Published online May 16, 2023. doi: 10.4253/wjge.v15.i5.368
Peer-review started: February 3, 2023
First decision: March 15, 2023
Revised: March 23, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 16, 2023
Processing time: 101 Days and 20.3 Hours
Rectal neuroendocrine tumours represent a rare colorectal tumour with a 10 fold increased prevalence due to incidental detection in the era of colorectal screening. Patient outcomes with early diagnosis are excellent. However endoscopic recognition of this lesion is variable and misdiagnosis can result in suboptimal endoscopic resection with subsequent uncertainty in relation to optimal long-term management. Endoscopic techniques have shown particular utility in managing this under-recognized neuroendocrine tumour.
Core Tip: Rectal neuroendocrine tumours (r-NETs) are increasingly detected during colorectal screening. Endoscopists may not accurately distinguish r-NETs from other polyps and inadvertent resection attempts result in significant post resection challenges. r-NETs have an unpredictable metastasis pattern, requiring appropriate pre-resection assessment. Accurate endoscopic assessment and resection provides an effective option in the management of r-NETs.
- Citation: Keating E, Bennett G, Murray MA, Ryan S, Aird J, O'Connor DB, O'Toole D, Lahiff C. Rectal neuroendocrine tumours and the role of emerging endoscopic techniques. World J Gastrointest Endosc 2023; 15(5): 368-375
- URL: https://www.wjgnet.com/1948-5190/full/v15/i5/368.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i5.368
Neuroendocrine tumours (NETs), previously described as carcinoid tumours, describes a classification of neoplastic cells originating from a neuroendocrine cell lineage. NETs can occur in multiple organ systems throughout the body and have a specific classification criteria according to the World Health Organization (WHO)[1]. These criteria include a grading of tumours based on mitotic counts and Ki-67 proliferation index (G1/G2/G3). The gastroenteropancreatic tract is the most common site for NETs (GEP-NETs), accounting for 73.7% of all NETs[2]. Overall, colonic NETs remain a rare occurrence compared to colorectal adenocarcinoma incidence rates, accounting for only 1.5% of all colorectal cancers[3].
Rectal neuroendocrine tumours (r-NETs) are one of the most frequent sites of GEP-NETs, representing 27% of GEP-NETs and 18% of all NETs[4]. The incidence of r-NETs is estimated to have risen 10 fold over the past 4 decades, attributed to increased incidental detection during colorectal cancer screening[5]. Weinstock et al[6] demonstrated that the majority of r-NETs are asymptomatic, with only a minority reporting symptoms such as altered bowel habit (12.8%), rectal bleeding (6.4%) or unexplained weight loss (2.1%). The carcinoid syndrome of flushing or diarrhoea is rarely associated with r-NETs[7].
The primary prognostic factor for r-NETs is driven by the disease stage at diagnosis. Five year survival for localised disease is excellent with rates of 94%-100%[8]. Regional and metastatic spread are uncommon as 75%-85% of r-NETs are localised at time of diagnosis[9]. According to the WHO grading criterion, r-NETs are predominantly G1 or G2 due to low proliferative activity.
The risk factors for nodal involvement or metastatic disease include lymphovascular invasion, muscularis propria involvement and tumour size/grade[8-10]. Pre-resection staging with endoscopic ultrasound (EUS) or magnetic resonance imaging (MRI) is necessary to adequately assess for regional or metastatic disease. Multiple approaches to achieve R0 resection may be utilised, primarily depending on lesion size, such as endoscopic mucosal resection (EMR, band or ligation approach), endoscopic submucosal dissection (ESD), combination approaches [e.g., knife-assisted snare resection (KAR)] or surgical approaches such as transanal resection (e.g., Transanal endoscopic microsurgery) or radical resections.
The classical described endoscopic appearance of r-NETs is of a small, typically < 20 mm, solitary nodule with a yellow coloration, embedded in the rectal submucosa. However, the correct endoscopic diagnosis of r-NET is not always achieved by the endoscopist, demonstrated by Fine et al[11], to be as low as 18%. As prognosis in r-NETs is dependent on the appropriate resection method, lack of recognition may result in a compromised initial resection, affecting patient prognosis.
The standard description of an r-NET at endoscopy is of a solitary nodular structure, appearing to be embedded in the normal rectal mucosa, and most often associated with a yellow coloration. r-NETs can be endoscopically differentiated from rectal adenomas by the presence of overlying normal rectal mucosa. The majority of r-NETs are < 20 mm in size[6] and increasing tumour size is also associated with increased risk of metastasis, especially once size exceeds 20 mm[12]. Lesion size is thus a primary consideration in planning excision strategies.
However, metastatic disease has been confirmed in small r-NETs of < 10 mm diameter[13], indicating that tumour size cannot be used in isolation. Therefore close inspection of the surface mucosa at endoscopy is required as the presence of overlying ulceration, depression or erosions is also associated with tumour metastasis[14].
If r-NET is suspected at time of endoscopy, the European Neuroendocrine Tumour Society (ENETS) guidelines, published in 2012 and revised in 2016, recommends completing a pre-intervention workup with a rectal EUS to establish tumour size, tumour depth (including involvement of muscularis propria) and evidence of lymphovascular involvement[5,9,15]. EUS accuracy of tumour depth is high, with rates of 91-100% correlation with post-resection findings reported[16,17]. Accurate, pre-intervention EUS is therefore essential in determining r-NET stage and selection of optimal resection strategy.
r-NETs of > 10 mm in size are also recommended to undergo MRI pelvis examination to assess for muscularis propria invasion and lymphovascular involvement. If muscularis propria involvement or nodal positivity is confirmed, a surgical approach with anterior resection and total mesenteric excision is recommended[9].
Beyond a lesion size of 20 mm, the risk of r-NET metastasis is significant and evaluation with CT thorax, abdomen and pelvis, in addition to MRI pelvis is required, prior to surgical management[8,9].
As outlined above, resection approach for r-NETs depends on accurate assessment of size, grade (if biopsies completed) and locoregional involvement. Complete excision of r-NETs < 10 mm is considered the gold standard and can be safely achieved using advanced endoscopic techniques[5,8].
Strategies to excise r-NETs of 10-19 mm in size have not reached consensus acceptance and there are no comparative studies of endoscopic resection vs surgical outcomes for this size cohort[9,15,18]. The requirement for a general anaesthetic for surgical approaches such as transanal endoscopic micr
The ENETS 2012 guideline stipulates that the only guaranteed curative option is complete resection in a localised lesion[9]. Pathological interpretation of r-NET margin clearance is therefore of primary importance to determine risk of locoregional spread. En-bloc resections are preferential to piecemeal resection to aid pathological assessment. The goal of achieving en-bloc pathology specimens influences the choice of advanced endoscopic technique employed to resect the r-NET (Table 1).
| Technique | Size limitation | En bloc resection rate (%) | R0 resection rate (%) | Procedural time (min) | Availability |
| C-EMR | < 10 mm | 36.4-80 | 27.3-76.8 | 3.3 ± 0.8 | Widely available |
| M-EMR | < 10 mm | 89.3-100 | 88.0-93.5 | 5.7 ± 1.2 | Available |
| EMR-C | < 10 mm | 87.0-100 | 69.4-94.1 | 4.2 ± 2.0 | Available |
| ESD | < 20 mm | 98.2-100 | 90.38-93.8 | 8.1 ± 9.4/19.8 ± 11.3 | Limited to experienced centres |
| KAR | < 10 mm | Limited to case series | Limited to case series | Limited to case series | Available |
EMR is widely used in the safe and successful resection of large non-pedunculated colorectal polyps (LNPCPs) by Western endoscopists[19]. Conventional EMR (C-EMR) en-bloc resection rates in Western centres, across all polyp sizes, approach 35%[20]. En-bloc resections have significantly lower rates of recurrence over piecemeal EMR[21]. In relation to r-NETs therefore, caution must be exercised in lesion assessment, to ensure that an en-bloc resection is feasible.
The ENETS guidelines endorse the use of C-EMR for r-NET lesions < 10 mm in size, once muscularis propria involvement has been out ruled with rectal EUS[15]. However, Nakamura et al[22] demon
In the same Nakamura study, modified EMR (M-EMR) strategies such as band ligation EMR or cap assisted EMR achieved significantly higher complete resection and curative resection rates, 88.0% and 69.4% respectively. Additionally, M-EMR achieved a 100% en-bloc resection rate. While this study was limited by a small number of EMRs (n = 11), its results are consistent with other studies regarding EMR resection of r-NETs outcomes and support the use of M-EMR over C-EMR for resection of r-NETs < 10 mm.
With regard to cap-assisted EMR (EMR-C), it is superior to EMR in complete histologic resection rates (94.1% vs 76.8%) without significant additional perforation or bleeding risks[23]. Similarly for band ligation EMR, complete resection rates outperform C-EMR, 93.3% vs 65.5% respectively, again without additional procedural times or complication rates[24].
M-EMR is restricted in its use to lesions < 10 mm in size, due to the specifications of the band or cap diameter of the equipment. M-EMR outcomes, for r-NETs < 10 mm in diameter, approach the resection results of ESD[5], and may be more accessible to Western endoscopists who lack suitable ESD exposure. A recent Japanese study demonstrated superior M-EMR complete resection rates and lower recurrence rates vs ESD, but this did not reach significance[25]. Yang et al[23] have also demonstrated that EMR-C histologic resection rates approach those of ESD (94.1% vs 93.8%) , but again, this did not reach significance.
The ENETS 2016 guidelines, factoring the improved en-bloc resection rates, recommend M-EMR, and specifically band ligation EMR, for r-NET resections of lesions < 10 mm[15].
ESD uses an endoscopically deployed thermal-knife to dissect the submucosal plane, facilitating en-bloc resection and aiding pathological interpretation. ESD was pioneered in Japan for the resection of gastric neoplasia and consequently, there are significant differences in the R0 outcomes and exposure to ESD practice between Asian and non-Asian countries[26].
ESD affords excellent r-NET en-bloc resection rates ranging from 98.2% to 100% and high R0 resection rates (90.38%-90.9%) for lesions < 20 mm[27,28]. Due to these superior outcomes, systematic reviews have recommended ESD over EMR for the resection of r-NETs < 10 mm and for ESD consideration in lesions < 20 mm[27,29].
Analysing the utility of ESD, there are several limitations to consider. Colonic ESD for LNPCPs is associated with higher complication rates including perforation and post-polypectomy bleeding (PPB) when compared to EMR[30]. Specifically considering r-NETs, there is a non-significant trend towards perforation and PPB but this is limited by small sample sizes[31]. Increased endoscopist experience is associated with a reduction in ESD complication rates[27]. Another consistent limitation of ESD is the increased procedural time required vs EMR[29,31].
Chen et al[27] also highlighted the coagulation or burn effect on normal tissues at time of ESD and potential effects on R0 pathologic interpretation. To counter this phenomenon, Yoshii et al[32] demonstrated an “underwater” ESD approach which afforded a heat sink effect, successfully limiting burn artefact.
ESD requires extensive training and procedural exposure to perform safely and effectively with a significant learning curve[33]. As demonstrated above, ESD outcomes differ between Asian and non-Asian endoscopists. Attempting to accelerate the learning curve of ESD for Western endoscopists has led to the development of a hybrid technique, combining familiar EMR practices with elements of ESD.
KAR described by Bhattacharya et al in the resection of LNPCPs incorporates standard submucosal injection, followed by circumferential submucosal dissection[34]. Once a circumferential margin has been established, a snare is deployed to facilitate en-bloc resection. The study achieved a 53% en- bloc resection rate in polyps < 50 mm in size and demonstrated a recurrence rate of 4.3% for en-bloc specimens. The KAR technique was subsequently shown to be effective in the management of scarred polyps with previous EMR[35].
Lisotti et al[36] applied the KAR technique for two < 5 mm r-NETs in a case series, successfully achieving en-bloc resections and negative resection margins. The following case report from our institution further illustrates the utility of KAR in this context.
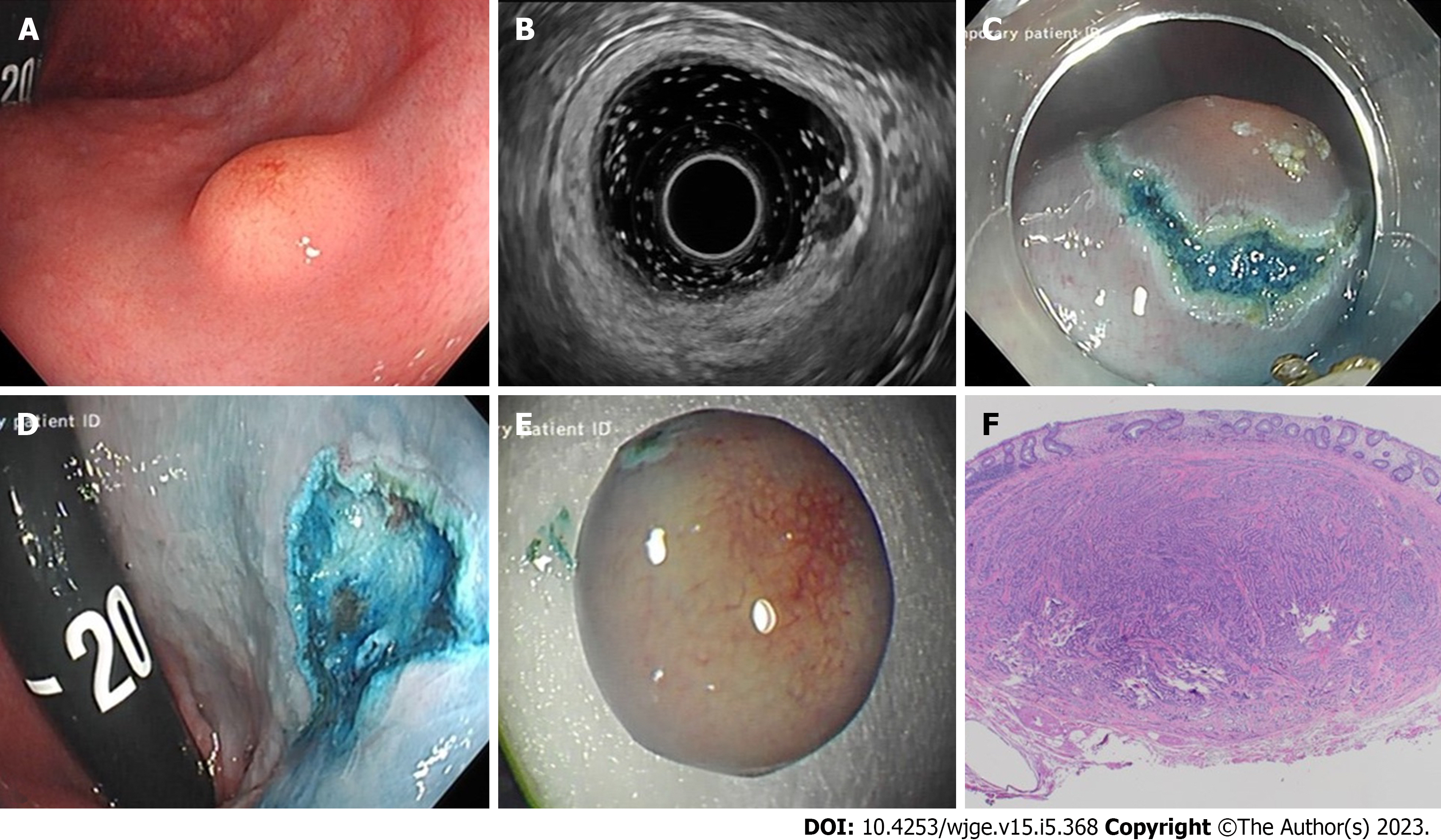
We present the case of a 33-year-old male with a background history of cystic fibrosis, referred for consideration for lung transplantation. During a pre-transplantation screening colonoscopy at a local hospital a 6mm submucosal lesion was identified 3 cm above the anorectal junction (Figure 1A). Re-evaluation at our institution included EUS, which confirmed a hypoechoic, homogenous, well circumscribed lesion, arising from the submucosa and consistent with a r-NET (Figure 1B). Submucosal injection (gelofusion and methylene blue) was followed by hybrid KAR to successfully achieve en-bloc resection (Figure 1C and D). Histopathological examination confirmed a grade 1, well-differentiated neuroendocrine tumour, with no evidence of lymphovascular involvement and negative margins (Figure 1F). After multidisciplinary discussion, and corresponding to 2012 ENETs guidelines on sub-centimetre r-NETs, surveillance was not considered necessary for this 6mm lesion and the patient has been listed for transplantation.
ENETS guidelines for surveillance post r-NET resection are determined by size, in addition to mitotic grade[9]. Follow-up modalities recommended include colonoscopy, rectal EUS and cross sectional imaging. G1 or G2 r-NETS, < 10 mm in size, with no evidence of lymphovascular invasion or muscularis propria involvement are not recommended for follow-up at present. All r-NETs 10-20 mm require annual endoscopic follow up. r-NETs > 20 mm require intensive follow-up due to the risk of metastasis.
The surveillance guidelines have generated debate, particularly for r-NETs of <10mm in diameter. The reported metastatic risk of these small r-NETs has varied from 0% to 10%[16,37,38]. Holinga et al[38], proposed an intensive EUS surveillance programme at 3 mo post resection, in addition to 6 moly EUS for the 3 years post resection.
Fine et al[11] confirmed that the real time endoscopic recognition of r-NETs is low at only 18%. Of 284 unsuspected r-NETs in the French study, 190 (67%) underwent attempted resection, primarily by standard polypectomy (n = 148/190, 78%)[11]. The successful R0 resection rate for patients who underwent polypectomy at initial colonoscopy was only 17%. As the prognosis of r-NETs depends on the successful complete excision of the lesion, salvage therapies such as EMR, ESD or trans-anal endoscopic microsurgery were required.
The retrospective diagnosis of r-NET poses a challenge in determining appropriate surveillance. Polypectomy or piecemeal EMR are often associated with R1 pathology as well as difficulty assessing for lymphovascular invasion, a key factor in surveillance algorithms. Therefore, appropriate surveillance for these cases is yet to be determined and results in local variation in practice. Such difficulties can largely be avoided by accurate index endoscopic assessment.
Rectal neuroendocrine tumours represent a rare colorectal tumour, with increasing prevalence due to incidental diagnosis during standard colorectal screening. Accurate endoscopic recognition rates of r-NETs are disappointing and the area requires increased focus in endoscopy training to improve specificity. Endoscopist education on the differentiation of rectal adenomas from r-NETs is a priority in this regard. Management strategies for diagnosed r-NETS are well established. Advanced endoscopic resection techniques have resulted in improved outcomes and can be an effective alternative for surgical resection for intermediate (10-19 mm) r-NETs but further studies are required. Newer techniques such as KAR may be valuable but require further study. International surveillance guidelines are clear but adherence to guidelines is variable and need to be more consistently applied.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ahmed M, United States; Tan KY, Singapore; Wang LH, China S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 481] [Article Influence: 160.3] [Reference Citation Analysis (2)] |
| 2. | Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 3. | Kang H, O'Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3247] [Article Influence: 191.0] [Reference Citation Analysis (0)] |
| 5. | Chablaney S, Zator ZA, Kumta NA. Diagnosis and Management of Rectal Neuroendocrine Tumors. Clin Endosc. 2017;50:530-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Weinstock B, Ward SC, Harpaz N, Warner RR, Itzkowitz S, Kim MK. Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology. 2013;98:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB 3rd, Pommier RF; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Shields CJ, Tiret E, Winter DC; International Rectal Carcinoid Study Group. Carcinoid tumors of the rectum: a multi-institutional international collaboration. Ann Surg. 2010;252:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Fine C, Roquin G, Terrebonne E, Lecomte T, Coriat R, Do Cao C, de Mestier L, Coffin E, Cadiot G, Nicolli P, Lepiliez V, Hautefeuille V, Ramos J, Girot P, Dominguez S, Céphise FV, Forestier J, Hervieu V, Pioche M, Walter T. Endoscopic management of 345 small rectal neuroendocrine tumours: A national study from the French group of endocrine tumours (GTE). United European Gastroenterol J. 2019;7:1102-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 12. | Koura AN, Giacco GG, Curley SA, Skibber JM, Feig BW, Ellis LM. Carcinoid tumors of the rectum. Cancer. 1997;79:1294-1298. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Heah SM, Eu KW, Ooi BS, Ho YH, Seow-Choen F. Tumor size is irrelevant in predicting malignant potential of carcinoid tumors of the rectum. Tech Coloproctol. 2001;5:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kim BN, Sohn DK, Hong CW, Han KS, Chang HJ, Jung KH, Lim SB, Choi HS, Jeong SY, Park JG. Atypical endoscopic features can be associated with metastasis in rectal carcinoid tumors. Surg Endosc. 2008;22:1992-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 16. | Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS, Jung SA, Park DI, Baek IH, Kim SH, Chang DK. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Ishii N, Horiki N, Itoh T, Maruyama M, Matsuda M, Setoyama T, Suzuki S, Uchida S, Uemura M, Iizuka Y, Fukuda K, Suzuki K, Fujita Y. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P, Rex DK. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 20. | Chandan S, Khan SR, Kumar A, Mohan BP, Ramai D, Kassab LL, Draganov PV, Othman MO, Kochhar GS. Efficacy and histologic accuracy of underwater versus conventional endoscopic mucosal resection for large (>20 mm) colorectal polyps: a comparative review and meta-analysis. Gastrointest Endosc. 2021;94:471-482.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 276] [Article Influence: 25.1] [Reference Citation Analysis (2)] |
| 22. | Nakamura K, Osada M, Goto A, Iwasa T, Takahashi S, Takizawa N, Akahoshi K, Ochiai T, Nakamura N, Akiho H, Itaba S, Harada N, Iju M, Tanaka M, Kubo H, Somada S, Ihara E, Oda Y, Ito T, Takayanagi R. Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol. 2016;51:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Yang DH, Park Y, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2016;83:1015-22; quiz 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Kim HH, Park SJ, Lee SH, Park HU, Song CS, Park MI, Moon W. Efficacy of endoscopic submucosal resection with a ligation device for removing small rectal carcinoid tumor compared with endoscopic mucosal resection: analysis of 100 cases. Dig Endosc. 2012;24:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Kamigaichi Y, Yamashita K, Oka S, Tamari H, Shimohara Y, Nishimura T, Inagaki K, Okamoto Y, Tanaka H, Yuge R, Urabe Y, Arihiro K, Tanaka S. Clinical outcomes of endoscopic resection for rectal neuroendocrine tumors: Advantages of endoscopic submucosal resection with a ligation device compared to conventional EMR and ESD. DEN Open. 2021;2:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Chen T, Yao LQ, Xu MD, Zhang YQ, Chen WF, Shi Q, Cai SL, Chen YY, Xie YH, Ji Y, Chen SY, Zhou PH, Zhong YS. Efficacy and Safety of Endoscopic Submucosal Dissection for Colorectal Carcinoids. Clin Gastroenterol Hepatol. 2016;14:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Wang XY, Chai NL, Linghu EQ, Li HK, Zhai YQ, Feng XX, Zhang WG, Zou JL, Li LS, Xiang JY. Efficacy and safety of hybrid endoscopic submucosal dissection compared with endoscopic submucosal dissection for rectal neuroendocrine tumors and risk factors associated with incomplete endoscopic resection. Ann Transl Med. 2020;8:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Zhong DD, Shao LM, Cai JT. Endoscopic mucosal resection vs endoscopic submucosal dissection for rectal carcinoid tumours: a systematic review and meta-analysis. Colorectal Dis. 2013;15:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 31. | Zhou X, Xie H, Xie L, Li J, Cao W, Fu W. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Yoshii S, Hayashi Y, Matsui T, Aoi K, Tsujii Y, Iijima H, Takehara T. "Underwater" endoscopic submucosal dissection: a novel technique for complete resection of a rectal neuroendocrine tumor. Endoscopy. 2016;48 Suppl 1:E67-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Marín-Gabriel JC, Fernández-Esparrach G, Díaz-Tasende J, Herreros de Tejada A. Colorectal endoscopic submucosal dissection from a Western perspective: Today's promises and future challenges. World J Gastrointest Endosc. 2016;8:40-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Bhattacharyya R, Chedgy FJ, Kandiah K, Longcroft-Wheaton G, Bhandari P. Knife-assisted snare resection (KAR) of large and refractory colonic polyps at a Western centre: Feasibility, safety and efficacy study to guide future practice. United European Gastroenterol J. 2016;4:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Chedgy FJ, Bhattacharyya R, Kandiah K, Longcroft-Wheaton G, Bhandari P. Knife-assisted snare resection: a novel technique for resection of scarred polyps in the colon. Endoscopy. 2016;48:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Lisotti A, Sadalla S, Cominardi A, Brighi N, Fusaroli P. Knife-assisted resection (KAR) for small rectal neuroendocrine neoplasia. Gastroenterol Rep (Oxf). 2020;8:479-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Holinga J, Khalid A, Fasanella K, Sanders M, Davison J, McGrath K. Metastatic risk of diminutive rectal carcinoid tumors: a need for surveillance rectal ultrasound? Gastrointest Endosc. 2012;75:913-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |