Published online Jun 16, 2023. doi: 10.4253/wjge.v15.i6.480
Peer-review started: April 17, 2023
First decision: May 19, 2023
Revised: May 20, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: June 16, 2023
Processing time: 57 Days and 10.8 Hours
Although esophageal candidiasis (EC) may manifest in immunocompetent individuals, there is a lack of consensus in the current literature about predisposing conditions that increase the risk of infection.
To determine the prevalence of EC in patients without human immunodeficiency virus (HIV) and identify risk factors for infection.
We retrospectively reviewed inpatient and outpatient encounters from 5 regional hospitals within the United States (US) from 2015 to 2020. International Classification of Diseases, Ninth and Tenth Revisions were used to identify patients with endoscopic biopsies of the esophagus and EC. Patients with HIV were excluded. Adults with EC were compared to age, gender, and encounter-matched controls without EC. Patient demographics, symptoms, diagnoses, medications, and laboratory data were obtained from chart extraction. Differences in medians for continuous variables were compared using the Kruskal-Wallis test and categorical variables using chi-square analyses. Multivariable logistic regression was used to identify independent risk factors for EC, after adjusting for potential confounding factors.
Of the 1969 patients who had endoscopic biopsies of the esophagus performed from 2015 to 2020, 295 patients had the diagnosis of EC. 177 of 1969 patients (8.99%) had pathology confirming the diagnosis of EC and were included in the study for data collection and further analysis. In comparison to controls, patients with EC had significantly higher rates of gastroesophageal reflux disease (40.10% vs 27.50%; P = 0.006), prior organ transplant (10.70% vs 2%; P < 0.001), immunosuppressive medication (18.10% vs 8.10%; P = 0.002), proton pump inhibitor (48% vs 30%; P < 0.001), corticosteroid (35% vs 17%; P < 0.001), Tylenol (25.40% vs 16.20%; P = 0.019), and aspirin use (39% vs 27.50%; P = 0.013). On multivariable logistic regression analysis, patients with a prior organ transplant had increased odds of EC (OR = 5.81; P = 0.009), as did patients taking a proton pump inhibitor (OR = 1.66; P = 0.03) or corticosteroids (OR = 2.05; P = 0.007). Patients with gastroesophageal reflux disease or medication use, including immunosuppressive medications, Tylenol, and aspirin, did not have a significantly increased odds of EC.
Prevalence of EC in non-HIV patients was approximately 9% in the US from 2015-2020. Prior organ transplant, proton pump inhibitors, and corticosteroids were identified as independent risk factors for EC.
Core Tip: While esophageal candidiasis (EC) is often associated with human immunodeficiency virus (HIV), the prevalence and clinical risk factors for infection in the non-HIV population are less well established. Our study found the prevalence of EC among patients without HIV in the United States to be higher than anticipated, approximately 9%, over a 5-year period. Independent risk factors for infection were prior organ transplant, proton pump inhibitor, or corticosteroid use. The findings of this study may aide clinicians in establishing an early diagnosis and treatment of EC, thereby preventing the later complications of more severe disease.
- Citation: Kimchy AV, Ahmad AI, Tully L, Lester C, Sanghavi K, Jennings JJ. Prevalence and clinical risk factors for esophageal candidiasis in non-human immunodeficiency virus patients: A multicenter retrospective case-control study. World J Gastrointest Endosc 2023; 15(6): 480-490
- URL: https://www.wjgnet.com/1948-5190/full/v15/i6/480.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i6.480
Infectious esophagitis is well known to occur in immunocompromised patients particularly those with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS). Candida is the most common pathogen isolated in cases of infectious esophagitis with the predominant species being Candida albicans[1]. Classic symptoms reported in patients with esophageal candidiasis (EC) include odynophagia, dysphagia, and chest discomfort[2]. Upper endoscopy plays a critical role in the evaluation of EC. It allows for direct visualization of the esophageal mucosa and may reveal the patchy white plaques most often described in cases of EC. In addition, brush or tissue biopsies of the esophagus have demonstrated the highest sensitivity and specificity for EC[3]. Cytology or histopathology findings of budding yeasts and pseudohyphae or hyphae with invasion of the epithelium provide confirmation of the diagnosis[1].
Persistent EC may result in severe complications such as esophageal hemorrhage, perforation, or strictures; therefore, early detection and eradication of infection are important to improve patient outcomes[4]. Systemic therapy is recommended for treatment of EC in immunosuppressed individuals as opposed to the topical agents used for oropharyngeal disease. Fluconazole is the standard therapy indicated for EC while other medications including echinocandins and amphotericin are typically reserved for refractory disease[1]. The treatment of EC in non-HIV patients does not differ for those who are symptomatic; however, guidelines are not well established for treatment of patients without symptoms[1,5].
While EC has most often been associated with HIV, a recent study showed a decreasing prevalence of EC among patients with HIV. This decline was attributed to the rise in the use of highly active antiretroviral therapy. In addition, there was an increase in the prevalence of EC among patients without HIV[4]. Although EC can develop in individuals without HIV, studies investigating clinical risk factors for infection in this patient population have been far less to date[1]. The current literature suggests that the use of corticosteroids may increase the risk of infection; however, there has been a lack of consensus about other risk factors including proton pump inhibitor use (PPI)[2,4,6-10].
It is important to identify patients at risk for EC who may benefit from early diagnosis and treatment with antimicrobial agents and avoid costly, invasive interventions in those without predisposing factors[4]. The aim of this study was to determine the prevalence of EC in patients without HIV and identify common clinical presentations and risk factors for EC in this patient population.
This retrospective case-control study received approval from the MedStar Health-Georgetown University Institutional Review Board. We retrospectively reviewed inpatient and outpatient encounters from 5 hospitals located in the District of Columbia and Maryland regions of the United States (US) from January 2015 through December 2020. All hospitals were a part of MedStar Health, a non-profit regional healthcare system. The hospitals included 2 academic tertiary care centers, Georgetown University Hospital and Washington Hospital Center, and 3 community hospitals, Franklin Square Medical Center, Good Samaritan Hospital, and Union Memorial Hospital.
The International Classification of Diseases, Ninth and Tenth Revisions were used to identify patients who had endoscopic biopsies of the esophagus (ICD-9-CM 42.24 and ICD-10-PCS 0DB58ZX, 0DB18ZX, 0DB28ZX, 0DB38ZX). Patients with a diagnosis of HIV/AIDS (ICD-9-CM 042 or ICD-10-CM B20) were excluded. In addition, vulnerable populations such as minors (age less than 18 years old), pregnant women, and prisoners were excluded. Cases of EC were then identified using the diagnostic codes ICD-9-CM 112.84 and ICD-10-CM B37.8. The diagnosis of EC was confirmed with cytology or histopathology from brush or tissue biopsies of the esophagus. Patients without pathology results confirming the diagnosis of EC were excluded. The control group was formed by patients who had endoscopic biopsies of the esophagus and did not have a diagnosis of EC that were propensity matched in a 1 to 1 ratio to cases of EC using age, gender, and encounter type (inpatient vs outpatient).
Patient demographics, symptoms, diagnoses, medications, and laboratory data were obtained from the documented outpatient or inpatient encounter preceding the upper endoscopy. The endoscopy, cytology, and histopathology findings were extracted from reports documented in patient charts. Immunosuppressive medications included the use of chemotherapy, immunomodulators, calcineurin inhibitors, or biological therapies. Medications listed in this study consisted of inhaled, oral, or intravenous formulations. Only patients with complete medical history information were included.
The prevalence of EC was defined as the number of patients with a confirmed diagnosis of EC out of all patients who had endoscopic biopsies of the esophagus obtained during the study period. We summarized the distribution of categorical and continuous variables using frequencies and percentages, and medians with interquartile ranges, respectively. We compared differences in medians for continuous variables using the Kruskal-Wallis test. Differences in categorical variables were evaluated using chi-square analyses. Multivariable logistic regression analyses were performed using EC as the outcome variable, which included adjustments for age, gender, race or ethnicity, body mass index (BMI), heavy alcohol use, gastroesophageal reflux disease (GERD), history of organ transplant, and use of medications such as immunosuppressive agents, PPI, corticosteroids, Tylenol, or aspirin. Statistical significance was defined as P value <0.05. The statistical methods of this study were reviewed by Kavya Sanghavi from MedStar Health Research institute.
Of the 1969 patients who had endoscopic biopsies of the esophagus performed from 2015-2020, 295 patients had the diagnosis of EC. There were 118 patients with the diagnosis of EC who were excluded due to a lack of pathology results confirming the diagnosis of EC or insufficient data for review. 177 of 1,969 patients (8.99%) had pathology confirming the diagnosis of EC and were included in the study for data collection and further analysis. The annual incidence of EC in patients who underwent endoscopy with esophageal biopsy ranged from 7.45% to 10.84% with no significant trend observed over the study period (P = 0.58). Patients with EC had a median age of 65 years old and BMI of 24.48 kg/m2. There were 91 females (51.40%) and 86 males (48.60%), and their racial and ethnic backgrounds were as follows: 74 White (41.80%), 90 Black (50.80%), and 13 others (7.30%), which included Hispanic, Latine, Asian, and not identified patients. Amongst the study population, there were 295 matched controls identified with 48 patients excluded due to insufficient data for review. Of the 247 matched controls included in the study, the median age was 63 years old, and the median BMI was 26.29 kg/m2. There were 134 females (54.30%) and 113 males (45.70%), and their racial and ethnic backgrounds were as follows: 127 White (51.40%), 109 Black (44.10%), and 11 others (4.50%). Median age, gender, race, and ethnicity did not significantly differ between EC cases and the controls. However, patients with EC were found to have a significantly lower median BMI than the control group (P = 0.003) (Table 1).
| Risk factor | EC1 patients,n = 177 | Matched controls, n = 247 | P value |
| Age (yr) | 65.00 | 63.17 | 0.637 |
| BMI1 (kg/m2) | 24.48 | 26.29 | 0.003 |
| Gender | 0.564 | ||
| Female | 91 (51.40) | 134 (54.30) | |
| Male | 86 (48.60) | 113 (45.70) | |
| Race and ethnicity | 0.104 | ||
| White | 74 (41.80) | 127 (51.40) | |
| Black | 90 (50.80) | 109 (44.10) | |
| Other1 | 13 (7.30) | 11 (4.50) | |
| Heavy alcohol use | 16 (9.00) | 42 (17.00) | 0.019 |
| Tobacco use | 97 (54.80) | 123 (49.80) | 0.309 |
| GERD1 | 71 (40.10) | 68 (27.50) | 0.006 |
| Diabetes | 63 (35.60) | 71 (28.70) | 0.135 |
| Liver cirrhosis | 9 (5.10) | 7 (2.80) | 0.23 |
| End stage renal disease | 24 (13.60) | 26 (10.60) | 0.347 |
| Transplant recipient | 19 (10.70) | 5 (2.00) | < 0.001 |
| Active Malignancy | 32 (18.10) | 32 (13.00) | 0.146 |
| Immunosuppressive medication use1 | 32 (18.10) | 20 (8.10) | 0.002 |
| TNF inhibitor use1 | 1 (0.60) | 1 (0.40) | 0.812 |
| Proton pump inhibitor use | 85 (48.00) | 74 (30.00) | < 0.001 |
| H2RA use | 21 (11.90) | 30 (12.10) | 0.93 |
| Corticosteroid use | 62 (35.00) | 42 (17.00) | < 0.001 |
| Tylenol use | 45 (25.40) | 40 (16.20) | 0.019 |
| NSAID use1 | 18 (10.20) | 21 (8.50) | 0.558 |
| Aspirin use | 69 (39.00) | 68 (27.50) | 0.013 |
| Bisphosphonate use | 3 (1.70) | 2 (0.80) | 0.405 |
| Antibiotic use | 22 (12.40) | 19 (7.70) | 0.104 |
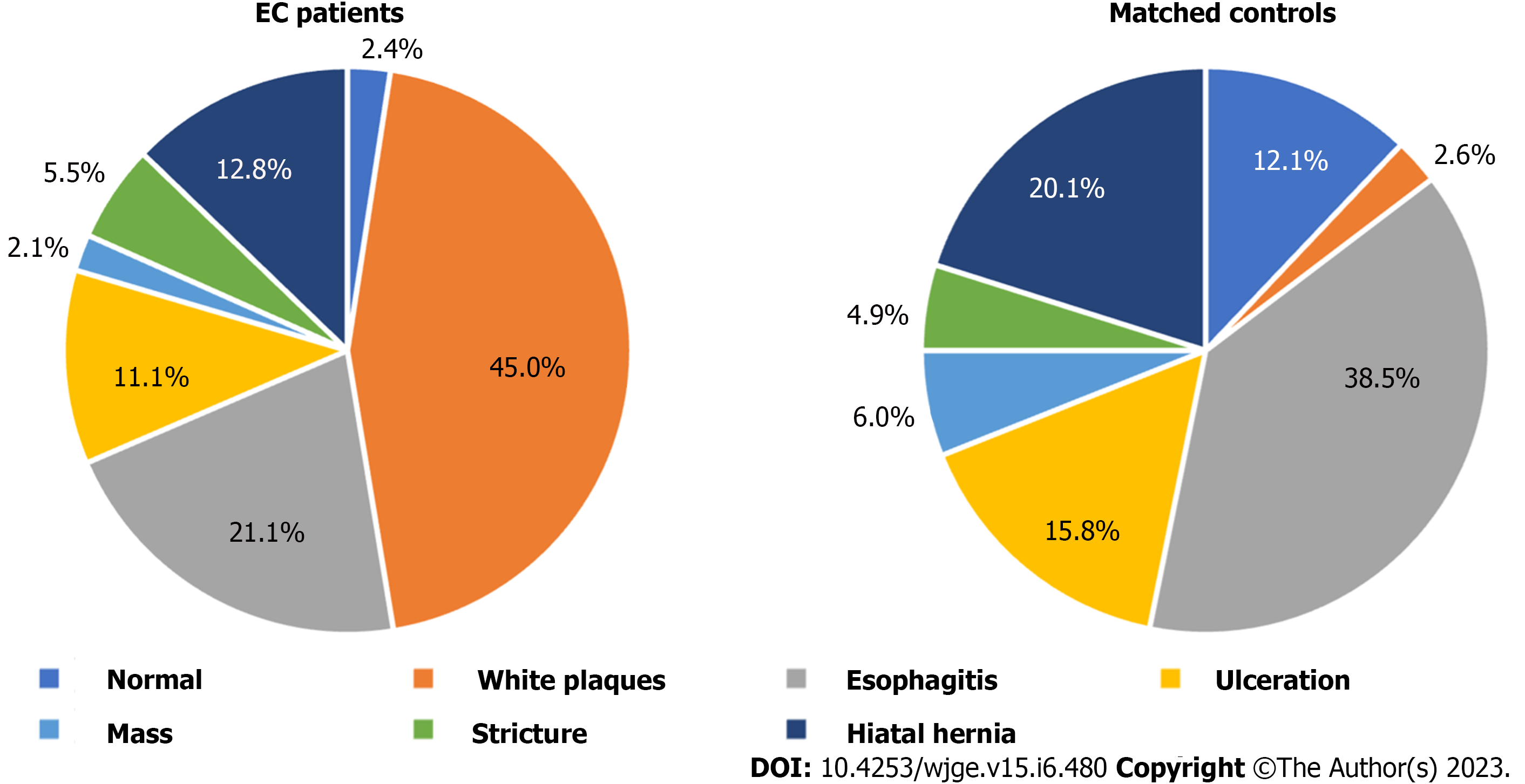
The most common clinical symptoms in patients with EC were dysphagia (16.94%), followed by nausea (14.98%), melena (14.66%), and vomiting (13.68%). Chest pain and odynophagia were present in only 3.91% and 3.26% of EC cases, which was comparable to the rate in patients without EC (4.14% and 2.53%). In the controls, the predominant symptoms were vomiting (18.16%), nausea (17.47%), melena (13.33%), and dysphagia (11.95%) (Table 2). There were few patients with EC who were asymptomatic on presentation representing only 3.58% of cases. Endoscopy findings frequently encountered among patients with EC included white/yellow plaques (44.98%) and esophagitis (21.11%). In 2.08% of EC patients, the esophagus was normal on endoscopy. The controls most often had esophagitis (38.51%), or a hiatal hernia (20.11%) found on endoscopy, with only 2.59% having white/yellow plaques (Figure 1).
| Presenting symptom | EC1 patients, n = 177 | Matched controls, n = 247 |
| Dysphagia | 52 (16.94) | 52 (11.95) |
| Nausea | 46 (14.98) | 76 (17.47) |
| Melena | 45 (14.66) | 58 (13.33) |
| Vomiting | 42 (13.68) | 79 (18.16) |
| Hematemesis | 26 (8.47) | 44 (10.11) |
| Epigastric pain | 21 (6.84) | 22 (5.06) |
| Weight loss | 18 (5.86) | 8 (1.84) |
| Non-specific | 17 (5.54) | 36 (8.28) |
| Abdominal pain | ||
| Diarrhea | 13 (4.23) | 7 (1.61) |
| Chest pain | 12 (3.91) | 18 (4.14) |
| Asymptomatic | 11 (3.58) | 11 (2.53) |
| Odynophagia | 10 (3.26) | 11 (2.53) |
| Dyspepsia | 6 (1.95) | 9 (2.07) |
| Acid reflux | 5 (1.63) | 2 (0.46) |
| Unknown | 0 (0.00) | 2 (0.46) |
In comparison to controls, patients with EC had significantly higher rates of GERD (40.10% vs 27.50%; P = 0.006), prior organ transplant (10.70% vs 2%; P < 0.001), immunosuppressive medication (18.10% vs 8.10%; P = 0.002), PPI (48% vs 30%; P < 0.001), corticosteroid (35% vs 17%; P < 0.001), Tylenol (25.40% vs 16.20%; P = 0.019), and aspirin use (39% vs 27.50%; P = 0.013). Heavy alcohol use was less frequent in patients with EC than those without EC (9% vs 17%; P = 0.19). There was no significant difference in the rates of tobacco use, diabetes, liver cirrhosis, end-stage renal disease, active malignancy, or medication use of tumor necrosis factor inhibitors, histamine type-2 receptor antagonists (H2RAs), non-steroidal anti-inflammatory drugs, and antibiotics between the two groups (Table 1). On multivariable logistic regression analysis, patients with a prior organ transplant had increased odds of EC (OR = 5.81; P = 0.009), as did patients taking a PPI (OR = 1.66; P = 0.03) or corticosteroids (OR = 2.05; P = 0.007). In addition, there was a significant association between patients of other races and ethnicities and EC when Whites were used as the reference group (OR = 2.55; P = 0.041). Patients with GERD or medication use, including immunosuppressive medications, Tylenol, and aspirin, did not have a significantly increased odds of EC (Table 3).
| Risk factor | Odds ratio | P value |
| Gender | ||
| Female | Reference | |
| Male | 1.28 | 0.269 |
| Race and ethnicity | ||
| White | Reference | |
| Black | 1.50 | 0.080 |
| Other1 | 2.55 | 0.041 |
| Age | 1.00 | 0.720 |
| BMI1 | 0.99 | 0.209 |
| Heavy alcohol use | 0.51 | 0.059 |
| GERD1 | 1.60 | 0.051 |
| Transplant recipient | 5.81 | 0.009 |
| Immunosuppressive medication use1 | 0.87 | 0.746 |
| Proton pump inhibitor use | 1.66 | 0.030 |
| Corticosteroid use | 2.05 | 0.007 |
| Tylenol use | 1.57 | 0.092 |
| Aspirin use | 1.45 | 0.115 |
Fluconazole monotherapy was the most common treatment used in patients with EC (77.97%). Other treatments used included nystatin monotherapy (1.69%), echinocandin monotherapy (1.13%), amphotericin monotherapy (0.56%), and fluconazole with nystatin (3.39%), or with echinocandin (2.26%). There were 12.99% of patients with EC who did not receive treatment with anti-fungal therapy. Of the 154 patients with EC who received anti-fungal therapy, 60 had follow-up data available for review. Approximately half of these patients (32/60) experienced symptom resolution after completing the prescribed treatment course.
Additional findings noted in the present study included the presence or absence of oral candidiasis, viral esophagitis, or esophageal carcinoma. Oral candidiasis was present in 5 patients with EC and 2 patients without EC. There was 1 patient with EC who had histopathology demonstrating coinfection with herpes simplex virus. In the controls, 3 patients had histopathology consistent with HSV infection alone. Esophageal squamous cell carcinoma was present on histopathology in 9 patients with EC and 5 patients without EC. There were 6 patients with esophageal adenocarcinoma on histopathology in the controls, while none of the patients with EC had esophageal adenocarcinoma on histopathology.
While EC has long afflicted patients with HIV, its presence among the non-HIV population along with the predisposing conditions for infection are less well known. In our study, the overall prevalence of EC in patients without HIV was approximately 9% with annual incidence ranging from 7.5% to 10.8% with no significant trend observed over the study period from 2015-2020. The prevalence/incidence of EC in our study was higher than rates previously reported in the literature, which ranged from 0.32% to 5.2%[4,8,9,11]. Studies with a lower prevalence/incidence of EC tended to be older and conducted in East Asian territories while a more recent study within the US had a higher overall incidence of 5.2%[4,8,9,11]. This may suggest an increasing prevalence of EC among patients without HIV or a greater predominance of EC within the US population. In addition, the prevalence of EC may have been underestimated in other studies due to methodological limitations such as including only patients undergoing routine health physicals or requiring the presence of white plaques on endoscopy for the diagnosis, which according to our results represents less than half of EC cases[4,8,11].
We found that most patients with EC in our study were elderly with a median age of 65 years old; however, increasing age was not found to be a significant risk factor for EC. Similar ages were reported in other studies ranging from 51 to 65 years. old, but unlike the present study, they demonstrated a significant association between increasing age and EC[4,11,12]. Due to the waning of epithelial cell immunity over time, older age has been thought to increase susceptibility to infections of the esophagus, which may include EC[7]. In our study, BMI was significantly lower in patients with EC (median 24.5) compared to controls (median 26.3), and increasing BMI was not associated with an increased risk of EC. A study by Ogiso et al[2] also found that patients with EC were less likely to have a high BMI. Patients with low BMIs are thought to be at an elevated risk for oropharyngeal candidiasis due to immune dysfunction related to malnutrition; however, further studies are needed to evaluate this relationship specifically in cases of EC[2].
The classic symptoms of infectious esophagitis have been described as dysphagia, odynophagia, and chest pain, although in some cases patients may remain asymptomatic[5]. We found that patients with EC presented most often with dysphagia (16.94%) while odynophagia and chest pain manifested in only a small percentage of patients (3.26% and 3.91%). In addition, there were few cases of EC that were asymptomatic (3.58%). Overall, greater than two thirds of patients with EC presented with non-specific gastrointestinal symptoms other than those typically attributed to infectious esophagitis. Chen et al[11] also reported a low number of EC cases that presented with dysphagia and chest pain (2.1% and 14.9%) and none of the patients had odynophagia. Another study found more than half of patients with EC were asymptomatic on presentation while only 11.7% of patients had dysphagia, chest pain, or odynophagia[8]. These findings suggest that EC may be discovered in non-HIV patients who have non-specific upper gastrointestinal symptoms, which may be attributed to EC or another co-existing gastrointestinal condition with asymptomatic infection. Mimidis et al[12] proposed that there could be a relationship between the presenting symptom of EC and the predisposing factor for infection. The authors found that corticosteroid and acid suppressor use correlated with chest discomfort on presentation. While odynophagia was common in patients with malignancy, dysphagia was more often found in those with motility disorders. Clinicians should remain vigilant for EC in those with risk factors for infection but without the classic symptoms associated with infectious esophagitis as atypical presentations are more common in non-HIV patients.
In patients with HIV, oral candidiasis often occurs concurrently with EC; however, this manifestation has not been fully evaluated in the non-HIV population[7]. It is thought that oral immunity is impaired early in the course of HIV infection due to salivary gland dysfunction allowing for the overgrowth of yeast[4]. One study conducted in a non-HIV patient population reported no cases of oral candidiasis among those diagnosed with EC[6]. In another study by Chocarro Martínez et al[13], there were only 3.9% of patients with EC who also had findings of oral candidiasis. In the present study, oral candidiasis was found in 2.8% of EC cases among patients without HIV. The lack of simultaneous oropharyngeal disease in non-HIV patients suggests that impaired oral immunity may not be required for the pathogenesis of EC in this patient population.
The usual endoscopic characteristics reported in cases of EC include creamy white plaques early in the disease course with later progression to friable mucosa with ulcerations and strictures[12]. In the HIV population, increased severity of esophageal infection has been shown to correlate with lower CD4 counts[12]. We found in our non-HIV population that nearly half of patients with EC had white/yellow plaques present on endoscopy. Fewer patients had findings of more severe disease such as esophagitis, ulcerations, or strictures and about 2% had a normal esophagus on endoscopy. In similar studies by Nassar et al[7] and Alsomali et al[9], most patients with EC had white plaques present on endoscopy with a limited number showing signs of severe infection and approximately 3%-5% had a normal esophagus. These findings indicate that EC may present with milder disease on endoscopic evaluation in non-HIV patients and in some cases without white plaques or with a normal esophagus, thus requiring biopsy for histopathologic diagnosis.
The present study showed that PPI use was a significant risk factor for the development of EC in patients without HIV. This association was not observed in patients with H2RA use, which may be attributed to the greater acid suppression achieved by PPIs. It has been proposed that hypochlorhydria may permit colonization of the stomach by oral cavity yeasts thereby increasing the risk for esophageal infection[12]. While GERD was more common in patients with EC than those without EC, we found that GERD was not associated with an increased risk of EC. This suggests that the acid suppression from PPIs is likely responsible for the observed association with EC rather than the disease this medication is used to treat. These findings are supported by the results of other studies in the current literature. Chocarro et al[13] also demonstrated a significant relationship between omeprazole use and EC, which was not seen with H2RA use. In a large retrospective cohort study, three-quarters of patients with EC were found to be taking a PPI at the time of diagnosis. In addition, there was a subset of 15 patients who had endoscopy confirmed EC following initiation or dose increase of PPI therapy[7]. Although there were two studies in our search of the literature that did not find a significant relationship between PPI use and EC, the present study adds to those that have identified PPIs as an independent risk factor for EC in patients without HIV[4,8].
We found that corticosteroid use significantly increased the risk of EC in patients without HIV. This was consistent with the results of prior studies that also demonstrated an association between corticosteroid use and the development of EC[6,8,11,13]. One large retrospective cohort study found that a higher-prednisone equivalent dose further increased the risk of EC, which they attributed to the greater impairment in cellular immunity achieved with higher doses of steroids[4]. The increased risk of EC with corticosteroid use is likely related to the suppression of immune cell function systemically or within the esophagus with swallowing of inhaled corticosteroids[12]; however, additional studies are needed to elucidate the risk of EC with systemic compared to inhaled formulations.
Fungal infections are known to be a significant cause of morbidity and mortality in solid organ transplant recipients. Candida is the most frequently isolated pathogen in this patient population with the greatest risk of infection early in the post-transplant period. Mucosal infections are common as seen with the increased frequency of oral candidiasis in these patients[14]. The susceptibility to fungal infections observed in transplant recipients has been attributed to the use of high-dose steroids, episodes of rejection, hyperglycemia, leukopenia, and old age[15]. While cases of EC have been reported in the literature, the association between EC and history of prior organ transplant has not been fully elucidated. A retrospective review of renal transplant recipients hospitalized for fungal infections in the US found that Candida was responsible for over 80% of infections with the esophagus being the most frequent site in 31.9% of cases. Of note, HIV status was not reported for the patients included in this study[16]. In our study, history of prior organ transplant was a significant risk factor for the development of EC independent of using corticosteroids or immunosuppressive medications, which included chemotherapeutics, immunomodulators, calcineurin inhibitors, and biologic agents.
Unlike in oropharyngeal disease, EC requires treatment with systemic rather than local antifungal therapy. Fluconazole is considered the first-line therapy for EC with dosages ranging from 200 to 400 mg daily for a total of 2 to 3 wk. Treatment failure has been observed in cases of fluconazole resistance, which is often related to the frequency and duration of prior therapy and the emergence of Candida species with greater azole resistance[5]. Fluconazole was the treatment of choice for most patients with EC in our study as few patients received treatment with other anti-fungal therapies. Interestingly, only about half of patients with EC experienced resolution of symptoms with anti-fungal treatment. Another study also reported fluconazole as the predominant therapy used for treatment of EC. In patients who had a follow-up endoscopy, approximately 24% had persistence of Candida infection and half of these patients had received anti-fungal treatment[8]. Given these findings, the prevailing Candida species and fluconazole resistance rates in patients without HIV may differ from those with HIV and further investigation is needed to guide future treatment recommendations.
Our results were subject to limitations of the retrospective observational study design. EC cases were identified from inpatient and outpatient encounters at hospital facilities, which did not include ambulatory surgery centers, and follow-up data was limited. As patients had various indications for endoscopy, it is unclear whether the presence of candida on histopathology was consistent with a clinically significant infection. A major strength of this study was the patient population, which included both inpatient and outpatient encounters as well as age, gender, and encounter-matched controls. In addition, the data was collected over the more recent years from multiple hospitals with differing levels of care, thus limiting referral bias. Patients with an established diagnosis of HIV were excluded, which has been performed in few studies of EC. We also confirmed that patients with a diagnosis of EC had histopathology demonstrating the presence of Candida.
In conclusion, this study determined the prevalence of EC in the non-HIV patient population to be approximately 9% from 2015 through 2020. EC should remain on the differential in patients with risk factors for infection presenting with non-specific gastrointestinal complaints as the classic symptoms of infectious esophagitis are less common in patients without HIV. Clinicians may consider esophageal biopsy in patients with risk factors for EC presenting with typical or atypical symptoms and a normal esophagus on endoscopy for histopathologic diagnosis. However, further studies are needed in patients with a histopathologic diagnosis of EC who are asymptomatic and have normal findings on endoscopy to determine if treatment is required for eradication of infection. Identification of high-risk patients is critical for the early diagnosis and treatment of EC to prevent later complications with progression to more severe disease. In our retrospective case-control study, prior organ transplant, PPIs, and corticosteroids were identified as independent risk factors for the development of EC in patients without HIV.
Infectious esophagitis is well known to occur in immunocompromised patients particularly those with human immunodeficiency virus with Candida being the most common pathogen isolated.
While esophageal candidiasis (EC) has most often been associated with human immunodeficiency virus (HIV), a recent study showed a decreasing prevalence of EC among patients with HIV and an increase in the prevalence of EC among patients without HIV. Although EC can develop in individuals without HIV, studies investigating clinical risk factors for infection in this patient population have been far less to date. We designed this study to determine the prevalence, clinical manifestations, and risk factors for EC in a non-HIV patient population.
The aim of this study was to determine the prevalence of EC in patients without HIV and identify common clinical presentations and risk factors for EC in this patient population.
This retrospective case-control study encompassed inpatient and outpatient encounters from 5 hospitals located in the District of Columbia and Maryland regions of the United States. Cases of EC were identified among patients who had endoscopic biopsies of the esophagus and the presence of EC on cytology and/or histopathology. Patients with HIV were excluded. Multivariable logistic regression was used to identify independent risk factors for EC, after adjusting for potential confounding factors.
This study determined the prevalence of EC in the non-HIV patient population to be approximately 9% from 2015 through 2020. We found that patients with EC presented most often with non-specific gastrointestinal complaints while odynophagia and chest pain manifested in only a small percentage of patients. Less than half of patients with EC had white/yellow plaques present on endoscopy. Prior organ transplant, proton pump inhibitors, and corticosteroids were identified as independent risk factors for EC.
The prevalence of EC in our study was higher than expected based upon rates reported in prior studies. Classic symptoms of infectious esophagitis are less common in patients without HIV. Clinicians may consider esophageal biopsy for histopathologic diagnosis in patients with risk factors for EC presenting with atypical symptoms and/or absence of white plaques on endoscopy. Significant risk factors for infection in our study were a history of organ transplant, proton pump inhibitor, or corticosteroids use.
Further studies are needed to evaluate for an increasing prevalence of EC and risk factors for infection in the non-HIV patient population.
We would like to acknowledge the biostatistical support provided by the MedStar Health Research Institute.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy; American College of Gastroenterology; American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Glumac S, Croatia; Meena DS, India; Sachdeva S, India S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Hoversten P, Kamboj AK, Katzka DA. Infections of the esophagus: an update on risk factors, diagnosis, and management. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ogiso H, Adachi S, Mabuchi M, Horibe Y, Ohno T, Suzuki Y, Yamauchi O, Kojima T, Takada E, Iwama M, Saito K, Iwashita T, Ibuka T, Yasuda I, Shimizu M. Risk factors for the development of esophageal candidiasis among patients in community hospital. Sci Rep. 2021;11:20663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 3. | Underwood JA, Williams JW, Keate RF. Clinical findings and risk factors for Candida esophagitis in outpatients. Dis Esophagus. 2003;16:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Takahashi Y, Nagata N, Shimbo T, Nishijima T, Watanabe K, Aoki T, Sekine K, Okubo H, Sakurai T, Yokoi C, Kobayakawa M, Yazaki H, Teruya K, Gatanaga H, Kikuchi Y, Mine S, Igari T, Takahashi Y, Mimori A, Oka S, Akiyama J, Uemura N. Long-Term Trends in Esophageal Candidiasis Prevalence and Associated Risk Factors with or without HIV Infection: Lessons from an Endoscopic Study of 80,219 Patients. PLoS One. 2015;10:e0133589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Ahuja NK, Clarke JO. Evaluation and Management of Infectious Esophagitis in Immunocompromised and Immunocompetent Individuals. Curr Treat Options Gastroenterol. 2016;14:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Yakoob J, Jafri W, Abid S, Jafri N, Islam M, Hamid S, Shah HA, Hussainy AS. Candida esophagitis: risk factors in non-HIV population in Pakistan. World J Gastroenterol. 2003;9:2328-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Nassar Y, Eljabbour T, Lee H, Batool A. Possible Risk Factors for Candida Esophagitis in Immunocompetent Individuals. Gastroenterology Res. 2018;11:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Choi JH, Lee CG, Lim YJ, Kang HW, Lim CY, Choi JS. Prevalence and risk factors of esophageal candidiasis in healthy individuals: a single center experience in Korea. Yonsei Med J. 2013;54:160-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Alsomali MI, Arnold MA, Frankel WL, Graham RP, Hart PA, Lam-Himlin DM, Naini BV, Voltaggio L, Arnold CA. Challenges to "Classic" Esophageal Candidiasis: Looks Are Usually Deceiving. Am J Clin Pathol. 2017;147:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Hoversten P, Otaki F, Katzka DA. Course of Esophageal Candidiasis and Outcomes of Patients at a Single Center. Clin Gastroenterol Hepatol. 2019;17:200-202.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Chen YH, Jao TM, Shiue YL, Feng IJ, Hsu PI. Prevalence and risk factors for Candida esophagitis among human immunodeficiency virus-negative individuals. World J Clin Cases. 2022;10:10896-10905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mimidis K, Papadopoulos V, Margaritis V, Thomopoulos K, Gatopoulou A, Nikolopoulou V, Kartalis G. Predisposing factors and clinical symptoms in HIV-negative patients with Candida oesophagitis: are they always present? Int J Clin Pract. 2005;59:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Chocarro Martínez A, Galindo Tobal F, Ruiz-Irastorza G, González López A, Alvarez Navia F, Ochoa Sangrador C, Martín Arribas MI. Risk factors for esophageal candidiasis. Eur J Clin Microbiol Infect Dis. 2000;19:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007;45:305-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:2058-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Abbott KC, Hypolite I, Poropatich RK, Hshieh P, Cruess D, Hawkes CA, Agodoa LY, Keller RA. Hospitalizations for fungal infections after renal transplantation in the United States. Transpl Infect Dis. 2001;3:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |