Published online Jun 16, 2023. doi: 10.4253/wjge.v15.i6.447
Peer-review started: February 24, 2023
First decision: March 24, 2023
Revised: April 8, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 16, 2023
Processing time: 110 Days and 5.2 Hours
Endoscopic ultrasound (EUS) stands as an accurate imaging modality for esop
To display the role of EUS in pre-intervention early esophageal cancer staging and how the index endoscopic features of invasive esophageal malignancy compare for prediction of depth of invasion and cancer management.
This was a retrospective study of patients who underwent pre-resection EUS after a diagnosis of esophageal cancer at a tertiary medical center from 2012 to 2022. Patient clinical data, initial esophagogastroduodenoscopy/biopsy, EUS, and final resection pathology reports were abstracted, and statistical analysis was conducted to assess the role of EUS in management decisions.
Forty nine patients were identified for this study. EUS T stage was concordant with histological T stage in 75.5% of patients. In determining submucosal involvement (T1a vs T1b), EUS had a specificity of 85.0%, sensitivity of 53.9%, and accuracy of 72.7%. Endoscopic features of tumor size > 2 cm and the presence of esophageal ulceration were significantly associated with deep invasion of cancer on histology. EUS affected management from endoscopic mucosal resecti
EUS was reasonably specific in ruling out submucosal invasion but had relatively poor sensitivity. Data validated endoscopic indicators suggested superficial cancers in the group with a tumor size < 2 cm and the lack of esophageal ulceration. In patients with these findings, EUS rarely identified a deep cancer that warranted a change in management.
Core Tip: This study aims to convey the role of endoscopic ultrasound (EUS) for early esophageal cancer considered for endoscopic or surgical resection and how the index endoscopic features of esophageal malignancy compare for prediction of depth of invasion and cancer management. This was a retrospective study of 49 patients who underwent pre-resection EUS after diagnosis of esophageal cancer. EUS was reasonably specific in ruling out submucosal invasion but had relatively poor sensitivity. Data validated endoscopic features suggesting superficial cancers including a tumor size < 2 cm and the lack of esophageal ulceration. In patients with these findings, EUS rarely identified a deep cancer that warranted a change in management.
- Citation: Kahlon S, Aamar A, Butt Z, Urayama S. Role of endoscopic ultrasound for pre-intervention evaluation in early esophageal cancer. World J Gastrointest Endosc 2023; 15(6): 447-457
- URL: https://www.wjgnet.com/1948-5190/full/v15/i6/447.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i6.447
Esophageal cancer is the eighth-most common cancer and sixth-most common cause of mortality globally[1]. In the United States, an estimated 20,640 cases of esophageal cancer are diagnosed in 2022, and 16,410 deaths are expected from the disease, highlighting the importance of its diagnosis and treatment[2,3].
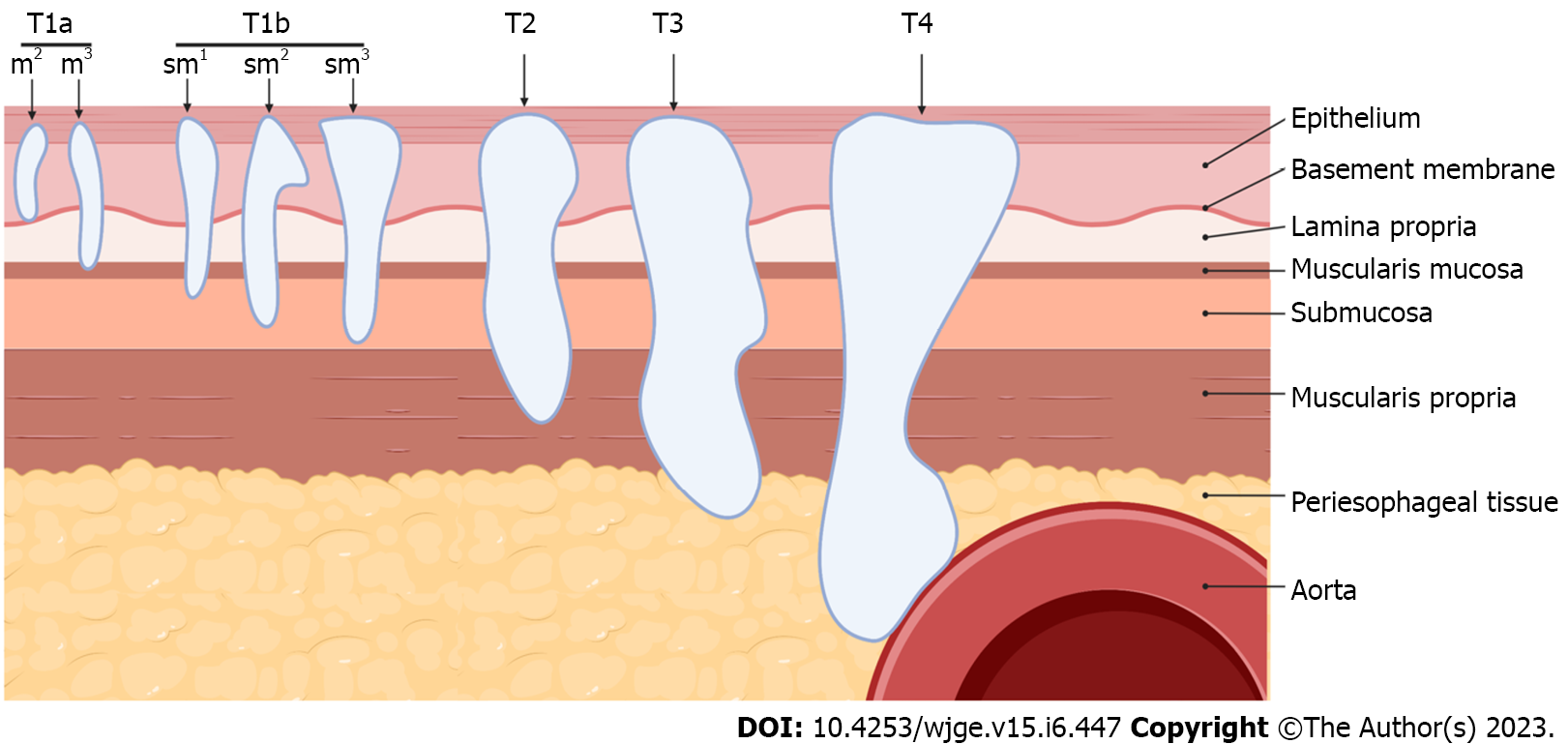
With the advent of less invasive interventions including endoscopic mucosal resection (EMR) or submucosal dissection (ESD) for superficial cancers, accurate clinical staging of esophageal cancer becomes critical in selecting appropriate treatment options[1]. Pre-intervention tumor depth staging (T staging) is vital in assessing which patients without an evidence of metastasis, would benefit from endoscopic or surgical intervention. Tumors limited to mucosa can be completely resected with endoscopic therapy due to lower risk of incomplete resection or lympho-vascular invasion3. NCCN guideline recommends endoscopic resection in the management of T1a lesions and superficial T1b lesions, or T1b-sm1 lesions that superficially invade the submucosa[4]. Tumors staged T1b-sm2 or sm3 have significant risk for recurrence and warrant evaluation for esophagectomy[5].
Endoscopic ultrasound (EUS) has been commonly utilized as the most accurate imaging study for staging primary esophageal cancer in comparison to other modalities[4]. Specifically, EUS has been shown to accurately assess T staging in the cancer (73.2%-80.6%), excelling in distinguishing T3/T4 Lesions from T1/T2[6-9]. EUS remains a key component of locoregional assessment to determine the depth of invasion and nodal involvement while also allowing the possibility of fine-needle aspiration sampling[10]. Classifying more superficial lesions into T1a, T1b-sm1, T1b-sm2, or T1b-sm3 lesions, however, has proven difficult via EUS[11]. Currently for superficial cancers, EUS is readily combined with EMR or ESD to optimize the clinical management. Specifically, EUS allows exclusion of the presence of a deeper cancer invasion, which makes an EMR or ESD potentially unsafe and/or lead to an incomplete intervention.
There are several studies delineating the correlation of endoscopic and biopsy assessments as evidence for deeper invasion in esophagus cancer in lieu of EUS[12-15]. These suggest that EUS may not provide additional information in situations where endoscopic or pathologic parameters sufficiently characterize esophageal cancers and fully dictate management. Thus, controversy remains in the utility of EUS in patients who have suspected early-stage esophageal cancer and how it can affect management. Current study aims to display the role of EUS for early esophageal cancer staging and how the index endoscopic indicators of invasive esophageal malignancy compare for assessment of depth of invasion and the cancer management.
This was a retrospective study of patients who underwent pre-intervention EUS with a diagnosis of esophageal cancer between January 2012 to January 2022 at a tertiary medical center. This study period was used to minimize the effect of incomplete data allocation from the period prior to establishment of electronic medical record. This study was approved on November 1, 2021 by the Institutional Review Board of the hospital in accordance with its ethical standards and assigned IRB protocol number 1816393-1.
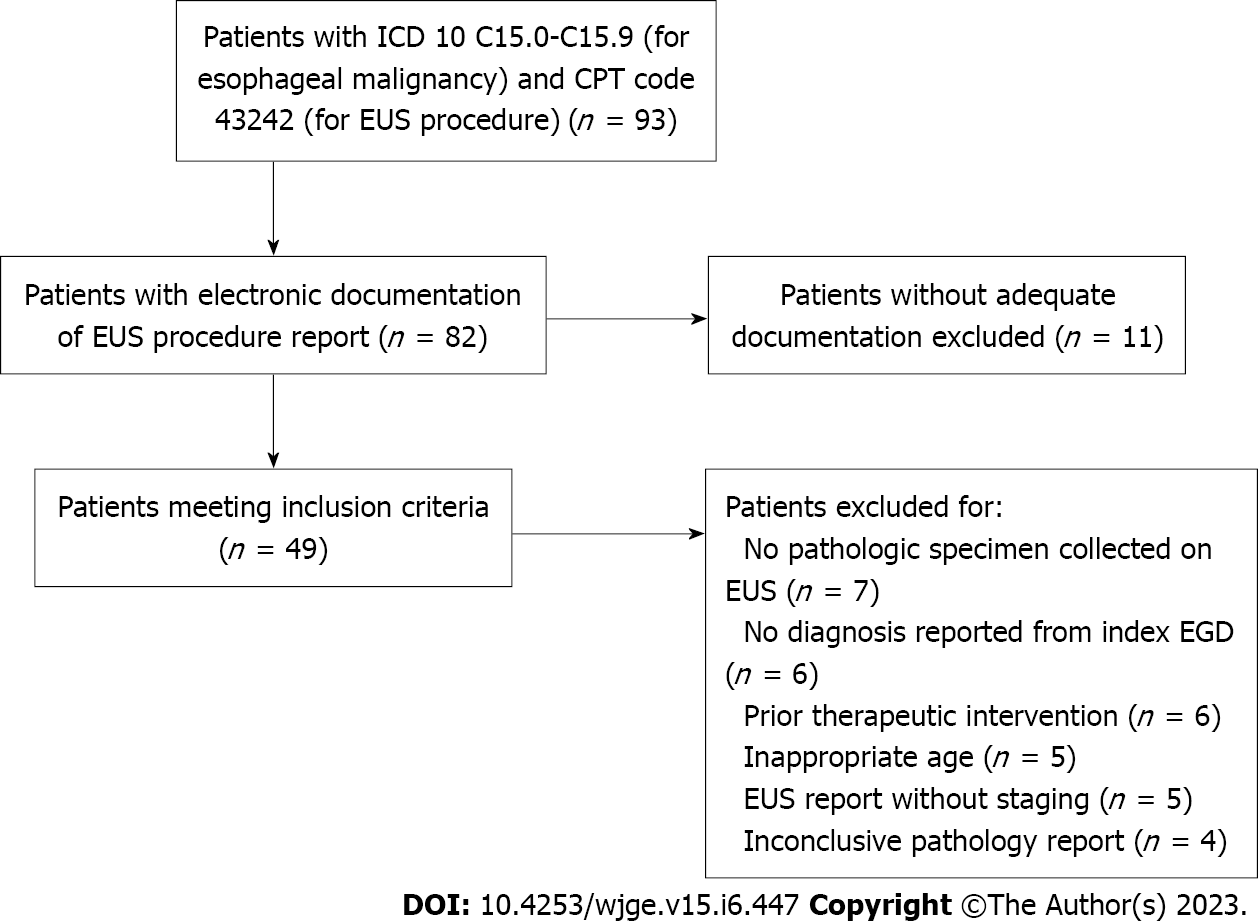
Ninety three patients were identified via EMR search conducted with assistance from the Clinical and Translational Science Center at University of California, Davis. The search was conducted at for patients with ICD-10 codes C15.0 to C15.9 logged for esophageal malignancies in their medical record and those with Current Procedural Terminology code 43242 logged for EUS procedures during the study period at our medical center. Patient’s without electronic documentation of EUS procedure reports were excluded from the study. From this population, patients were ascertained who met the inclusion criteria of age over 18 years, established diagnosis from biopsies collected during index esophagogastroduodenoscopy (EGD), EUS conducted prior to any therapeutic intervention such as endoscopic/surgical resection. Exclusion criteria included: EUS was not conducted prior to any therapeutic interventions, EUS did not indicate staging, EUS did not yield a pathologic specimen, and patients treated with neoadjuvant treatment before esophagectomy. Forty nine patients met criteria for analysis. This is summarized in Figure 1.
EUS was performed with an Olympus radial echoendoscope (GF-UE160, Olympus America, Penn Valley, United States). EGD and EUS procedures were performed by a single endosonographer with over 10 years of experience at the beginning of the study period. Pre-operative T staging was made in accordance with TNM staging system for esophageal cancer with the 8th edition of the American Joint Committee on Cancer (AJCC) classifications for staging of epithelial cancers of the esophagus and esophagogastric junction[16]. As this classification was updated in 2017 to differentiate T1a from T1b lesions, cases conducted prior to 2017 were staged in this study per the updated criteria based on findings present in EUS and pathology reports[17]. The level of tumor invasion was consistently described in both types of reports, allowing for pre-2017 to be classified using the 8th edition TNM staging. Descriptions of submucosal invasion as “irregularities between the mucosal and submucosal border” were used to determine T1b or beyond staging in written reports. The presence of notable para-esophageal lymph nodes on EUS was also denoted in reports including comments regarding diagnostic value. Pathologic diagnosis was determined by pathologists’ interpretation of tissue sample taken during endoscopy either by EMR, ESD, esophagectomy or forceps biopsy. For the purposes of this study, deep invasion (DI) was defined as a T2 lesion or more (Figure 2).
Patient characteristics and clinical data were extracted from chart review including birth date, sex, ethnicity, type of esophageal cancer (adenocarcinoma, squamous cell carcinoma or other cancer), diagnosis of Barrett’s esophagus. EGD/EUS written procedure reports were used to extract data for the following characteristics: Presence of esophageal ulceration, size of tumor, presence of notable para-esophageal lymph nodes, and T staging per EUS. If unavailable in the EUS report, the presence of ulceration and size of tumor was reported via an initial EGD report if done less than 3 mo prior to EUS procedure date. Either biopsy or resection method after EUS was recorded as well. Data from pathology after EMR, ESD, esophagectomy, or forceps biopsy included size and grade of tumor, lateral and deep margins status, the presence of lympho-vascular invasion, and TNM-staging identified on the specimen.
IBM SPSS Statistics 20 was used for all statistical analysis. Frequencies and percentages were calculated for all nominal and ordinal variables. Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value and Accuracy of EUS in identifying sub-mucosal invasion in histological verified T1 tumors were calculated. Moreover, DI of tumor on histology (defined as T2 or beyond) and clinical characteristics significantly associated with DI were identified by using chi-square test. P value < 0.05 was considered significant for all comparisons.
A total of 49 patients were identified for the study. Table 1 summarizes the demographics and clinical characteristics of all patients. Majority of them were males and white, 85.7% and 87.5%, respectively. Adenocarcinoma was the predominant type of cancer (89.8%) among all patients. Prior diagnosis of Barrett’s esophagus was present in 65.3% patients. 30.6% of patients were noted to have esophageal ulceration during endoscopy. 39.6% of the patients had tumor size of > 2 cm on visual inspection. EUS identified non-diagnostic lymphadenopathy in 50% of patients, of which none had reported findings for diagnostic lymph node assessment per EUS criteria (i.e. size, shape, border, echogencity)[18]. On EUS, 48.9%, 20.4%, 8.2% and 22.4% of patients had T1a, T1b, T2 and T3 tumors, respectively. Subsequently, patients underwent EMR (51%), ESD (10.2%), esophagectomy (30.6%), and diagnostic biopsy (8.2%). On histological examination, 40.8%, 26.5%, 10.2% and 22.4% of patients had T1a, T1b, T2 and T3 tumors. Lympho-vascular invasion was found in 24.4% of all patients.
| Variable | Number/Total (n/N) | Percentage (%) | |
| Gender | Males | 42/49 | 85.7 |
| Ethnicity | Caucasian | 42/48 | 87.5 |
| Hispanic | 1/48 | 2.1 | |
| Asian | 5/48 | 10.4 | |
| Type of cancer | Adenocarcinoma | 44/49 | 89.8 |
| SCC | 5/49 | 10.2 | |
| Degree of differentiation | Invasive well differentiated | 18/39 | 46.2 |
| Invasive moderately differentiated | 19/39 | 48.7 | |
| Invasive poorly differentiated | 4/39 | 10.3 | |
| History of Barrett’s esophagus | Yes | 32/49 | 65.3 |
| Esophageal ulceration | Yes | 15/49 | 30.6 |
| Tumor size | < 1 cm | 6/48 | 12.5 |
| 1 – < 1.5 cm | 12/48 | 25 | |
| ≥ 1.5 - < 2 cm | 11/48 | 22.9 | |
| ≥ 2 cm | 19/48 | 39.6 | |
| Lymphadenopathy | Yes (only non-diagnostic EUS features) | 24/48 | 50.0 |
| EUS stage | T1a | 24/49 | 48.9 |
| T1b | 10/49 | 20.4 | |
| T2 | 4/49 | 8.2 | |
| T3 | 11/49 | 22.4 | |
| T4 | 0/49 | 0 | |
| Specimen collection method | Biopsy | 4/49 | 8.2 |
| EMR | 25/49 | 51.0 | |
| ESD | 5/49 | 10.2 | |
| Esophagectomy | 15/49 | 30.6 | |
| Lympho-vascular invasion | Yes | 12/49 | 24.4 |
| Pathological staging | T1a | 20/49 | 40.8 |
| T1b | 13/49 | 26.5 | |
| T2 | 5/49 | 10.2 | |
| T3 | 11/49 | 22.4 | |
| Tumor recurrence | Yes | 5/44 | 11.4 |
Table 2 summarizes T stages on EUS against the stage found on final histology. Among all patients with histological T1a (n = 20), 85.0% were correctly labeled as T1a by EUS (17/20), while 15.0% (3/20) were labeled as T1b. Among 13 histologically verified T1b patients, only 46.2% (6/13) were correctly identified as T1b on EUS. Similarly, among 5 T2 patients, only 3 were correctly identified as T2 by EUS. All 11 T3 patients were correctly identified as T3 by EUS. Overall EUS T stage was concordant with histological T stage in 75.5% of patients (37/49).
| Pathologic stage | ||||||
| T1a, N = 20 | T1b, N = 13 | T2, N = 5 | T3, N = 11 | |||
| EUS stage | T1a | n/N (%) | 17/20 (85.7) | 6/13 (46.2) | 1/5 (20) | 0/11 (0) |
| T1b | n/N (%) | 3/20 (14.2) | 6/13 (46.2) | 1/5 (20) | 0/11 (0) | |
| T2 | n/N (%) | 0/20 (0) | 1/13 (7.7) | 3/5 (60) | 0/11 (0) | |
| T3 | n/N (%) | 0/20 (0) | 0/13 (0) | 0/5 (0) | 11/11 (100) | |
Among these cancer patients, 33 out of 49, had either T1a or T1b cancer on histology. Table 3 shows sensitivity, specificity, PPV, NPV, and diagnostic accuracy of EUS in identifying sub-mucosal invasion (T1b) in T1 Cancers. Although EUS was reasonably specific in ruling out sub-mucosal invasion when it was not present (85.0%), it had a poor sensitivity to identify sub-mucosal invasion when it truly was present (53.9%). EUS had an overall accuracy of 72.7% in identifying sub-mucosal invasion in T1 cancers.
| Submucosal invasion on path | ||||||||
| Yes (T1b), N = 13 | No (T1a), N = 20 | Sensitivity | Specificity | PPV | NPV | Accuracy | ||
| Submucosal invasion on EUS | Yes | 7 | 3 | 53.9% | 85.0% | 70% | 73.9% | 72.7% |
| No | 6 | 17 | ||||||
DI of tumor on histology was defined as T2 or beyond and endoscopic characteristics significantly associated with DI are depicted in Table 4. Proportions of patients with DI having the significant endoscopic parameters were compared to patients without DI. Tumor size ≥ 2 cm on visual inspection was significantly associated with DI of cancer on histology. 50% of DI cancers and 21.2% of superficial cancers had ulceration on EGD. Similarily, pathologic factors associated with DI are also noted. As the tumors’ degree of differentiation went from well- to poor-, likelihood of DI also significantly increased (P = 0.0392).
| Deep invasion on pathology | Endoscopic parameter | |||
| Tumor size ≥ 2 cm on visual inspection | Presence of esophageal ulceration | Tumor size ≥ 2 cm on visual inspection & presence of esophageal ulceration | ||
| Yes (T2 and beyond) | n1/N1 (%) | 13/16 (81.2) | 8/16 (50.0) | 7/16 (43.8) |
| No (T1a and T1b) | n2/N2 (%) | 6/32 (18.8) | 7/33 (21.2) | 2/33 (6.1) |
| P valuea | < 0.001 | 0.0403 | 0.0014 | |
| Deep invasion on pathology | Degree of differentiation on pathology | |||
| Well-Differentiated | Moderately to poorly differentiated | |||
| Yes (T2 and beyond) | n1/N1 (%) | 2/12 (16.7) | 10/12 (83.3) | |
| No (T1a and T1b) | n2/N2 (%) | 14/27 (53.6) | 13/27 (46.4) | |
| P valuea | 0.0392 | 0.0392 | ||
The EUS parameter associated with DI was the presence of notable (non-diagnostic) para-esophageal lymph node, as depicted in Table 5. Importantly, the presence of notable para-esophageal lymph nodes, whether characterized as lymphadenopathy or described as “prominent”, was typically without significant diagnostic findings including size, shape, border, or echogencity. Thus, none of the reported notable lymph nodes met EUS criteria predictive for lymph node metastasis[18].
| Deep invasion on pathology | EUS parameter | ||
| Presence of notable (but non-diagnostic) para-esophageal lymph nodes on EUS | Presence of positive lymph nodes by EUS criteria | ||
| Yes (T2 and beyond) | n1/N1 (%) | 13/16 (81.2) | 0/16 (0) |
| No (T1a and T1b) | n2/N2 (%) | 11/33 (33.3) | 0/33 (0) |
| P valuea | < 0.001 | N/A | |
Several studies indicate that endoscopic findings of tumor size ≥ 2 cm and the presence of ulceration are associated with deep invasive tumors that are staged T2 and beyond[13-15]. Thus, the lack of these findings on endoscopy would suggest more superficial cancers. Cases without these findings on endoscopy were assessed to identify if the addition of EUS identified DI, when a superficial cancer is suspected. This is critically important as DI warrants esophagectomy over EMR/ESD.
The utility of pre-intervention EUS of the esophageal cancer is influenced by its accuracy in T staging. Early studies have reported the accuracy at 84%[19]. Additional studies reported the EUS accuracy ranging from 75%-82% for T1 esophageal cancer as compared to 88-100% for T4 lesions[20]. In current study including the sub-classification of T1a and T1b, EUS T staging was found to be concordant with histology 75.5% of the time.
In a study focusing on early-stage esophageal cancer subset, the lower accuracy of EUS reflects on the imprecision of distinguishing T1a and T1b lesions, which in turn reflects on its limitation of subclassifying a lesion into superficial (sm1) vs deep submucosal invasion (sm2 and sm3) cancer. In a systematic review and subsequent meta-analysis, Thosani et al[21] reported sensitivities and specificities for EUS in determining T1a and T1b staging. For T1b, the sensitivity and specificity were both 0.86. In staging T1b lesions, our study indicated EUS was reasonably specific (0.83) in ruling out sub-mucosal invasion; however, it had relatively poor sensitivity (0.54) in identifying the invasion. Overall accuracy of EUS in staging T1b lesions in our study was 72.7%. Similar issues were highlighted by another retrospective cohort study involving 131 cases of patient undergoing EUS for early esophageal cancer staging. In the study, EUS found no submucosal involvement in 80% of cases, however, histopathological evaluation after EMR determined either submucosal invasion, positive resection margin for cancer, or lympho-vascular invasion in 24% of these cases[11].
The value of pre-intervention EUS evaluation in suspected early-stage cancer relies on whether it provides change-of-management information for endoscopic intervention such as EMR or ESD. Clear evidence suggestive of deep muscular involvement (i.e. DI) or presence of significant adenopathy would preclude such endoscopic intervention.
Established endoscopic predictive signs of DI (i.e. T2 and beyond) include size ≥ 2 cm, moderate to poorly differentiated cancer, and the presence of ulceration[13-15]. In our study, 81.2% of lesions with deep invasion were ≥ 2 cm, validating this parameter association with deep invasion. The presence of esophageal ulceration had a similar trend with 50.0% of lesions with deep invasion having ulceration, significantly more than the 21.2% of superficial cancers with ulceration. Both endoscopic parameters of a tumor size ≥ 2 cm and the presence of esophageal ulceration were present in 43.8% of cases with DI and only 6.1% of cases without DI. The association between the investigated endoscopic features with deep invasive esophageal lesions is further cemented through these results. It was also found that the presence of moderate to poor differentiation was associated with deep invasion in 83.3% of cases. The presence of these parameters indicates a higher likelihood for deep invasion and EUS is warranted as prior studies and our study indicate its accuracy in staging lesions that are T2 and beyond. Particularly, size, ulceration, and degree of differentiation can be determined on initial diagnostic EGD with biopsy, highlighting their presence as determining indicators to pursue an EUS staging procedure. Differentiating between superficial and deep cancer helps to determine intervention and has significant implications downstream in survival, complications, and cost-saving measures[22].
If endoscopic parameters of a tumor size ≥ 2 cm and ulceration are not present, it could be inferred that the relevant lesion is more likely superficial. Thus, we reviewed the cases with lesions < 2 cm or ulceration among our group to see if EUS noted DI. Of 29 patients with tumors < 2 cm in size, EUS identified DI and suggested esophagectomy in 2. Of 34 patients without ulceration, EUS identified DI cancer and suggested esophagectomy in 8 of them. Seven of these patient’s had other signs of deep invasion including tumor size ≥ 2 cm or moderately to poorly differentiated cancer. Of 21 patients without esophageal ulceration and with a tumor size < 2 cm, EUS identified 1 case of DI and changed management to esophagectomy, as noted in Table 6. Given the small sample size of these subgroups, significance is difficult to determine, however, we observed that EUS only infrequently changed the outcome in the patients based on prior endoscopic features.
| Endoscopic Parameter(s) Associated with superficial cancer | Cases of EUS revealing superficial cancer (leading to EMR or ESD) | Cases of EUS revealing DI (Esophagectomy performed) | Frequency EUS changes management (%) |
| Tumor size < 2 cm | 27 | 2 | 6.9 |
| Lack of ulceration | 26 | 8 | 23.5 |
| Tumor size < 2 cm & lack of ulceration | 20 | 1 | 4.8 |
The finding of any notable (non-diagnostic) para-esophageal lymph nodes on EUS was significantly associated with DI cancers per data presented in Table 5 above. In both deep and superficial cancers, all notable para-esophageal lymph nodes described in procedure reports were not malignant by EUS criteria (size great than 10 mm, round appearance, well-demarcated, and homogeneous hypoechogenic appearance) and did not significantly alter clinical management[18,23]. Among these patients, no lymph nodes were noted on the staging computed tomography imaging. In the 11 superficial cancers with non-diagnostic para-esophageal lymph nodes, the finding did not alter management after undergoing endoscopic intervention based on EUS findings. On follow up, all 11 patients had no additional treatment for esophageal cancer and no evidence of recurrence from the date of the studied EUS procedure (ranging from 01/2005 to 03/22022) until present day. In all 13 patients who had non-diagnostic para-esophageal lymph nodes in addition to deep invasion on pathology, endoscopic parameters associated with deep invasion (tumor size ≥ 2 cm, presence of ulceration, and moderate to poorly differentiated cancer) were present as well. All 13 patients were considered for esophagectomy, with a majority undergoing surgical resection. While non-diagnostic para-esophageal lymph nodes are more often present in deeper cancers, their presence does not appear to change management decisions.
The present study presents a limitation of a single-center retrospective study with a study population that lacks external validity. The volume of patients included in this study may not adequately depict the population of patients undergoing EUS procedures. Patients were predominantly white males, and as discussed, esophageal cancer occurs globally at higher rates in certain subpopulations throughout the world. Additionally, cases were analyzed using written reports of EUS procedures without any validation of the imaging findings directly. Written reports of submucosal invasion are limited by endoscopist interpretation without reviewing all imaging findings, which was not possible in all cases. Cases where EUS did not determine staging were excluded, thus limiting analysis of instances where EUS was not able to assess depth of invasion at all; however, the vast majority of cases where EUS did not yield staging did not visualize cancerous lesions on endoscopy. A majority of patients had T1a lesions, adding selection bias to our study limited by the types of patients referred to our single academic medical. Our study selects for patients living in the US with adequate access to care to undergo the aforementioned procedures.
To further substantiate our findings, a prospective multi-center analyses would be ideal to verify operability and accuracy. To improve on the limitation of endoscopic ultrasonography precision in detecting the subtle submucosal invasion further investigation may require applications of technologies such as photoacoustic or scanning laser acoustic microscopy or optical coherence tomography, which could provide higher axial resolution than ultrasonography at meaningful penetration depths of a few millimeters[24,25].
In conclusion, EUS has limited effectiveness in distinguishing sublayer involvement of superficial esophageal lesions. Since pre-intervention EUS in evaluation of endoscopically and imaging suggested superficial cancer may be limited, we suggest that the role of EUS in this setting may be assessed with careful endoscopic examination and approached in the following way: When initial endoscopic indicators suggest deep invasion, EUS has utility in investigating the DI cancer. In cases where deep cancer is not suspected based on the endoscopic parameters, one may consider directly proceeding with endoscopic intervention as it is cost effective and provides more accurate T staging by histology.
Endoscopic ultrasound (EUS) has been utilized as the most accurate imaging modality for primary tumor staging in esophageal cancer. Primary tumor staging is key in management as cancers with submucosal invasion warrant esophagectomy while more superficial cancers are managed with endoscopic interventions like endoscopic muscoal resection (EMR) and endoscopic submucosal dissection (ESD). Studies exist that correlate endoscopic parameters with biopsy assessments to identify esophageal cancers with deep invasion in lieu of EUS.
EUS has proven to be useful in identifying advances stage tumors. Its usefulness in early-stage cancers has been more controversial. We wanted to assess how EUS influences management in early-stage esophageal cancers as the presence of submucosal invasion warrants surgery instead of endoscopic intervention.
The objectives of this study included evaluating the diagnostic capabilities of EUS in primary staging of esophageal cancers. We also sought to identify if EUS could reliably discriminate between early-stage cancers with and without submucosal invasion. The study aimed to substantiate endoscopic parameters associated with deep esophageal cancer vs superficial esophageal cancer. Finally, our objective was to determine how often EUS changed management by identifying submucosal invasion in cancers with endoscopic parameters associated with superficial esophageal cancers.
A retrospective cohort study was utilized to assess patients who had undergone primary staging of esophageal cancer via EUS at a tertiary medical center. Case data was gathered via chart review and statistical analysis was conducted to assess the accuracy of EUS, endoscopic parameters associated with deep invasion, and the frequency EUS findings changed management when endoscopic parameters suggested a superficial cancer.
In staging T1b lesions, EUS was specific in ruling in submucosal invasion but had relatively poor sensitivity in ruling out T1b lesions. Endoscopic parameters of tumor size > 2 cm and ulceration were associated with deep invasion (T2 and beyond). The EUS parameter of notable para-esophageal lymph was associated with deep invasion, while on pathology, moderate to poorly differentiated cancers were associated with deep invasion. When known endoscopic signs of deep invasion were not present, EUS altered management from EMR/ESD to esophagectomy in < 5% of cases.
EUS is accurate in staging deep invasive cancers (T2 or beyond) and reliably excludes deep invasive cancers from T1 Lesions. EUS is limited in distinguishing between T1a and T1b lesions. We reinforced that tumor size > 2 cm, lymph node involvement and poor differentiation are endoscopic parameters associated with deep invasion (T2 or beyond). EUS infrequently changes the outcome in the patients based on prior endoscopic features. While EUS may improve accuracy, our data indicates that it rarely finds deep submucosal invasion to warrant esophagectomy over EMR/ESD when endoscopic features suggest a superficial cancer (T1a or more superficial).
Future directions should focus on expanding the external validity of this study through either a larger sample size or prospective cohort analysis. This study also warrants further investigation on modalities for detecting the subtlety of submucosal invasion, including applications of technologies such as photoacoustic or scanning laser acoustic microscopy or optical coherence tomography.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association, No. 101013.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Binda C, Italy; Edholm D, Sweden; Martino A, Italy S-Editor: Liu JH L-Editor: A P-Editor: Fan JR
| 1. | Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 499] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (4)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11422] [Article Influence: 3807.3] [Reference Citation Analysis (4)] |
| 3. | Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 682] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 5. | Othman MO, Lee JH, Wang K. Clinical Practice Update on the Utility of Endoscopic Submucosal Dissection in T1b Esophageal Cancer: Expert Review. Clin Gastroenterol Hepatol. 2019;17:2161-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Heidemann J, Schilling MK, Schmassmann A, Maurer CA, Büchler MW. Accuracy of endoscopic ultrasonography in preoperative staging of esophageal carcinoma. Dig Surg. 2000;17:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Hu Y, Fu JH, Rong TH, Xu GL, Li XD, Zhang PY, Yang H, Zhu ZH, Zhang SY. [Application of endoscopic ultrasonography to preoperative clinical staging of esophageal cancer]. Ai Zheng. 2005;24:1358-1362. [PubMed] |
| 8. | Mennigen R, Tuebergen D, Koehler G, Sauerland C, Senninger N, Bruewer M. Endoscopic ultrasound with conventional probe and miniprobe in preoperative staging of esophageal cancer. J Gastrointest Surg. 2008;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Shimpi RA, George J, Jowell P, Gress FG. Staging of esophageal cancer by EUS: staging accuracy revisited. Gastrointest Endosc. 2007;66:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer? World J Gastroenterol. 2017;23:751-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (3)] |
| 11. | Pouw RE, Heldoorn N, Alvarez Herrero L, ten Kate FJ, Visser M, Busch OR, van Berge Henegouwen MI, Krishnadath KK, Weusten BL, Fockens P, Bergman JJ. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc. 2011;73:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Young PE, Gentry AB, Acosta RD, Greenwald BD, Riddle M. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Gamboa AM, Kim S, Force SD, Staley CA, Woods KE, Kooby DA, Maithel SK, Luke JA, Shaffer KM, Dacha S, Saba NF, Keilin SA, Cai Q, El-Rayes BF, Chen Z, Willingham FF. Treatment allocation in patients with early-stage esophageal adenocarcinoma: Prevalence and predictors of lymph node involvement. Cancer. 2016;122:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Krill T, Baliss M, Roark R, Sydor M, Samuel R, Zaibaq J, Guturu P, Parupudi S. Accuracy of endoscopic ultrasound in esophageal cancer staging. J Thorac Dis. 2019;11:S1602-S1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Ruan R, Chen S, Tao Y, Yu J, Zhou D, Cui Z, Shen Q, Wang S. Retrospective analysis of predictive factors for lymph node metastasis in superficial esophageal squamous cell carcinoma. Sci Rep. 2021;11:16544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 529] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 17. | Zhang D, Zheng Y, Wang Z, Huang Q, Cao X, Wang F, Liu S. Comparison of the 7th and proposed 8th editions of the AJCC/UICC TNM staging system for esophageal squamous cell carcinoma underwent radical surgery. Eur J Surg Oncol. 2017;43:1949-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Saunders HS, Wolfman NT, Ott DJ. Esophageal cancer. Radiologic staging. Radiol Clin North Am. 1997;35:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Dhupar R, Rice RD, Correa AM, Weston BR, Bhutani MS, Maru DM, Betancourt SL, Rice DC, Swisher SG, Hofstetter WL. Endoscopic Ultrasound Estimates for Tumor Depth at the Gastroesophageal Junction Are Inaccurate: Implications for the Liberal Use of Endoscopic Resection. Ann Thorac Surg. 2015;100:1812-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, Swisher SG, Hofstetter WL, Guha S, Bhutani MS. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Thakkar S, Kaul V. Endoscopic Ultrasound Staging of Esophageal Cancer. Gastroenterol Hepatol (N Y). 2020;16:14-20. [PubMed] |
| 23. | Badreddine RJ, Prasad GA, Lewis JT, Lutzke LS, Borkenhagen LS, Dunagan KT, Wang KK. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol. 2010;8:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Zhang K, Qiu J, Yang F, Wang J, Zhao X, Wei Z, Ge N, Chen Y, Sun S. Photoacoustic endoscopy and EUS: Shaking the future of multimodal endoscopy. Endosc Ultrasound. 2022;11:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 25. | Tsai TH, Fujimoto JG, Mashimo H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics (Basel). 2014;4:57-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |