Published online May 16, 2023. doi: 10.4253/wjge.v15.i5.319
Peer-review started: September 20, 2022
First decision: November 11, 2022
Revised: January 12, 2023
Accepted: April 6, 2023
Article in press: April 6, 2023
Published online: May 16, 2023
Processing time: 238 Days and 5.4 Hours
The development and clinical application of new diagnostic endoscopic technologies such as endoscopic ultrasonography with biopsy, magnification endoscopy, and narrow-band imaging, more recently supplemented by artificial intelligence, have enabled wider recognition and detection of various gastric neoplasms including early gastric cancer (EGC) and subepithelial tumors, such as gast
Core Tip: Minimally invasive and advanced endoscopic procedures have reduced the need for extensive and invasive surgical procedures for early gastric cancer and subepithelial tumors. These novel techniques have decreased side effects, duration of hospitalization, and sedation requirements. The possibilities evolve constantly from improved diagnosis to better therapeutic techniques. This review discusses current endoscopic techniques for the diagnosis and treatment of gastric neoplasms, with special focus on guidelines and newly developed tools and methods.
- Citation: Cheema HI, Tharian B, Inamdar S, Garcia-Saenz-de-Sicilia M, Cengiz C. Recent advances in endoscopic management of gastric neoplasms. World J Gastrointest Endosc 2023; 15(5): 319-337
- URL: https://www.wjgnet.com/1948-5190/full/v15/i5/319.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i5.319
Since the advent of gastroscopy in 1868, the evolution of endoscopy has dramatically changed the natural history of several gastrointestinal pathologies. Since the initial challenge of fitting a light source with the scope of adding artificial intelligence (AI)-guided probes, the evolution has been exponential. Wolff and Shinya[1] performed the daunting task of removing colonic polyps endoscopically. In the era of laparotomy and colotomy for polyp removal, 303 polyps were removed endoscopically with minor bleeding in four patients[1]. In 2004, Kalloo et al[2] reported a “novel” endoscopic peroral transgastric approach for the peritoneal cavity. During this trial, the peritoneal cavity was accessed by a needle-like puncture of the gastric wall, the peritoneal cavity was examined, and a liver biopsy was performed. Gastric wall defects were closed using clips. This successful endeavor in 50 pigs led to what is now known as natural orifice transluminal endoscopic surgery[2].
Since the introduction of endoscopic ultrasonography (EUS) in 1980, its clinical role has continuously expanded from a diagnostic imaging approach for various gastric neoplasms to EUS-guided fine-needle aspiration (FNA) or fine-needle biopsy (FNB) to facilitate a cytological or histological diagnosis with locoregional staging of malignant gastric tumors. Treating gastric cancer by radical gastrectomy and lymph node dissection has several side effects, including internal hernias and dumping syndrome. With the development of endoscopic mucosal resection (EMR) in Japan over the past 20 years, the need for radical surgery has declined. Endoscopic submucosal dissection (ESD), which is organ-preserving and avoids surgical risks such as bleeding, leakage, and postoperative stenosis, is now a standard practice for early gastric cancer (EGC) resection. These minimally invasive techniques have provided advanced endoscopy a unique status. Our review aims to discuss these latest endoscopic diagnostic and therapeutic measures for managing epithelial and subepithelial gastric neoplasia.
Adenoma: Adenomas account for 10% of all gastric polyps in most Western countries[3]. Malignant transformation, which is common in flat adenomas, is also directly related to adenoma size. Lesions larger than 2 cm have a 40%-50% chance of malignant transformation, whereas smaller lesions (< 2 cm) have a 2% risk of malignant transformation[4]. Moreover, the irregular surface and microvascular pattern under magnification endoscopy (ME) with NBI (ME-NBI) and the color change from pale to red under white-light endoscopy (WLE) suggest the transition of adenoma to early adenocarcinoma.
Adenocarcinoma: Gastric cancer remains the second most common cancer worldwide, accounting for 60% of all cases in East Asian countries, such as China, Japan, and Korea[5]. It is the third leading cause of cancer-related deaths worldwide. According to the World Health Organization (WHO) database, gastric cancer is uncommon in North America[6]. The probabilities of acquiring and dying of gastric cancer were 1.5% and 1%, respectively. Several case-control studies from South Korea and Japan showed that the odds ratio for death from gastric cancer among subjects who underwent gastric endoscopic examinations was significantly decreased compared to those who did not undergo such screening, revealing that endoscopic screening reduces gastric cancer mortality rates[7,8]. The overall case fatality rate for gastric cancer is 81.6% among countries with limited focus on screening and, hence, typically late diagnoses of gastric cancer. In countries such as Japan, where EGC is being diagnosed promptly, the case fatality rate is 58.3%[9]. This striking difference forms the basis of this review article, which makes early cancer detection and treatment using advanced and sophisticated endoscopic procedures non-negotiable.
Adenocarcinoma is the most common histological type of gastric cancer. An estimated 95% of all gastric malignancies are adenocarcinoma[10]. Adenocarcinoma can be divided by anatomic location into non-cardia (distal) gastric adenocarcinoma and gastric cardia (proximal) adenocarcinoma. The incidence of non-cardia gastric adenocarcinoma has declined worldwide because of better eradication regimens for Helicobacter pylori (H. pylori), reduced smoking rates, and positive lifestyle changes. In contrast, the incidence of gastric cardia cancer has increased in the Western world and is primarily related to gastroesophageal reflux disease owing to increasing obesity rates[11].
Early detection is the gold standard for the management of all types of cancers, including EGC. The 2014 edition of the Japanese Guidelines for Gastric Cancer Screening recommended routine endoscopy screening every 2 years for individuals 50 years and older[12]. The Japan Gastroenterological Endoscopy Society (JGES) 2020 guidelines recommend a 1-3-year interval surveillance endoscopy for patients with clinical and endoscopic risk factors for gastric cancer[13]. The European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group, European Society of Pathology, and the Sociedade Portuguesa de Endoscopia Digestiva in a joint commission reached the consensus that WLE alone is insufficient for the diagnosis of precancerous gastric lesions. They recommended the use of magnification chromoendoscopy, NBI, or ME-NBI for the surveillance and diagnosis of these lesions[14].
It is essential to determine the depth of invasion of EGC as mucosal (cT1a) or submucosal (cT1b) to enable rational decisions regarding therapeutic strategies. Conventional WLE is the most common modality used to determine invasion depth. Indicators of cancer invasion deeper than 500 μm from the submucosa (pT1b2) on WLE include hypertrophy or fusion of concentrated folds, tumor size at least 30 mm, marked redness, irregular surface, marginal elevation, submucosal tumor-like raised margins, and non-extension sign[15-20]. The positive predictive value for diagnosing cT1b2 cancer using these indicators is reported to be 63%-89%[20,21]. EGC can be successfully managed using advanced endoscopic procedures such as EMR and ESD.
Oxyntic gland adenoma and gastric adenocarcinoma of the fundic gland type: First reported in 2007, gastric adenocarcinoma of the fundic gland type (GAFG) is an extremely rare variant of gastric adenocarcinoma composed of columnar cells with differentiation to chief and/or parietal cells[22,23]. It is more common in elderly people aged ≥ 60 years. Considering the benign biological behavior of this tumor, Singhi et al[24] proposed the term oxyntic gland adenoma/polyp, as it is usually confined to the mucosa with minimal infiltration of the submucosa and no reported lymphovascular invasion or metastasis. In the latest version of the classification of gastric neoplasms issued by the WHO, a neoplasm confined to the mucosa is called an oxyntic gland adenoma, while a neoplasm with submucosal invasion is classified as GAFG[25].
Oxyntic gland adenomas and GAFG are located in the upper third of the stomach (i.e., fundus, cardia, and upper third of the body) and originate from a deeper area of the gastric mucosa[22-24]. Therefore, they may mimic fundic gland polyps or gastric neuroendocrine tumors (NETs) on endoscopy. The four most common endoscopic features of oxyntic gland adenoma/GAFG are a submucosal tumor shape, whitish color, dilated vessels with branch architecture, and background mucosa without atrophic changes[26]. However, immunohistochemical staining is essential for the differential diagnosis. A recent multicenter study from Japan has suggested that endoscopic resection using EMR or ESD is a suitable initial treatment strategy for oxyntic gland adenoma and GAFG without reported recurrence[27].
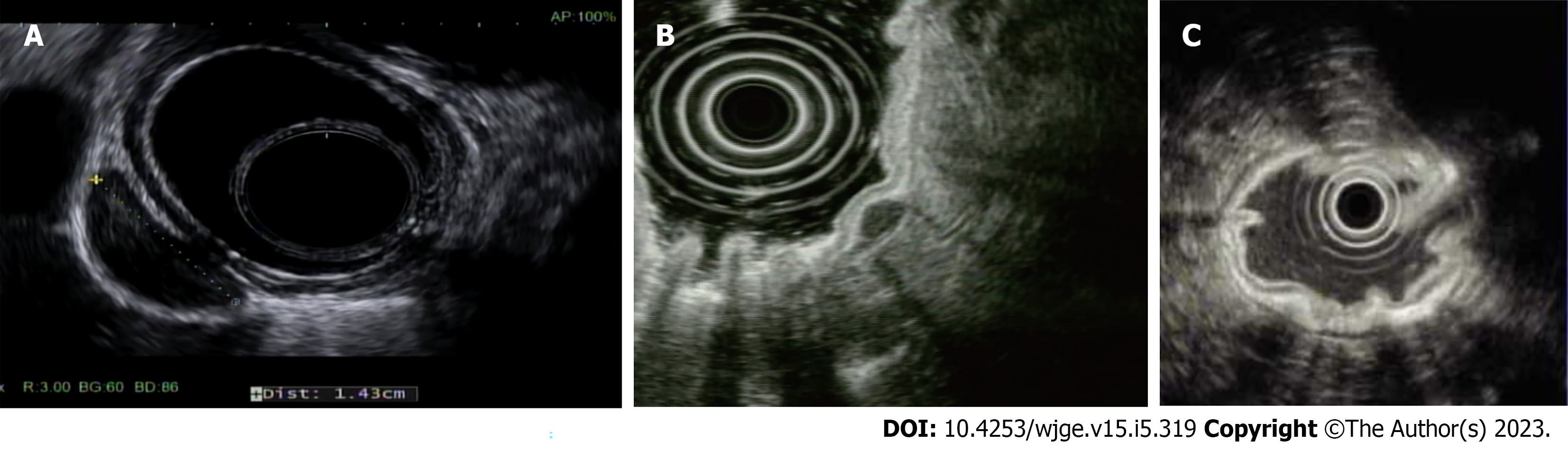
Gastric subepithelial tumors (SETs) usually arise from the submucosa or muscularis propria (MP) and exhibit distinct EUS features (Table 1). EUS can be used to identify subepithelial lesions based on the originating layer, characteristic echo patterns, and echo levels. EUS with FNB can facilitate histological evaluation, especially for smaller lesions.
| Subepithelial tumor | Endoscopic ultrasound layer | Histological layer | Echogenicity | Shape | Other features |
| Gastrointestinal stromal tumor | 4th | Muscularis propria | Hypo | Irregular or round | Heterogenous, marginal halo, cystic spaces, lymphadenopathy |
| Leiomyoma | 2nd or 4th | Deep mucosa or muscularis propria | Hypo | Round | Homogenous, fine margins |
| Neuroendocrine tumor (carcinoid) | 2nd | Deep mucosa | Hypo or iso | Round, sessile or polypoid | Erythematous depression or ulceration, smooth margin |
| Lipoma | 3rd | Submucosa | Hyper | Round | Homogenous |
| Schwannoma | 4th | Muscularis propria | Hypo | Round | Heterogenous, exophytic |
| Granular cell tumor | 2nd | Deep mucosa | Hypo | Round | Homogenous, fine margins |
| Inflammatory fibroid polyp | 2nd | Deep mucosa | Hypo | Irregular | Heterogenous, diffuse margins |
| Ectopic pancreas | 2nd, 3rd or 4th | Depending on layer | Mixed | Irregular | Ductal structure, anechoic microcysts |
Gastrointestinal stromal tumor: Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the GI tract. A recent systematic review of the global epidemiology of GISTs reported an incidence of 10-15 per million per year, with the highest incidence reported in China, Taiwan, and Norway[28]. GISTs appear as hypoechoic tumors of the fourth layer (MP), usually round on EUS (Figure 1A)[29]. EUS with FNB has a high yield of approximately 86% for the diagnosis of GISTs[30]. Although these are mostly benign tumors, tumor size and mitotic count are prognostic factors for malignancy potential. The National Comprehensive Cancer Network (NCCN) recommends the surgical resection of all GISTs ≥ 2 cm and symptomatic GISTS ≤ 2 cm[31]. In recent years, studies have shown that many SETs, including GISTs originating from the submucosa and even the MP, can be resected endoscopically using techniques such as ESD, submucosal tunneling endoscopic resection (STER), endoscopic full-thickness resection (EFTR), and endoscopic submucosal excavation (ESE) with admissible complication rates[32-36].
NETs: NETs account for 0.5% of all malignancies, with over half found in the GI tract (62%-70%)[37]. Carcinoid tumors, the most common type of NET, are primarily observed in the stomach and rectum. Its overall worldwide incidence has been increasing, likely due to improved diagnostic modalities and extensive use of acid-suppressive medications, leading to secondary hypergastrinemia, enterochromaffin-like cell hyperplasia, and ultimately neoplasia. They appear as small, round, sessile, or polypoid lesions on endoscopy. They also have dilated vessels, central depressions, or ulcerations. It is more common in women after their fifth decade of life. EUS shows hypo-or isoechoic lesions, originating from the third layer (submucosa) (Figure 1B). The NCCN recommends surveillance for tumors ≤ 20 mm in size and surgical resection for larger lesions[31]. The American Society for Gastrointestinal Endoscopy (ASGE) recommends endoscopic resection of types 1, 2, and 3 gastric carcinoids ≤ 1 cm and surgical removal of type 3 gastric carcinoids ≥ 1 cm and all type 4 carcinoids, regardless of size, given the high risk of lymph node invasion and metastasis. Although the optimal surveillance interval is not currently clear, some experts recommend endoscopic surveillance every 1-2 years post-resection[38].
Leiomyoma: Leiomyomas are usually benign tumors that arise from the muscularis mucosa or MP. EUS can be used to identify and distinguish them from other more sinister growths[29], particularly GISTs.
Schwannoma: The stomach is the most common location for gastrointestinal schwannomas. Schwannomas account for 0.2% of all gastric tumors[39]. They can be identified using computed tomography and contrast-enhanced harmonic EUS (CEH-EUS). These usually appear as exophytic, moderately homogenous, or heterogeneous enhancements on EUS[29].
Gastric lymphoma: Although the stomach is the most frequent site of gastrointestinal lymphoma, gastric lymphoma is a rare malignancy, accounting for only 3% of all gastric cancer cases. Gastric lymphoma may be primarily confined to the stomach and regional lymph nodes or secondary, as part of a systemic disease. More than 95% of gastric lymphomas are non-Hodgkin’s lymphomas, mostly the B-cell type. Marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) is the most common form in Western populations, followed by diffuse large B-cell lymphomas. MALT lymphoma is usually a low-grade lymphoma that is strongly associated with H. pylori infection.
On endoscopy, gastric lymphomas present in a localized or diffuse pattern with a variety of appearances, ranging from ulcers (single or multiple), nodules, polypoid, exophytic masses, or submucosal tumors to diffuse or localized thickening of gastric folds or irregular cobblestone-type mucosa with or without ulcerations, most commonly involving the distal half of the stomach. Of note, standard superficial biopsies may not be diagnostic, therefore, deeper biopsies obtained by the endoscopic snare technique, mucosal resection, or jumbo forceps are often needed for a pathologic diagnosis due to frequent submucosal involvement. EUS is a part of the work-up for local tumor staging in which depth and regional lymph node infiltration are investigated using FNA, as needed. The accuracy of EUS for T-staging of gastric lymphoma was 59% in a prospective multicenter study[40]. Because the disease is frequently multifocal within the stomach, treated patients require endoscopic follow-up to identify local recurrences.
Intestinal metaplasia and dysplasia: Correa et al[41] described the gastric cancer cascade as a series of changes from non-atrophic gastritis (AG) to AG, intestinal metaplasia (IM), dysplasia, and eventually adenocarcinoma. IM is a precancerous lesion of the stomach associated with H. pylori infection. If left unidentified or untreated, IM may transform into low-grade dysplasia, high-grade dysplasia (irreversible), and eventually carcinoma.
More recently, image-enhanced endoscopy and ME-NBI have shown higher diagnostic sensitivity than conventional WLE for the surveillance of IM[42,43]. The morphological appearance of dysplasia could be polyploid or flat, with a reddish or discolored mucosa. Japanese data suggests an overall 5-year cumulative gastric cancer incidence of 1.9%-10% in AG and 5.3%-9.8% in IM[44]. The risk of progression of low-grade dysplasia and high-grade dysplasia to gastric cancer in different populations is reportedly 2.8%-11.5% and 10%-68.8%, respectively; therefore, guidelines recommend endoscopic resection of a defined lesion with any degree of biopsy-proven dysplasia, especially considering a meta-analysis showing the upstaging of gastric low-grade dysplasia in 25% of lesions, with 7% being upstaged to malignant following endoscopic resection[45-49].
Consensus is lacking on the interval and methodology of surveillance for IM among societies, primarily due to the variation in gastric cancer prevalence with background genotypic and phenotypic differences among various geographical regions. In a significant study from the Netherlands, IM was integrated with the previously proposed histologic scoring system, Operative Link on Gastritis Assessment, to stage IM by replacing AG to create a more consistent and accurate staging system to estimate gastric cancer risk - Operative Link on Gastric Intestinal Metaplasia (OLGIM), because histological evaluation of atrophy is subject to poor inter-or intra-observer agreement[50]. The high-risk lesions showing significant IM in the antrum and corpus corresponding to stage III-IV have an odds ratio of 2.41 and 3.99 for gastric cancer. Accordingly, the ESGE recommends endoscopic surveillance with protocol biopsies taken from at least two topographic sites (lesser and greater curvature of the antrum and corpus) and labeled in two separate vials every 3 years for patients with IM and gastric adenocarcinoma at the gastric antrum and corpus (OLGIM stage III-IV)[14].
Patients with advanced AG and a family history of gastric cancer may benefit from more intensive follow-up (e.g., every 1 - 2 years after diagnosis). The ESGE has also set out a weak recommendation of 3-5 years of endoscopic follow-up for autoimmune gastritis, which is a chronic progressive inflammatory condition leading to corpus-predominant AG, due to the lack of large cohort data. The JGES 2020 guidelines linked atrophy, IM, enlarged folds, and xanthomas to a risk of gastric cancer[13]. They recommend that nonspecific IM be diagnosed using image-enhanced endoscopy, particularly NBI, or confocal laser endomicroscopy (CLE).
On the other hand, the Japanese and Korean guidelines do not routinely recommend risk stratification using biopsy-proven gastric adenocarcinoma or IM because of its policy of screening endoscopy at 2-year intervals for all individuals aged ≥ 40 years because of the high prevalence of H. pylori infection and gastric cancer. However, they generally recommend 1-3-year intervals for endoscopic surveillance of IM, with preferably shorter intervals for the detection of endoscopically resectable EGC. In the United States, a country with a low incidence of gastric cancer, the ASGE and American Gastroenterological Association (AGA), recommend a surveillance strategy for high-risk patients that considers the risk factors (i.e., complete vs incomplete IM, extensive vs limited IM, family history, immigration from endemic areas, ethnic minority race, and longstanding history of H. pylori)[51,52]. For high-risk patients with an incidental diagnosis of IM, a repeat endoscopy may be warranted within 1 year to determine the anatomic extent (gastric mapping), histologic subtype of IM, and possible high-risk stigmata such as nodularity. Overall, further population-based studies are needed to establish clear guidelines on this vague topic.
EUS: EUS is the most reliable non-surgical method for evaluating primary gastric tumor depth and invasion as part of loco-regional tumor staging. The ASGE recommends the use of EUS for locally staging gastric cancer[53]. The NCCN and European Society for Medical Oncology recommend pretreatment in all cases of non-metastatic gastric cancers[31,54]. CEH-EUS and elastography have improved the diagnostic performance of EUS. It can define SET size, layer of origin, margins, and echogenicity. Sakamoto et al[55] utilized CEH-EUS to evaluate GIST vascularity. It identified vascular irregularities and diagnosed GISTs with a sensitivity of 100%, specificity of 63%, and accuracy of 83%. Kim et al[56] utilized elastography with EUS to identify SETs. In this study, EUS elastography differentiated GISTs from leiomyomas with a sensitivity and specificity of 100% and 94.1%, respectively. EUS showed a 79% tumor staging accuracy in a case series of 126 patients with gastric cancer. For nodal staging, EUS has a sensitivity of 82.8% and specificity of 74.2%[57]. Dittler and Siewert[58] evaluated 254 patients with gastric adenocarcinoma using preoperative EUS. EUS was correct in determining the tumor (T) and nodal (N) stages in 83% and 66% of cases, respectively, compared with postoperative histopathological staging. Moreover, the actual complete resection rate (R0)(78%) was approximately equal to the preoperative rate predicted by EUS (81%).
Accurate assessments of gastric cancer depth and complete resection are directly related to patient prognosis and disease management. EUS helps evaluate otherwise operable diseases. This makes it a unique tool that increases physician confidence when planning a treatment course. However, the data are insufficient to recommend its routine use as part of the endoscopic work-up for EGC. Several reports have indicated the usefulness of EUS for determining EGC invasion depth, although other observational studies have not confirmed this[16,21,59-61]. Therefore, conventional WLE should be used to determine EGC invasion depth and EUS should be used as an auxiliary method for lesions diagnosed as cT1b on conventional endoscopy (Figure 1C)[16,18]. In addition, EUS is generally indicated for a suspicious lesion without a diagnosis by conventional biopsy.
Dye-based image-enhanced chromoendoscopy: The use of dye highlights differences in mucosal elevation, changes in surface structure and color, and lesion borders (demarcation line). WLE combined with indigo carmine chromoendoscopy has been widely used to determine the borders between cancerous and non-cancerous mucosa and the invasion extent of EGC. Identifying the horizontal lesion border before endoscopic resection increases the complete resection rate and reduces the local recurrence risk. Chromoendoscopy using indigo carmine more accurately estimated lesion borders in EGC than WLE (75.9% vs 50.0%), while chromoendoscopy using indigo carmine and acetic acid estimated lesion borders with a 90.7% accuracy[62]. A Korean study also reported that chromoendoscopy using indigo carmine and acetic acid more accurately estimated lesion borders in EGC than WLE (84.1% vs 66.9%)[63]. Sakai et al[64] examined 53 EGC lesions and gastric adenomas and compared the diagnostic performances of different endoscopic modalities. WLE had a performance rate of 17.0%, chromoendoscopy combined with indigo carmine dye was 52.8%, acetic acid was 41.5%, and indigo carmine dye added to acetic acid was 94.3%. They also showed that routine endoscopy missed 20 lesions of EGC. In a recent study of 104 patients, the diagnostic accuracies of WLE, indigo carmine, and acetic acid-indigo carmine mixture were 50%, 75.9%, and 90.7%, respectively[62]. However, indigo carmine chromoendoscopy reportedly fails to accurately diagnose the horizontal extent of invasion in approximately 20% of cases, even when modern high-resolution endoscopy is used to determine the circumferential borders of EGC[65-68]. Of note, Kang et al[69] showed that margin biopsy prior to ESD with onsite frozen histopathological examination of EGCs with obscure margins, despite chromoendoscopy using acetic acid and indigo carmine, enables significantly better prediction of lateral extent; therefore, frozen section biopsy can be used to perform more accurate ESD in these patients.
NBI and ME: NBI is a useful technique, particularly for evaluating vascularized lesions. NBI enhances blood vessels using green and blue light to aid in the identification of mucosal patterns and lesion margins that are otherwise difficult to detect using standard endoscopy. Dysplasia and EGC can present as subtle mucosal changes that can be missed on routine endoscopy. A recent multicenter randomized control trial showed that NBI improved mucosal surface contrast and increased the detection rate of EGC and dysplastic lesions[70]. Magnification of the fine mucosal patterns of the gastric pits aids the preliminary evaluation of suspected lesion. These findings were used in conjunction with the histological results to achieve a diagnosis.
ME can highlight patterns, such as coarse and irregular mucosa, in elevated-type cancers or a finer pattern in depressed-type cancers. It can also show changes to the vascular microstructure. A multicenter randomized controlled trial to distinguish between depressed gastric cancer and non-cancerous lesions measuring ≤ 1 cm reported that the accuracy rate of diagnosis, sensitivity, and specificity of ME-NBI for small depressed gastric lesions were 90.4%, 60.0%, and 94.3%, respectively, with the diagnostic accuracy and specificity rates being significantly better than those of WLE[71]. A recent meta-analysis also showed that the rate of presumptive diagnosis of small gastric cancers was significantly higher when ME was used than when conventional endoscopy was used. In this study, the sensitivity and specificity of ME as a diagnostic method were 96% and 95.5%, respectively[72]. In a prospective study, 165 patients with depressed-type EGCs were evaluated. These lesions were examined without magnification, magnification, or ME-NBI. The results showed that ME-NBI can predict the histological features of EGC[73]. Moreover, several studies have compared the accuracy of border prediction between ME-NBI and chromoendoscopy using indigo carmine, reporting the superiority of the former technique in estimating the horizontal borders of lesions more accurately than the latter[65-68]. Overall, both chromoendoscopy and image-enhanced endoscopy are recommended to determine the extent of resection before endoscopic resection of EGC. ME-NBI is reportedly useful for differentiating elevated lesions of gastric cancer from adenomas[74,75]. Other narrow-band light methods include blue laser imaging (BLI), linked color imaging (LCI), and i-scan optical enhancement. The BLI system involves two types of lasers with wavelengths of 410 and 450 nm as the light source and fluorescent light, respectively, which is useful for examining mucosal surface patterns. LCI is a color enhancement technology that provides slight color differences in mucosal color, which are easy to recognize with sufficient brightness compared to BLI. Randomized controlled trials for the detection of EGC using BLI and LCI are also underway. However, several studies have shown that magnifying BLI endoscopy is useful for the qualitative diagnosis of EGC similar to magnifying NBI endoscopy[76,77].
CLE: CLE is a combination of endoscopy and electron microscopy for immediate tissue and vessel analysis during ongoing endoscopy. CLE has been studied in several upper and lower GI tract diseases, including Barrett’s esophagus, gastric cancers, celiac disease, and colorectal adenoma and carcinoma[78]. This enables the endoscopist to obtain real-time in vivo magnification of the tissue in question and leads to targeted sampling. Two approved versions are currently available. One was incorporated into the distal tip of a high-resolution endoscope, while the other was a standalone probe introduced via the instrument channel during endoscopy. The probe was placed in contact with the mucosa, and the tissue in question was magnified 1000-fold[79]. Visual analysis of microarchitecture and subsurface imaging is a powerful diagnostic tool. Several studies demonstrated that CLE successfully distinguished between normal and regenerative or neoplastic tissues[80,81]. CLE has a sensitivity of 98% for the diagnosis of GIM[82]. Its sensitivity of diagnosing gastric dysplasia is 89%, while that for EGC is 91%[83,84]. In a recent study by Jeon et al[85], the accuracy of CLE diagnosis was compared with that of ESD biopsy and histopathology. The results showed a higher diagnostic accuracy of CLE vs traditional biopsy for EGC. This may help reduce the need for unnecessary interventions and invasive procedures.
AI: Gastric cancer diagnosis is largely dependent on the clinical expertise of endoscopists, radiologists, and pathologists. The miss rate of gastric cancer by EGD can be as high as 25.8%, depending on the experience of endoscopists[86-88]. The use of AI eliminates ‘human errors’ and can help identify lesions that can be missed by the human eye. AI has been used for the diagnosis, screening, and surveillance of gastric cancer. AI utilizes computer algorithms known as machine learning and deep learning on convolutional neural network (CNN) and computer-aided diagnosis, which range from automatically identifying endoscopic images of gastric cancers to analyzing pathological images with remarkable accuracy. This self-learning computer-cognition is now being used to identify precancerous conditions, such as AG[89], and detect EGC[90,91], and working cooperatively with ME-NBI[92,93]. AI-assisted CNN computer-aided diagnostic systems help endoscopists detect and confirm WLE and chromoendoscopic characteristics of gastric cancer, reduce diagnostic error rates, and choose optimal treatment. Moreover, a recent study from Japan showed that AI has reached even higher sensitivity than experts with similar specificity detecting EGC[94]. Several studies have highlighted the role of AI in recognizing the depth of invasion of EGC using CNN-based models[95-97]. Notably, Ling et al[96] showed that their CNN-based AI model outperformed endoscopists in assessing the depth of invasion. Zhu et al[98] developed an algorithm to differentiate between submucosal lesions < 500 μm (Sm1) and > 500 μm (Sm2). AI has a sensitivity of 76% and specificity of 96% in identifying lesions that can otherwise be missed on visual inspection. AI also assists pathologists with whole-slide imaging[99], identification of tumor-infiltrating lymphocytes[100], and computer-assisted identification of gastric tumors[101]. Overall, AI works like a peripheral brain that increases the diagnostic accuracy of EGC and is valuable even to the most experienced endoscopist and pathologist.
EMR: Rosenberg first reported using EMR for rectal and sigmoid polyp resections[102]. It has become an effective method for removing precancerous and cancerous lesions from the GI tract. EGC (1-2 cm in size) without risk of lymph node metastases can be successfully managed using EMR[103]. Different techniques are used for EMR. Injection-assisted EMR and lift-assisted polypectomy were first introduced in 1955 for rigid sigmoidoscopy. Normal saline (NS) solution was injected into the submucosal space to create a cushion, and the lesion was resected using a snare.
Multiple submucosal injection solutions are available on the market that provide long-lasting cushion vs NS. Hyaluronic acid (0.13%-0.4%) is most commonly used in the East, such as Japan and South Korea, and has the longest duration, making it more advantageous, especially for ESD, despite its high expense. Hydroxypropyl methyl cellulose (0.3%-0.8%), succinylated gelatin, glycerol, and hydroxyethyl starch (6% in NS) are other less expensive options with a shorter duration. In addition, the United States Food and Drug Administration approved pre-filled, pre-dyed, ready-to-use syringe Eleview (Cosmo Pharmaceuticals, NV, United States) and newly released EndoClot SIS (EndoClot Plus Inc., CA, United States) which is a starch-based powder of absorbable modified polymer distributed by Olympus are commercially available in the United States, offering a long cushion duration but at high cost. Another commonly used pre-filled syringe, Orise gel (Boston Scientific, MA, United States), has recently been recalled from the market due to higher incidence of adverse events of foreign body granulomatous reactions.
Cap-assisted EMR also begins with a submucosal injection. A specialized endoscope with a prefixed cap at the tip is positioned over the lesion, and the lifted mucosa is suctioned into the cap, after which it is resected using standard snare excision and electrocautery. Ligation-assisted EMR can be performed with or without a submucosal injection. The target lesion is suctioned into a banding cap, and a band is deployed at the base of the lesion to create a pseudopolyp. This pseudopolyp is then resected with an electrocautery snare.
Another useful technique, especially for patients undergoing repeat gastric EMR or who underwent previous partial resections, biopsies, or recurrent EGC, is underwater EMR[104]. Conventional EMR is difficult to perform for such lesions because severe submucosal fibrosis prevents mucosal lifting during submucosal injection. In this technique, the GI lumen is suctioned, and air is removed; it is then filled with water or NS to immerse the target lesion. This raises the target lesion, including the mucosa and submucosa, from deeper layers without requiring a submucosal injection[105]. There are several benefits of this method, such as facilitating the capture of flat lesions and eliminating the possible risk of seeding neoplastic cells into deeper layers and the peritoneum.
The intraprocedural bleeding rate during gastric EMR ranges from 0% to 11.5%, and it can be managed using standard endoscopic hemostasis techniques[106,107]. Delayed bleeding after gastric EMR occurs in approximately 5% of cases, with intraprocedural bleeding being the best predictor of delayed bleeding[108]. According to a systematic review, the risk of perforation due to gastric EMR was 1%[109]. The main disadvantage of EMR techniques is lesion size, which precludes en bloc resection. En bloc resection by EMR is limited to lesions less than 20 mm, and piecemeal EMR performed for larger lesions carries a higher risk of local recurrence and problems in the accurate evaluation of tumor depth by pathologists. Therefore, a thorough evaluation of lesions for submucosal invasion is of paramount importance in addition to the use of ESD vs EMR if invasion is identified.
ESD: ESD was developed for en bloc resection of EGC with lesions greater than 20 mm in size. First described in 1988 by Hirao et al[110] for the resection of EGC, ESD gained popularity in Japan in the 1990s due to the high prevalence of gastric cancer and avoidance of gastrectomy due to its complications and decreased postoperative quality of life. In a multicenter retrospective study by Oda et al[111] that evaluated the results of EMR and ESD for EGC, ESD was superior to EMR in terms of major outcomes. For 714 lesions, the en bloc resection rate was significantly higher with ESD than with EMR (92.7% vs 56%). Similarly, the complete resection rate was significantly higher with ESD (73.6%) vs EMR (61.1%). The 3-year residual/recurrence-free rate was also significantly higher in the ESD group (97.6%) than the EMR group (92.5%). However, the incidence of perforation was significantly more common than that with EMR (3.6% vs 1.2%). In addition, the longer procedure time associated with gastric ESD vs EMR makes the latter more attractive for patients with significant comorbid conditions.
The most recent (2nd edition) guidelines of the JGES, in collaboration with the Japanese Gastric Cancer Association for ESD for EGC, defined absolute indications for endoscopic therapy for lesions that are considered to have less than 1% risk of lymph node metastasis and long-term outcomes similar to those of surgical gastrectomy[13]. Accordingly, the absolute indications include lesions that are: (1) cT1a (clinically intramucosal) differentiated-type carcinomas with a long diameter greater than 2 cm and no ulcer; (2) cT1a differentiated-type carcinomas with a long diameter measuring 3 cm or less and an ulcer; and (3) cT1a undifferentiated-type carcinomas with a long diameter of 2 cm or less.
Previously recommended expanded indications have been integrated into absolute indications for ESD in recent guidelines based on the results of multicenter prospective studies. Thus, only lesions that can be considered as expanded indications for ESD are those differentiated-type absolute indication lesions that locally recur as intramucosal cancer after initial ESD/EMR, as they were either not resected en bloc or had a positive horizontal margin. They recommended surveillance endoscopy 6 mo after complete resection of EGC by ESD[112]. The AGA 2021 guidelines also advise a repeat exam during the first year. If unremarkable, repeat endoscopy can be performed on an annual basis[113]. The indications for ESD have expanded in later years for the treatment of gastric SETs, which were conventionally resected surgically. A growing body of evidence suggests that ESD is a feasible, effective, and relatively safe method with the potential to preserve the stomach with a less invasive nature, and reduced cost compared to surgical resection for gastric SETs[114-118].
The growth pattern of SET is an important predictor of complete resection and risk of perforation. Intramural and subserosa (extraluminal) SETs are more likely to be incompletely resected. In contrast, the complete resection rates of tumors originating from the submucosal layer were significantly higher than those of tumors originating from the MP[33]. Some studies suggested that a tumor size ≥ 2 cm and tumor location in the upper third of the stomach as predictors of incomplete resection[115,118]. However, a large Chinese study reported a high en bloc complete resection rate of ESD (92.4%) with no recurrence during follow-up for gastric SETs originating in the MP with diameters of up to 5 cm[119]. Major complications include perforation, which is more often seen with tumors of the MP, and bleeding, which can be managed endoscopically in most cases[33,119].
The success rate of endoscopic treatment is considerably affected by gastric SET location and is technically more challenging with significantly higher perforation risk in the retroflexion position for those located at the fundus and high gastric body[120,121]. Of note, intramural-type schwannomas, which are the most common gastric schwannomas, have been reported to be difficult to resect from the MP because they are not encapsulated, in contrast to soft tissue schwannomas; therefore, perforation occurs more frequently[120]. ESD has evolved significantly with the development of new tools and techniques and has become a more common practice now in the West. The most commonly used endoscopic knives for gastric ESD are listed in Table 2.
| Endo knife type | Name (manufacturer) | Advantages | Disadvantages |
| Insulated tip knife | IT knife (Olympus, Tokyo, Japan) | Less risk of muscle layer injury and perforation due to ceramic insulated tip, more suitable for submucosal dissection. Can be used for hemostasis | Cannot be used for marking, precutting or injection. More difficult to maneuver. Pull-cut limits direction of incision. Cutting performance tends to deteriorate in cases with severe fibrosis such as ulcer scars. Lateral cutting is difficult as the ceramic tip at the distal end catches in the mucosa. Laying the knife down too much increases the risk of perforation |
| IT knife 2 (Olympus, Tokyo, Japan) | Improved incision and cutting performance in lateral cutting and fibrotic tissue with three blades attached to the back of the insulated ceramic tip. Faster incision and cutting, shorter operating time compared to IT knife. Safer than dual knife for beginners | Needle knife for marking, precutting and injection. Difficult to manipulate in cardia and greater curvature of upper body. Sharper than IT knife which may increase the risk of perforation if firm pressure or too much downward angle is used. Needs more gentle manipulation than IT knife | |
| Needle knife | Dual knife (Olympus, Tokyo, Japan) | Easy to maneuver. Can be used for all steps of ESD: Marking, injection, incision, dissection and hemostasis. Offers more precise fine incision with better cutting performance on fibrotic tissue and ulcer scar | Higher risk of perforation when dissecting close to the muscularis propria, especially since the tip of the electrode is exposed (not insulated) |
| Scissor knife | SB knife (Sumitomo Bakelite, Tokyo, Japan) | External insulation, curved blades to protect muscle layer with reduced risk of perforation for gastric lesions. Superior safety profile. Rotatable to adjust cutting line. Useful to cut the fibrotic tissue. Sufficient coagulation before incision to minimize bleeding. Suitable for trainees | Cannot control severe bleeding. Discontinuous cutting |
| Clutch cutter (Fujifilm, Japan) | Scissor-type knife similar to SB knife. More secure incision. Serrated cutting edge enables more efficient bleeding control than SB knife. Better self-completion rates and shorter procedure times for gastric ESD by nonexperts than IT2, probably due to hemostatic efficacy | Thicker than SB knife, cannot make a sharp mucosal incision as SB knife | |
| Waterjet knife | Hybrid knife (Erbe, Germany) | Waterjet knife with needleless injection. Multi-function probe, can be used for all steps of ESD. Shorter procedure time compared to non-waterjet knives. Lower risk of bleeding by water cushion. Three types with different functionalities | Requires ERBEJET® 2 hydro surgery system. More costly |
| RFA knife | Speedboat (Creo Medical, United Kingdom) | Multi-function probe, integrated injection needle, able to complete the entire procedure with a single instrument. Only bipolar RFA knife in the market, no grounding needed. RF cutting with lower voltage and minimal bleeding. Microwave coagulation with possibly lower rates of post polypectomy syndrome. Potentially faster procedure | Requires therapeutic scope with at least 3.7 mm accessory channel |
Modified ESD techniques such as ESE and, more recently, the pocket creation method (PCM) and the tunneling technique, which will be detailed below, using the principles of third space or intramural endoscopy have been applied to improve outcomes of ESD for endoscopic resection of SETs. The major difference between ESD and ESE procedures is the endoscopic resection depth. As deep excavation is necessary during ESE, the use of an insulated-tip knife, such as an IT knife2 or TT knife, is usually recommended during excavation to avoid or reduce thermal injury. Several studies have demonstrated the efficacy of ESE for gastric GISTs, with a favorable complete resection rate and low recurrence rate, but at the expense of a higher perforation rate of up to 50%[119,120]. The PCM technique utilizes a minimal incision instead of a traditional circumferential mucosal incision. This further improves outcomes, reduces the risk of submucosal fibrosis, and decreases perforation rates[122].
Tumors located in the lesser curvature, posterior or anterior wall of the gastric body, or cardia are technically challenging to approach and resect. A multibanding, two-channel scope with two inde
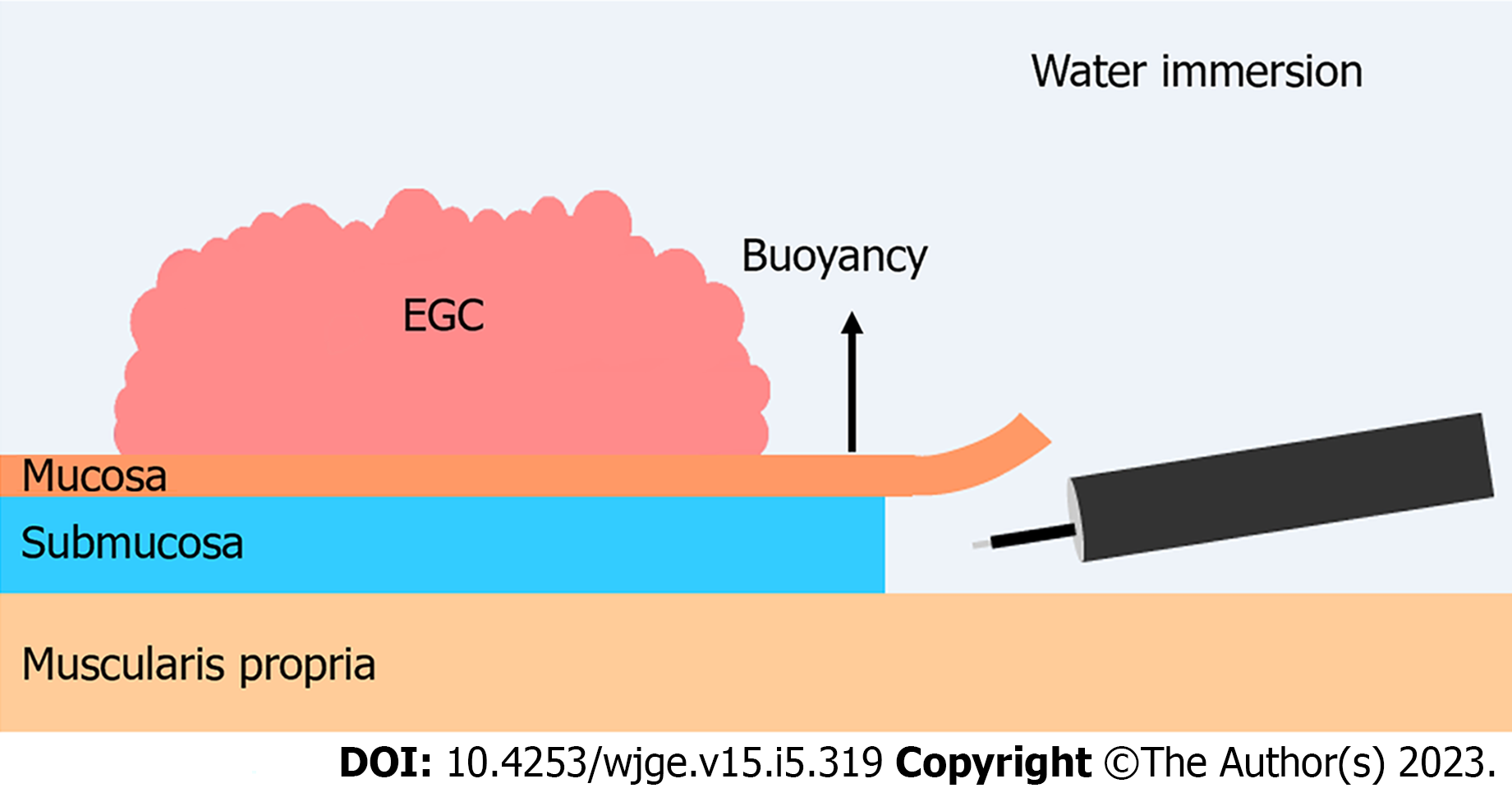
Two reports recently demonstrated that underwater ESD (U-ESD) is a promising novel gastric ESD method. Yoshii et al[124] reported using the first gastric U-ESD procedure to facilitate en bloc resection of a challenging EGC located on the greater curvature of the gastric body. Another comparative study on EGC and gastric adenoma showed that U-ESD had shorter procedural times (27.5 vs 41 min, P < 0.001) than and similar en bloc resection rates (97.9% vs 95.8%, P > 0.99) to standard ESD[125]. However, U-ESD was used with the PCM and compared to standard ESD; therefore, it is unclear whether the benefits could be attributed to the underwater technique, PCM, or their combined use. The advantages of U-ESD are twofold. First, during standard ESD, the borderline between air and water obstructs the visual field, while during U-ESD, enhanced visualization of the submucosal space can be achieved by the obliteration of any gas/fluid interface within the distal attachment and maintaining a clear view of the lumen with transparent NS solution. Second, U-ESD leverages the “buoyancy effect”. In standard ESD, the patient’s position is changed to facilitate lifting of the mucosal flap using gravity, which may be difficult for the lesions located on the greater curvature of the gastric body. In contrast, with the U-ESD technique, creation of the mucosal flap is assisted by buoyancy (Figure 2). Further comparative studies are required to confirm the advantages of U-ESD over standard ESD techniques.
Bleeding is a common complication associated with ESD. The risk of immediate and delayed bleeding associated with gastric ESD is reportedly 22% and 4.5%-5.5%, respectively[109,126,127]. Intraprocedural bleeding interferes with precise endoscopic resection by obstructing the operating field, which can lead to longer procedure times or increased perforation frequencies. Different types of lasers have been used for surgery owing to their precise excision capability and reduced risk of bleeding. In recent years, to mitigate the risk of bleeding and perforation, the feasibility of laser systems such as CO2 laser[128,129], Nd:YAG laser[130], thulium laser[131], and diode laser[132,133] has been evaluated for upper and lower gastrointestinal ESD in several animal and human studies. Cho et al[131] reported a very high technical success rate, with a 100% en bloc resection rate and 90% complete resection rate for EGC in a human study using a thulium laser. Moreover, there were no cases of active intraprocedural or delayed bleeding or perforation with minimal or no thermal injury to the MP with adjustment of power and wavelength and use of a submucosal injection to limit ablation to the superficial layers. However, further human studies are required to optimize the settings and compare laser ESD with conventional electrosurgical knives.
STER: Third space endoscopy is a unique concept that involves the mucosal flap valve principle with creation of a tunnel in the submucosal layer. Deeper layers of the GI tract are accessed by a proximally placed mucosal incision. Desired interventions such as myotomy and tumor resection can then be performed, followed by endoscopic closure of the mucosal incision using clips or sutures[134]. This methodology was first described for the treatment of achalasia cardia using peroral endoscopic myotomy 12 years ago. It has since rapidly evolved, and several new procedures such as STER, gastric and Zenker peroral endoscopic myotomy, and recanalization for complete esophageal obstruction are now being performed.
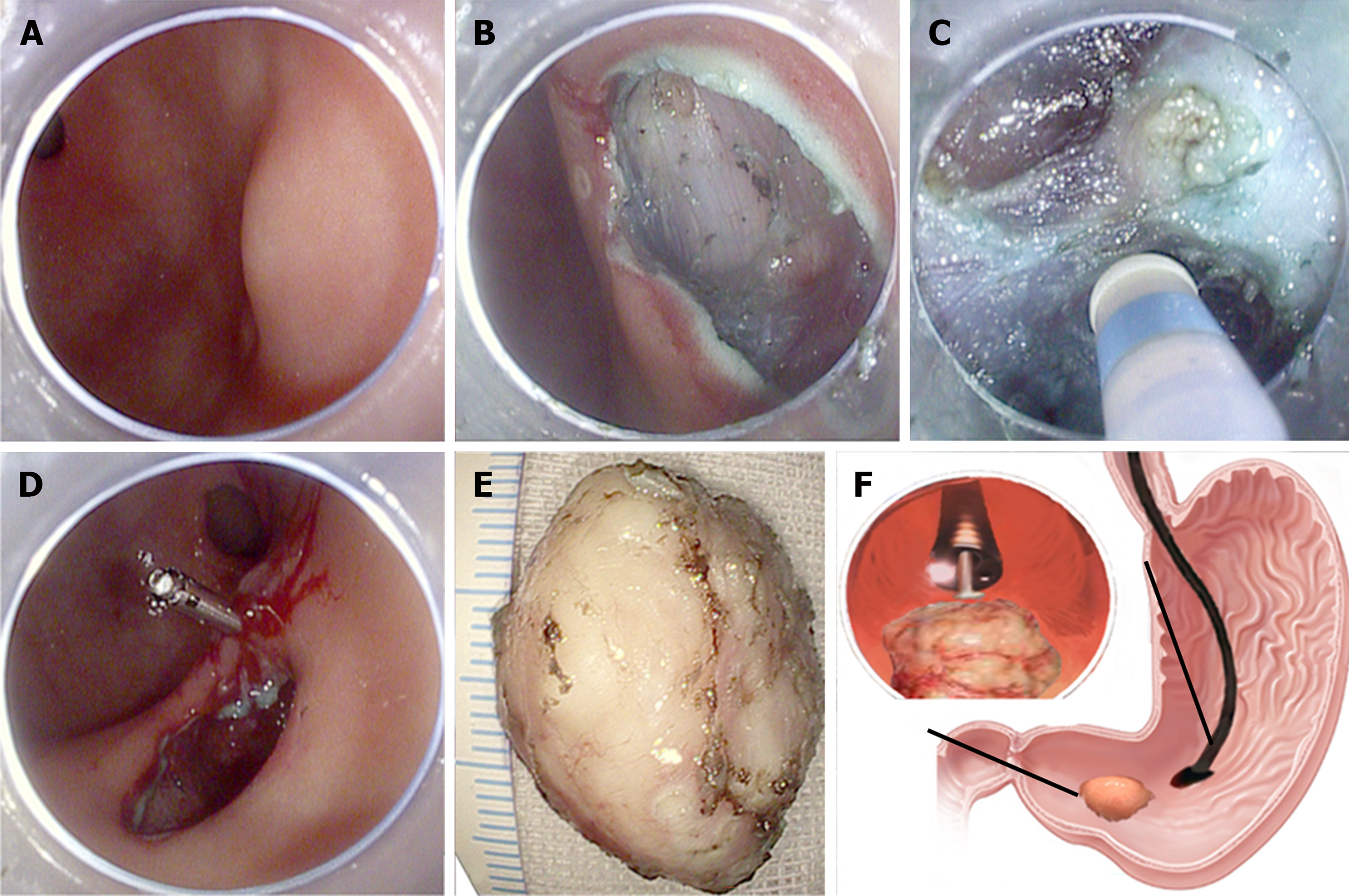
SETs with malignant potential such as GISTs and NETs can be endoscopically removed using STER. First, a 2-cm longitudinal mucosal incision is made 3-5 cm from the proximal margin of the lesion after submucosal injection and lifting, and a submucosal tunnel is created between the mucosal and MP layers by repetitive lifting and dissection to allow the endoscope to advance inside the tunnel. Then, meticulous dissection is performed with an endoknife until the tumor is completely exposed and resected. Finally, the mucosal incision is closed using endoclips (Figure 3). This is a safer technique than standard ESD with a very low risk for full-thickness perforation as the overlying mucosa of the SET is untouched, and the defects in the muscle and mucosa are at different locations[34,135]. However, STER has its limitations, with technical difficulties encountered at certain anatomic positions, such as the gastric fundus or lesser curvature. For malignant or premalignant lesions, complete resection margins can also be challenging for STER. EFTR is used to address this issue.
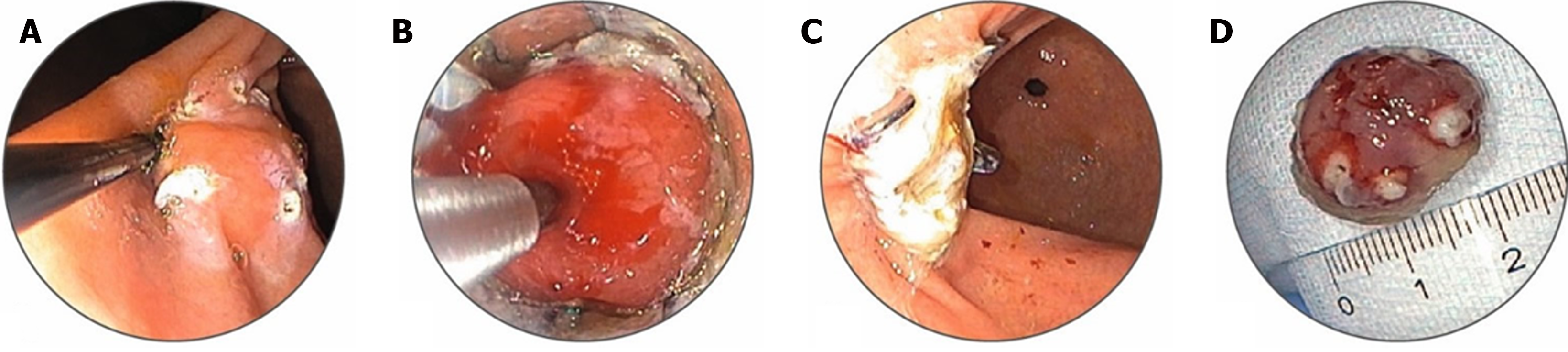
EFTR: Although EMR and ESD are established procedures for endoscopic resection of larger lesions, both have limitations that make EFTR a viable alternative. EFTR can be classified as “exposed” or “nonexposed” indictive of an intentional perforation in the GI tract lumen to the peritoneal cavity. Exposed EFTR in the stomach can be performed using a tunneled or non-tunneled approach, with subsequent closure of the defect. The defects or perforations can be closed using over-the-scope clips (OTSC) or an endoscopic suturing device (Apollo suturing device). Nonexposed EFTR relies on pre-resection apposition of the serosa to prevent full-thickness perforation[136]. An important aid to EFTR is the full-thickness resection device (FTRD), which combines resection and closure using a full-thickness OTSC in a single device. In this procedure, the lesion is grasped and pulled into a transparent hood, followed by the application of a clip to its base and resection above the clip using the inbuilt snare (Figure 4). Although FTRD is indicated for the resection of lesions ≤ 25 mm in the stomach and duodenum, the resection size for SETs is limited by the 12.1-mm inner cap diameter. Moreover, insertion of the Ovesco FTRD requires a scope with a larger accessory channel (at least 3.7 mm) and assistance with a stiff guidewire to pass an enclosed insertion balloon to dilate the esophagus and pylorus to compensate for the FTRD cap diameter. The positive outcomes of EFTR for gastric lesions include high technical success, complete resection rates, low recurrence, and adverse events[137]. EFTR has been especially useful in the management of gastric SETs, including GISTs, NETs, leiomyomas, adenomas, and EGCs with submucosal invasion. EFTR allows definite histological diagnosis, including risk stratification, in cases of GIST or NET, as opposed to conventional biopsy. Complete resection is possible in most cases and may obviate the need for further surveillance endoscopies in selected patients[138,139].
Lee et al[140] recently reported a new method called mechanical spray lumpectomy as a modified technique for EFTR to remove gastric SETs originating from the MP layer. In this method, mucosectomy is first performed using a standard snare, followed by repeated injections in the subserosal layer. The lesion is then mechanically pushed to separate the MP from the serosa using an endoscopic cap. Finally, the SET with the MP is completely dissected using the spray coagulation mode, and the defect is closed by clipping. The study showed a 100% en bloc resection rate and only one small perforation among 13 cases, which was successfully closed using hemostatic clips with no serious intra- or post-operative complications. Although this was a small study, its results are promising regarding its feasibility and safety[140]. Comparative studies are required to reveal which EFTR method might work better for individual cases, considering different variables, including lesion size and location.
Argon plasma coagulation (APC) is an established measure of tissue coagulation. APC is effective at treating EGC in patients who cannot undergo EMR or surgery. In a retrospective pilot study, Kitamura et al[141] showed that intestinal-type intramucosal carcinoma was successfully eradicated after one or two APC sessions. The more resistant types were locally controlled by follow-up APC sessions.
Gastric outlet obstruction is a debilitating sequela of gastric, duodenal, and pancreatobiliary malignancies. Gastrojejunostomy is usually the traditional treatment; however, it is an extensive surgical procedure with high morbidity rates. EUS-guided gastrojejunostomy with placement of a lumen-apposing metal stent is now an accepted alternative to invasive surgery[142]. Endoscopically-placed self-expandable metallic stents are an excellent alternative. A meta-analysis analyzed the outcomes of 307 procedures from nine studies. Endoscopic stenting was associated with higher clinical success (P = 0.007), a shorter time from the procedure to starting oral intake (P < 0.001), lower morbidity (P = 0.02), a lower incidence of delayed gastric emptying (P = 0.002), and a shorter hospital stay (P < 0.001) than surgical gastroenterostomy[143].
Gastrointestinal endo-surgery is the future of advanced and minimally invasive flexible endoscopic procedures. Like any other cancer, the most important prognostic factors for SETs and EGC are timely detection and early treatment. Sophisticated endoscopic procedures are assisting gastroenterologists to detect early changes in the gastrointestinal tract and identify malicious lesions on time. Endoscopic ultrasound, AI, chromoendoscopy, and image-enhanced endoscopy improve diagnostic precision. More refined guidelines for the endoscopic surveillance of premalignant gastric lesions are required. Minimally invasive procedures help remove gastric neoplasms at an early stage. Interventions such as EMR and ESD are becoming a standard practice universally with the addition of new tools and accessories to the armamentarium alongside novel methods. Comparative studies are required to determine the optimal method and tool for the endoscopic treatment of a variety of gastric neoplasms. There is still scope to incorporate palliative measures for the benefit of gastric cancer patients, as they are being used for other GI malignancies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen SY, China; Delsa H, Morocco S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Wolff WI, Shinya H. Polypectomy via the fiberoptic colonoscope. Removal of neoplasms beyond reach of the sigmoidoscope. N Engl J Med. 1973;288:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 127] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 903] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 3. | Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 136] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Carl-McGrath S, Ebert M, Röcken C. Gastric adenocarcinoma: epidemiology, pathology and pathogenesis. Cancer Therapy. 2007;5:877-894. |
| 5. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Organization WH; Organization WH. Global Cancer Observatory. Cancer surveillance database. Available at: https://gco.iarc.fr. |
| 7. | Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, Jung KW, Lee CW, Choi IJ, Park EC, Lee D. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology. 2017;152:1319-1328.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 8. | Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8:e79088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 189] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Schwartz GK. Invasion and metastases in gastric cancer: in vitro and in vivo models with clinical correlations. Semin Oncol. 1996;23:316-324. [PubMed] |
| 11. | Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 12. | Hamashima C; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol. 2018;48:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 13. | Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, Yoshida M, Miyashiro I, Fujimoto K, Tajiri H. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32:663-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 14. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 15. | Sano T, Okuyama Y, Kobori O, Shimizu T, Morioka Y. Early gastric cancer. Endoscopic diagnosis of depth of invasion. Dig Dis Sci. 1990;35:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Abe S, Oda I, Shimazu T, Kinjo T, Tada K, Sakamoto T, Kusano C, Gotoda T. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer. 2011;14:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Tsujii Y, Kato M, Inoue T, Yoshii S, Nagai K, Fujinaga T, Maekawa A, Hayashi Y, Akasaka T, Shinzaki S, Watabe K, Nishida T, Iijima H, Tsujii M, Takehara T. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc. 2015;82:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Yao T. Clinicopathological study for accurate endoscopic diagnosis of submucosal invasion by early cancer of depressed-type. Stomach Intest (Tokyo). 2008;43:1109-1125. |
| 20. | Nagahama T, Yao K, Imamura K, Kojima T, Ohtsu K, Chuman K, Tanabe H, Yamaoka R, Iwashita A. Diagnostic performance of conventional endoscopy in the identification of submucosal invasion by early gastric cancer: the "non-extension sign" as a simple diagnostic marker. Gastric Cancer. 2017;20:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 23. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Yao T, Vieth M. Oxyntic gland adenoma. Digestive system tumours. In: World Health Organization Classification of Tumours Editorial Board. World Health Organization of Tumours. 5. International Agency for Research on Cancer. Geneva: World Health Organization, 2019: 83-84. |
| 26. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Iwamuro M, Kusumoto C, Nakagawa M, Kobayashi S, Yoshioka M, Inaba T, Toyokawa T, Hori S, Tanaka S, Matsueda K, Tanaka T, Okada H. Endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma or gastric adenocarcinoma of the fundic gland type. Sci Rep. 2021;11:7375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 29. | Okai T, Minamoto T, Ohtsubo K, Minato H, Kurumaya H, Oda Y, Mai M, Sawabu N. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003;28:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Lee JH, Cho CJ, Park YS, Ahn JY, Kim DH, Na HK, Choi KD, Song HJ, Lee GH, Jung HY. EUS-guided 22-gauge fine needle biopsy for the diagnosis of gastric subepithelial tumors larger than 2 cm. Scand J Gastroenterol. 2016;51:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastrointestinal Stromal Tumors (GISTs). NCCN.org. [cited 15 August 2022]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf. |
| 32. | An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, Wang D, Li ZS, Shi XG. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017;31:4522-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, Baek IH, Kim JH, Park CK, Kwon MJ. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271-3279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Li QL, Chen WF, Zhang C, Hu JW, Zhou PH, Zhang YQ, Zhong YS, Yao LQ, Xu MD. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc. 2015;29:3640-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Shi D, Li R, Chen W, Zhang D, Zhang L, Guo R, Yao P, Wu X. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc. 2017;31:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Li Y, Luo H, Yu H. [Efficacy analysis of endoscopic submucosal excavation for gastric gastrointestinal stromal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:352-355. [PubMed] |
| 37. | Fang JM, Li J, Shi J. An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol. 2022;28:1009-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (12)] |
| 38. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Drago J, Fuente I, Cavadas D, Beskow A, Wright F. Gastric Schwannoma. J Gastrointest Surg. 2019;23:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Fischbach W, Goebeler-Kolve ME, Greiner A. Diagnostic accuracy of EUS in the local staging of primary gastric lymphoma: results of a prospective, multicenter study comparing EUS with histopathologic stage. Gastrointest Endosc. 2002;56:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer. 2004;7:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Zhang Q, Wang F, Chen ZY, Wang Z, Zhi FC, Liu SD, Bai Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer. 2016;19:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1183] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 44. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 45. | Rugge M, Cassaro M, Di Mario F, Leo G, Leandro G, Russo VM, Pennelli G, Farinati F; Interdisciplinary Group on Gastric Epithelial Dysplasia (IGGED). The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 48. | Park SY, Jeon SW, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Zhao G, Xue M, Hu Y, Lai S, Chen S, Wang L. How Commonly Is the Diagnosis of Gastric Low Grade Dysplasia Upgraded following Endoscopic Resection? A Meta-Analysis. PLoS One. 2015;10:e0132699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 51. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J, Baron TH, Faigel DO; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 52. | Gupta S, Li D, El Serag HB, Davitkov P, Altayar O, Sultan S, Falck-Ytter Y, Mustafa RA. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology. 2020;158:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 53. | ASGE Standards of Practice Committee; Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 54. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1118] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 55. | Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, Dote K, Kudo M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2011;73:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Kim SH, Yoo IK, Kwon CI, Hong SP, Cho JY. Utility of EUS elastography in the diagnosis of gastric subepithelial tumors: a pilot study (with video). Gastrointest Endosc. 2020;91:172-177.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Ganpathi IS, So JB, Ho KY. Endoscopic ultrasonography for gastric cancer: does it influence treatment? Surg Endosc. 2006;20:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in gastric carcinoma. Endoscopy. 1993;25:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Matsumoto Y, Yanai H, Tokiyama H, Nishiaki M, Higaki S, Okita K. Endoscopic ultrasonography for diagnosis of submucosal invasion in early gastric cancer. J Gastroenterol. 2000;35:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, Okita K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Kawahara Y, Takenaka R, Okada H, Kawano S, Inoue M, Tsuzuki T, Tanioka D, Hori K, Yamamoto K. Novel chromoendoscopic method using an acetic acid-indigocarmine mixture for diagnostic accuracy in delineating the margin of early gastric cancers. Dig Endosc. 2009;21:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Lee BE, Kim GH, Park DY, Kim DH, Jeon TY, Park SB, You HS, Ryu DY, Kim DU, Song GA. Acetic acid-indigo carmine chromoendoscopy for delineating early gastric cancers: its usefulness according to histological type. BMC Gastroenterol. 2010;10:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, Suzuki T, Kobayashi M, Matsumura T, Bekku D, Ito K, Nakamoto S, Tanaka T, Yokosuka O. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Asada-Hirayama I, Kodashima S, Sakaguchi Y, Ono S, Niimi K, Mochizuki S, Tsuji Y, Minatsuki C, Shichijo S, Matsuzaka K, Ushiku T, Fukayama M, Yamamichi N, Fujishiro M, Koike K. Magnifying endoscopy with narrow-band imaging is more accurate for determination of horizontal extent of early gastric cancers than chromoendoscopy. Endosc Int Open. 2016;4:E690-E698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Kiyotoki S, Nishikawa J, Satake M, Fukagawa Y, Shirai Y, Hamabe K, Saito M, Okamoto T, Sakaida I. Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol. 2010;25:1636-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011;74:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 68. | Nonaka K, Namoto M, Kitada H, Shimizu M, Ochiai Y, Togawa O, Nakao M, Nishimura M, Ishikawa K, Arai S, Kita H. Usefulness of the DL in ME with NBI for determining the expanded area of early-stage differentiated gastric carcinoma. World J Gastrointest Endosc. 2012;4:362-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Kang EJ, Cho JY, Lee TH, Jin SY, Cho WY, Bok JH, Kim HG, Kim JO, Lee JS, Lee IH. Frozen Section Biopsy to Evaluation of Obscure Lateral Resection Margins during Gastric Endoscopic Submucosal Dissection for Early Gastric Cancer. J Gastric Cancer. 2011;11:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Ang TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol. 2015;27:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017-2025.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 72. | Tajiri H, Doi T, Endo H, Nishina T, Terao T, Hyodo I, Matsuda K, Yagi K. Routine endoscopy using a magnifying endoscope for gastric cancer diagnosis. Endoscopy. 2002;34:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 74. | Tsuji Y, Ohata K, Sekiguchi M, Ohno A, Ito T, Chiba H, Gunji T, Fukushima J, Yamamichi N, Fujishiro M, Matsuhashi N, Koike K. Magnifying endoscopy with narrow-band imaging helps determine the management of gastric adenomas. Gastric Cancer. 2012;15:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Maki S, Yao K, Nagahama T, Beppu T, Hisabe T, Takaki Y, Hirai F, Matsui T, Tanabe H, Iwashita A. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between low-grade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer. 2013;16:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 76. | Dohi O, Yagi N, Majima A, Horii Y, Kitaichi T, Onozawa Y, Suzuki K, Tomie A, Kimura-Tsuchiya R, Tsuji T, Yamada N, Bito N, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Yanagisawa A, Itoh Y. Diagnostic ability of magnifying endoscopy with blue laser imaging for early gastric cancer: a prospective study. Gastric Cancer. 2017;20:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 77. | Dohi O, Yagi N, Yoshida S, Ono S, Sanomura Y, Tanaka S, Naito Y, Kato M. Magnifying Blue Laser Imaging versus Magnifying Narrow-Band Imaging for the Diagnosis of Early Gastric Cancer: A Prospective, Multicenter, Comparative Study. Digestion. 2017;96:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Goetz M, Watson A, Kiesslich R. Confocal laser endomicroscopy in gastrointestinal diseases. J Biophotonics. 2011;4:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388-392, 392.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 80. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 81. | Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am. 2008;18:451-466, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, Guo YT, Liu H. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y, Hashizume M. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, Shimoyama Y, Nagasaka T, Maeda O, Ando T, Ohmiya N, Itoh A, Hirooka Y, Goto H. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 2006;38:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc. 2011;74:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Hosokawa O, Hattori M, Douden K, Hayashi H, Ohta K, Kaizaki Y. Difference in accuracy between gastroscopy and colonoscopy for detection of cancer. Hepatogastroenterology. 2007;54:442-444. [PubMed] |
| 87. | Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Voutilainen ME, Juhola MT. Evaluation of the diagnostic accuracy of gastroscopy to detect gastric tumours: clinicopathological features and prognosis of patients with gastric cancer missed on endoscopy. Eur J Gastroenterol Hepatol. 2005;17:1345-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 89. | Guimarães P, Keller A, Fehlmann T, Lammert F, Casper M. Deep-learning based detection of gastric precancerous conditions. Gut. 2020;69:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 90. | Sakai Y, Takemoto S, Hori K, Nishimura M, Ikematsu H, Yano T, Yokota H. Automatic detection of early gastric cancer in endoscopic images using a transferring convolutional neural network. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:4138-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 91. | Luo H, Xu G, Li C, He L, Luo L, Wang Z, Jing B, Deng Y, Jin Y, Li Y, Li B, Tan W, He C, Seeruttun SR, Wu Q, Huang J, Huang DW, Chen B, Lin SB, Chen QM, Yuan CM, Chen HX, Pu HY, Zhou F, He Y, Xu RH. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 92. | Niu PH, Zhao LL, Wu HL, Zhao DB, Chen YT. Artificial intelligence in gastric cancer: Application and future perspectives. World J Gastroenterol. 2020;26:5408-5419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 93. | Horiuchi Y, Aoyama K, Tokai Y, Hirasawa T, Yoshimizu S, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Convolutional Neural Network for Differentiating Gastric Cancer from Gastritis Using Magnified Endoscopy with Narrow Band Imaging. Dig Dis Sci. 2020;65:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 94. | Ikenoyama Y, Hirasawa T, Ishioka M, Namikawa K, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Tsuchida T, Takeuchi Y, Shichijo S, Katayama N, Fujisaki J, Tada T. Detecting early gastric cancer: Comparison between the diagnostic ability of convolutional neural networks and endoscopists. Dig Endosc. 2021;33:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 95. | Nam JY, Chung HJ, Choi KS, Lee H, Kim TJ, Soh H, Kang EA, Cho SJ, Ye JC, Im JP, Kim SG, Kim JS, Chung H, Lee JH. Deep learning model for diagnosing gastric mucosal lesions using endoscopic images: development, validation, and method comparison. Gastrointest Endosc. 2022;95:258-268.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 96. | Ling T, Wu L, Fu Y, Xu Q, An P, Zhang J, Hu S, Chen Y, He X, Wang J, Chen X, Zhou J, Xu Y, Zou X, Yu H. A deep learning-based system for identifying differentiation status and delineating the margins of early gastric cancer in magnifying narrow-band imaging endoscopy. Endoscopy. 2021;53:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 97. | Nagao S, Tsuji Y, Sakaguchi Y, Takahashi Y, Minatsuki C, Niimi K, Yamashita H, Yamamichi N, Seto Y, Tada T, Koike K. Highly accurate artificial intelligence systems to predict the invasion depth of gastric cancer: efficacy of conventional white-light imaging, nonmagnifying narrow-band imaging, and indigo-carmine dye contrast imaging. Gastrointest Endosc. 2020;92:866-873.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 98. | Zhu Y, Wang QC, Xu MD, Zhang Z, Cheng J, Zhong YS, Zhang YQ, Chen WF, Yao LQ, Zhou PH, Li QL. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest Endosc. 2019;89:806-815.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 99. | Yoshida H, Shimazu T, Kiyuna T, Marugame A, Yamashita Y, Cosatto E, Taniguchi H, Sekine S, Ochiai A. Automated histological classification of whole-slide images of gastric biopsy specimens. Gastric Cancer. 2018;21:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Garcia E, Hermoza R, Castanon CB, Cano L, Castillo M, Castanñeda C. Automatic Lymphocyte Detection on Gastric Cancer IHC Images Using Deep Learning. CBMS. 2017;. [DOI] [Full Text] |
| 101. | Li Y, Li X, Xie X, Shen L. Deep learning based gastric cancer identification. Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018); 2018: 182-185. [DOI] [Full Text] |
| 102. | Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg. 1955;70:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Inoue H, Fukami N, Yoshida T, Kudo SE. Endoscopic mucosal resection for esophageal and gastric cancers. J Gastroenterol Hepatol. 2002;17:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Iwagami H, Kanesaka T, Ishihara R, Uedo N. Underwater endoscopic mucosal resection for remaining early gastric cancer after endoscopic submucosal dissection. Endoscopy. 2019;51:E229-E230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | ASGE Technology Committee; Hwang JH, Konda V, Abu Dayyeh BK, Chauhan SS, Enestvedt BK, Fujii-Lau LL, Komanduri S, Maple JT, Murad FM, Pannala R, Thosani NC, Banerjee S. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 106. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 107. | Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D, Ito K, Tanaka T, Yokosuka O. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Okano A, Hajiro K, Takakuwa H, Nishio A, Matsushita M. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc. 2003;57:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 110. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 266] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 111. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, Hamada T, Inoue H, Gotoda T, Yoshida S. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 112. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 113. | Wang AY, Hwang JH, Bhatt A, Draganov PV. AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary. Gastroenterology. 2021;161:2030-2040.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 114. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 115. | Lee JS, Kim GH, Park DY, Yoon JM, Kim TW, Seo JH, Lee BE, Song GA. Endoscopic Submucosal Dissection for Gastric Subepithelial Tumors: A Single-Center Experience. Gastroenterol Res Pract. 2015;2015:425469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Bang CS, Baik GH, Shin IS, Suk KT, Yoon JH, Kim DJ. Endoscopic submucosal dissection of gastric subepithelial tumors: a systematic review and meta-analysis. Korean J Intern Med. 2016;31:860-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Bhagat VH, Kim M, Kahaleh M. A Review of Endoscopic Full-thickness Resection, Submucosal Tunneling Endoscopic Resection, and Endoscopic Submucosal Dissection for Resection of Subepithelial Lesions. J Clin Gastroenterol. 2021;55:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Soh JS, Kim JK, Lim H, Kang HS, Park JW, Kim SE, Moon SH, Kim JH, Park CK, Cho JW, Lim MS, Kim KO. Comparison of endoscopic submucosal dissection and surgical resection for treating gastric subepithelial tumours. Scand J Gastroenterol. 2016;51:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, Chen X, Liu W, Wang B. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 120. | Jeong ID, Jung SW, Bang SJ, Shin JW, Park NH, Kim DH. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc. 2011;25:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 121. | Li L, Wang F, Wu B, Wang Q, Wang C, Liu J. Endoscopic submucosal dissection of gastric fundus subepithelial tumors originating from the muscularis propria. Exp Ther Med. 2013;6:391-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Sakamoto H, Hayashi Y, Miura Y, Shinozaki S, Takahashi H, Fukuda H, Okada M, Ino Y, Takezawa T, Sunada K, Lefor AK, Yamamoto H. Pocket-creation method facilitates endoscopic submucosal dissection of colorectal laterally spreading tumors, non-granular type. Endosc Int Open. 2017;5:E123-E129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 123. | Hamada K, Horikawa Y, Koyanagi R, Shiwa Y, Techigawara K, Nishida S, Nakayama Y, Honda M. Usefulness of a multibending endoscope in gastric endoscopic submucosal dissection. VideoGIE. 2019;4:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 124. | Yoshii S, Hayashi Y, Tsujii Y, Takehara T. Underwater endoscopic submucosal dissection: a novel resection strategy for early gastric cancer located on the greater curvature of the gastric body. Ann Gastroenterol. 2017;30:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Harada H, Murakami D, Suehiro S, Nakahara R, Ujihara T, Sagami R, Katsuyama Y, Hayasaka K, Amano Y. Water-pocket endoscopic submucosal dissection for superficial gastric neoplasms (with video). Gastrointest Endosc. 2018;88:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 126. | Goto O, Fujishiro M, Oda I, Kakushima N, Yamamoto Y, Tsuji Y, Ohata K, Fujiwara T, Fujiwara J, Ishii N, Yokoi C, Miyamoto S, Itoh T, Morishita S, Gotoda T, Koike K. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci. 2012;57:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 127. | Jang JS, Choi SR, Graham DY, Kwon HC, Kim MC, Jeong JS, Won JJ, Han SY, Noh MH, Lee JH, Lee SW, Baek YH, Kim MJ, Jeong DS, Kim SK. Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol. 2009;44:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Noguchi T, Hazama H, Nishimura T, Morita Y, Awazu K. Enhancement of the safety and efficacy of colorectal endoscopic submucosal dissection using a CO(2) laser. Lasers Med Sci. 2020;35:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Obata D, Morita Y, Kawaguchi R, Ishii K, Hazama H, Awazu K, Kutsumi H, Azuma T. Endoscopic submucosal dissection using a carbon dioxide laser with submucosally injected laser absorber solution (porcine model). Surg Endosc. 2013;27:4241-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Saccomandi P, Quero G, Costamagna G, Diana M, Marescaux J. Effects of Nd:YAG laser for the controlled and localized treatment of early gastrointestinal tumors: Preliminary in vivo study. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:4533-4536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 131. | Cho JH, Cho JY, Kim MY, Jeon SR, Lee TH, Kim HG, Jin SY, Hong SJ. Endoscopic submucosal dissection using a thulium laser: preliminary results of a new method for treatment of gastric epithelial neoplasia. Endoscopy. 2013;45:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | Tang J, Ye S, Ji X, Li J, Liu F. Comparison of synchronous dual wavelength diode laser versus conventional endo-knives for esophageal endoscopic submucosal dissection: an animal study. Surg Endosc. 2018;32:5037-5043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Jung Y, Baik GH, Ko WJ, Ko BM, Kim SH, Jang JS, Jang JY, Lee WS, Cho YK, Lim SG, Moon HS, Yoo IK, Cho JY. Diode Laser-Can It Replace the Electrical Current Used in Endoscopic Submucosal Dissection? Clin Endosc. 2021;54:555-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 134. | Bapaye A, Korrapati SK, Dharamsi S, Dubale N. Third Space Endoscopy: Lessons Learnt From a Decade of Submucosal Endoscopy. J Clin Gastroenterol. 2020;54:114-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 136. | ASGE Technology Committee; Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 137. | Liu AQ, Chiu PWY. Third space endoscopy: Current evidence and future development. Inter J Gastro Interven. 2020;9:42-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 138. | Hajifathalian K, Ichkhanian Y, Dawod Q, Meining A, Schmidt A, Glaser N, Vosoughi K, Diehl DL, Grimm IS, James T, Templeton AW, Samarasena JB, Chehade NEH, Lee JG, Chang KJ, Mizrahi M, Barawi M, Irani S, Friedland S, Korc P, Aadam AA, Al-Haddad M, Kowalski TE, Smallfield G, Ginsberg GG, Fukami N, Lajin M, Kumta NA, Tang SJ, Naga Y, Amateau SK, Kasmin F, Goetz M, Seewald S, Kumbhari V, Ngamruengphong S, Mahdev S, Mukewar S, Sampath K, Carr-Locke DL, Khashab MA, Sharaiha RZ. Full-thickness resection device (FTRD) for treatment of upper gastrointestinal tract lesions: the first international experience. Endosc Int Open. 2020;8:E1291-E1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 139. | Meier B, Schmidt A, Glaser N, Meining A, Walter B, Wannhoff A, Riecken B, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial). Surg Endosc. 2020;34:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 140. | Lee JM, Yoo IK, Hong SP, Cho JY, Cho YK. A modified endoscopic full thickness resection for gastric subepithelial tumors from muscularis propria layer: Novel method. J Gastroenterol Hepatol. 2021;36:2558-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 141. | Kitamura T, Tanabe S, Koizumi W, Mitomi H, Saigenji K. Argon plasma coagulation for early gastric cancer: technique and outcome. Gastrointest Endosc. 2006;63:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 142. | Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, Peñas I, de la Serna C, Shah J, Binmoeller K, Gaidhane M, Grimm I, Baron T, Kahaleh M. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4:E276-E281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 143. | Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |