD, Tran HH, Urgessa NA, Geethakumari P, Kampa P, Parchuri R, Bhandari R, Alnasser AR, Akram A, Kar S, Osman F, Hamid P. The Correlation Between Otitis Media With Effusion and Adenoid Hypertrophy Among Pediatric Patients: A Systematic Review. Cureus. 2022;14:e30985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Sung MW, Lee WH, Wee JH, Lee CH, Kim E, Kim JW. Factors associated with hypertrophy of the lingual tonsils in adults with sleep-disordered breathing. JAMA Otolaryngol Head Neck Surg. 2013;139:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ashraf A, Menon I, Gupta R, Arora V, Ahsan I, Das D. Oral findings as predictors of obstructive sleep apnea- A case-control study. J Family Med Prim Care. 2022;11:5263-5267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (5)] |
| 28. | Jayasree P, Kaliyadan F, Ashique KT. Black Hairy Tongue. JAMA Dermatol. 2022;158:573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Shangguan Y, Ji Z, Guo W, Hu W, Li X, Xu K. Oral Bacteria Dysbiosis in Patients with Linezolid-Induced Black Hairy Tongue: A Case Series. Infect Drug Resist. 2022;15:5449-5454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral Candidiasis: A Disease of Opportunity. J Fungi (Basel). 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 31. | Lu SY. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Cho E, Park Y, Kim KY, Han D, Kim HS, Kwon JS, Ahn HJ. Clinical Characteristics and Relevance of Oral Candida Biofilm in Tongue Smears. J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Hall AH, Jacquemin D, Henny D, Mathieu L, Josset P, Meyer B. Corrosive substances ingestion: a review. Crit Rev Toxicol. 2019;49:637-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Gelu-Simeon M, Chuong AP, Saliba F, Thiery G, Laurent M, Vilain C, Borel M, Amaral L, Alexis M, Saint-Georges G, Saillard E. Submucosal hematoma: a new distinctive sign during emergency upper digestive endoscopy for ammonia ingestion. BMC Gastroenterol. 2018;18:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Kluger Y, Ishay OB, Sartelli M, Katz A, Ansaloni L, Gomez CA, Biffl W, Catena F, Fraga GP, Di Saverio S, Goran A, Ghnnam W, Kashuk J, Leppäniemi A, Marwah S, Moore EE, Bala M, Massalou D, Mircea C, Bonavina L. Caustic ingestion management: world society of emergency surgery preliminary survey of expert opinion. World J Emerg Surg. 2015;10:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part I. Acute ulcers. Clin Exp Dermatol. 2009;34:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part II. Chronic ulcers. Clin Exp Dermatol. 2009;34:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Kawano S, Hiraoka S, Okada H, Akita M, Iwamuro M, Yamamoto K. Clinical Features of Intestinal Behçet's Disease Associated with Myelodysplastic Syndrome and Trisomy 8. Acta Med Okayama. 2015;69:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Changela K, Reddy M. Smoker's melanosis: Isolated pigmented lesion in the laryngopharynx and esophagus. Turk J Gastroenterol. 2017;28:524-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Yokoyama A, Mizukami T, Omori T, Yokoyama T, Hirota T, Matsushita S, Higuchi S, Maruyama K, Ishii H, Hibi T. Melanosis and squamous cell neoplasms of the upper aerodigestive tract in Japanese alcoholic men. Cancer Sci. 2006;97:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Panucci BZM, Ferrisse TM, Bufalino A, León JE. Concomitant endogenous and exogenous etiology for gingival pigmentation. Dermatol Online J. 2021;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Iwamuro M, Kawahara Y, Okada H. Oral Tattoos Associated with Dental Alloys. Intern Med. 2020;59:1331-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 43. | Dubach P, Caversaccio M. Images in clinical medicine. Amalgam tattoo. N Engl J Med. 2011;364:e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Ahmed ME, Ahmed ME, El Batawi AM, Abdelfattah HM, Jelassi N. Internal Hypopharyngeal Cyst: A Review of Literature. Dysphagia. 2019;34:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Woodfield CA, Levine MS, Rubesin SE, Laufer I, Mirza N. Pharyngeal retention cysts: radiographic findings in seven patients. AJR Am J Roentgenol. 2005;184:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Ding D, Yin G, Guo W, Huang Z. Analysis of lesion location and disease characteristics of pharyngeal and laryngeal papilloma in adult. Eur Arch Otorhinolaryngol. 2023;280:289-295. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Hirai R, Makiyama K, Higuti Y, Ikeda A, Miura M, Hasegawa H, Kinukawa N, Ikeda M. Pharyngeal squamous cell papilloma in adult Japanese: comparison with laryngeal papilloma in clinical manifestations and HPV infection. Eur Arch Otorhinolaryngol. 2012;269:2271-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Sroussi HY, Epstein JB, Bensadoun RJ, Saunders DP, Lalla RV, Migliorati CA, Heaivilin N, Zumsteg ZS. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6:2918-2931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 410] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 49. | Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, Birkeland AC, Brizel DM, Busse PM, Cmelak AJ, Colevas AD, Eisele DW, Galloway T, Geiger JL, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Mell LK, Mittal BB, Pinto HA, Rocco JW, Rodriguez CP, Savvides PS, Schwartz D, Shah JP, Sher D, St John M, Weber RS, Weinstein G, Worden F, Yang Bruce J, Yom SS, Zhen W, Burns JL, Darlow SD. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J Natl Compr Canc Netw. 2022;20:224-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 322] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 50. | de Bree R, Rinaldo A, Genden EM, Suárez C, Rodrigo JP, Fagan JJ, Kowalski LP, Ferlito A, Leemans CR. Modern reconstruction techniques for oral and pharyngeal defects after tumor resection. Eur Arch Otorhinolaryngol. 2008;265:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Zhang S, Wu S, Liu L, Zhu D, Zhu Q, Li W. Assessment of Quality of Life of Free Anterolateral Thigh Flap for Reconstruction of Tissue Defects of Total or Near-Total Glossectomy. J Oncol. 2020;2020:2920418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Liao LJ, Wang CT. Management of Unilateral Vocal Fold Paralysis after Thyroid Surgery with Injection Laryngoplasty: State of Art Review. Front Surg. 2022;9:876228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Ha JF. Unilateral vocal fold palsy & dysphagia: A review. Auris Nasus Larynx. 2020;47:315-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Hamdan AL, Ghanem A, Natout TE, Khalifee E. Diagnostic Yield of Office-Based Laryngeal Biopsy in Patients With Leukoplakia; A Case Study With Review of the Literature. J Voice. 2023;37:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Botini DS, Rodrigues SA, Castilho GL, Mercuri G, Martins RHG. Vocal Folds Leukoplakia: The Efficacy of Vitamin A in the Initial Treatment. Int Arch Otorhinolaryngol. 2023;27:e97-e103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 56. | Campo F, Ralli M, Di Stadio A, Greco A, Pellini R, de Vincentiis M. Role of Narrow Band Imaging Endoscopy in Preoperative Evaluation of Laryngeal Leukoplakia: A Review of the Literature. Ear Nose Throat J. 2022;101:NP403-NP408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Burkhardt A. Morphological assessment of malignant potential of epithelial hyperplastic lesions. Acta Otolaryngol Suppl. 1997;527:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Klimza H, Pietruszewska W, Rosiak O, Morawska J, Nogal P, Wierzbicka M. Leukoplakia: An Invasive Cancer Hidden within the Vocal Folds. A Multivariate Analysis of Risk Factors. Front Oncol. 2021;11:772255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |