Published online Apr 16, 2023. doi: 10.4253/wjge.v15.i4.273
Peer-review started: December 22, 2022
First decision: February 8, 2023
Revised: February 17, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 16, 2023
Processing time: 112 Days and 11.2 Hours
Solid pseudopapillary neoplasm (SPN) is an uncommon pathology of the pancreas with unpredictable malignant potential. Endoscopic ultrasound (EUS) assessment plays a vital role in lesion characterization and confirmation of the tissue diagnosis. However, there is a paucity of data regarding the imaging assessment of these lesions.
To determine the characteristic EUS features of SPN and define its role in preoperative assessment.
This was an international, multicenter, retrospective, observational study of prospective cohorts from 7 large hepatopancreaticobiliary centers. All cases with postoperative histology of SPN were included in the study. Data collected included clinical, biochemical, histological and EUS characteristics.
One hundred and six patients with the diagnosis of SPN were included. The mean age was 26 years (range 9 to 70 years), with female predominance (89.6%). The most frequent clinical presentation was abdominal pain (80/106; 75.5%). The mean diameter of the lesion was 53.7 mm (range 15 to 130 mm), with the slight predominant location in the head of the pancreas (44/106; 41.5%). The majority of lesions presented with solid imaging features (59/106; 55.7%) although 33.0% (35/106) had mixed solid/cystic characteristics and 11.3% (12/106) had cystic morphology. Calcification was observed in only 4 (3.8%) cases. Main pancreatic duct dilation was uncommon, evident in only 2 cases (1.9%), whilst common bile duct dilation was observed in 5 (11.3%) cases. One patient demonstrated a double duct sign at presentation. Elastography and Doppler evaluation demonstrated inconsistent appearances with no emergence of a predictable pattern. EUS guided biopsy was performed using three different types of needles: Fine needle aspiration (67/106; 63.2%), fine needle biopsy (37/106; 34.9%), and Sonar Trucut (2/106; 1.9%). The diagnosis was conclusive in 103 (97.2%) cases. Ninety-seven patients were treated surgically (91.5%) and the post-surgical SPN diagnosis was confirmed in all cases. During the 2-year follow-up period, no recurrence was observed.
SPN presented primarily as a solid lesion on endosonographic assessment. The lesion tended to be located in the head or body of the pancreas. There was no consistent characteristic pattern apparent on either elastography or Doppler assessment. Similarly SPN did not frequently cause stricture of the pancreatic duct or common bile duct. Importantly, we confirmed that EUS-guided biopsy was an efficient and safe diagnostic tool. The needle type used does not appear to have a significant impact on the diagnostic yield. Overall SPN remains a challenging diagnosis based on EUS imaging with no pathognomonic features. EUS guided biopsy remains the gold standard in establishing the diagnosis.
Core Tip: Solid pseudopapillary neoplasm (SPN) presented primarily as a solid lesion on endosonographic assessment. The lesion tended to be located in the head or body of the pancreas. There was no consistent characteristic pattern apparent on either elastography or Doppler assessment. Similarly SPN did not frequently cause stricture of the pancreatic duct or common bile duct. Importantly, we confirmed that Endoscopic ultrasound (EUS) -guided biopsy was an efficient and safe diagnostic tool. The needle type used does not appear to have a significant impact on the diagnostic yield. Overall SPN remains a challenging diagnosis based on EUS imaging with no pathognomonic features. EUS guided biopsy remains the gold standard in establishing the diagnosis.
- Citation: Pawlak KM, Tehami N, Maher B, Asif S, Rawal KK, Balaban DV, Tag-Adeen M, Ghalim F, Abbas WA, Ghoneem E, Ragab K, El-Ansary M, Kadir S, Amin S, Siau K, Wiechowska-Kozlowska A, Mönkemüller K, Abdelfatah D, Abdellatef A, Lakhtakia S, Okasha HH. Role of endoscopic ultrasound in the characterization of solid pseudopapillary neoplasm of the pancreas. World J Gastrointest Endosc 2023; 15(4): 273-284
- URL: https://www.wjgnet.com/1948-5190/full/v15/i4/273.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i4.273
Solid pseudopapillary neoplasm is a rare tumor of the pancreas which may demonstrate both solid and cystic imaging characteristics. In contrast to other cystic tumors such as serous or mucinous cystic neoplasms that contain a true epithelial lining or intraductal papillary mucinous neoplasm with cystically dilated pancreatic duct or branches filled with mucin, Solid pseudopapillary neoplasm (SPN) is a low-grade malignant tumor, histologically forming solid and pseudopapillary structures with an absence of specific line pancreatic epithelial differentiation[1].
Historically, most SPN were detected in patients presenting with abdominal pain or non-specific abdominal symptoms. At present, due to the wider application of advanced imaging techniques, the majority of these lesions are recognized incidentally[2]. As a result, the incidence of SPN is increasing, now equating to approximately 6% of all exocrine pancreatic neoplasms[2]. Although SPN usually demonstrates indolent behavior, higher grades of malignancy may be encountered and metastases have been reported in up to 20% of cases[2]. Therefore, detection and diagnosis of SPN mandate surgical referral, for consideration of resection. Importantly, SPN are cured by complete surgical resection alone[3].
Despite advances in imaging, pseudocysts, cystic neuroendocrine tumors and other cystic neoplasms may demonstrate similar imaging characteristic, making a pre-operative diagnosis challenging[4]. Furthermore, differentiation of SPN from other pancreatic neoplasms, such as pancreatic neuroendocrine tumors, acinar cell carcinomas, or ductal adenocarcinomas is important because SPN have a significantly improved prognosis compared with other malignant pancreatic tumors[5].
Traditionally computed tomography and magnetic resonance imaging have been considered the key preliminary diagnostic imaging tools for SPN. However, obtaining a final diagnosis remains dependent on cytohistological analysis[6]. The proximity of the pancreas to the stomach and duodenum facilitates endoscopic ultrasound (EUS) examination and the ability to obtain tissue through the fine needle aspiration/biopsy (FNA/FNB), in assessment of SPN. However, given the relative rareness of SPN, there remains a relative paucity of data regarding the role of EUS-guided biopsy rather than pre-operative assessment of the imaging features[7-9]. Therefore, we sought to define the characteristic EUS findings and their role in the preoperative assessment of SPN.
This was an international, multicenter, retrospective observational, open-label study involving seven endoscopy units from India, Egypt, Poland, United Kingdom, France, Romania, and Pakistan. The data has been collected by high-volume endoscopy centers, performing in the region of 1000 diagnostic and interventional EUS procedures per year, including EUS-guided biopsy. In all centers, the evaluation was performed by an expert endosonographer who was defined as having performed at least 1000 hepatopancreaticobiliary (HPB) EUS procedures.
All patients who underwent EUS during a ten year period who ultimately were diagnosed with SPN, (2010-2022), confirmed by histopathological assessment were enrolled in the study. Anonymized data was collected including patient demographics, symptoms, endosonographic features and histological results including EUS-guided biopsy result and surgical confirmation.
All patients were referred to EUS evaluation due to the non-metastatic, growing locally pancreatic tumor recognized in computed tomography for establishing the diagnosis. Information on EUS, images, EUS-guided biopsy including the number of passes, type of needle and fluid biochemistry analysis (amylase, CA 19.9 and mucin stain) from cystic component were recorded using a collective database. In all cases, surgical resection was the treatment of choice, providing definitive histological SPN confirmation.
The study was conducted and carried out in accordance with the Helsinki declaration as revised in 1989. Based on the anonymized data collection, the Institutional Review Board of Pomeranian Medical University in Szczecin granted approval. The study was conducted in the line with the STROBE guidelines.
Data management and analysis were performed using Statistical Package for Social Sciences (SPSS) version 28. Numerical data was summarized using mean and standard deviations or medians and/or ranges as appropriate. Categorical data was summarized as numbers and percentages. Estimates of the frequency were done using the numbers and percentages. Numerical data was explored for normality using Kolmogrov-Smirnov test and Shapiro-Wilk test. Chi square or Fisher’s tests were used to compare between the independent groups with respect to categorical data as appropriate. Comparisons between two groups for normally distributed numeric variables were done using the Student’s t-test while for non-normally distributed numeric variables, comparisons were done by Mann-Whitney test. Comparison between more than 2 groups was performed by Kruskal-Wallis for non-normally distributed variables. All tests were two tailed & Probability (P value) ≤ 0.05 was considered significant.
One hundred and six cases with SPN were included. The median age was 26 years (range 9 to 70), with similar incidence in both genders when compared by age, but with general female predominance (95 females, 89.6%) (Table 1). The majority of patients presented symptomatically (82.1%), among which the most frequent was abdominal pain (75.5%). However, a history of previous acute pancreatitis episodes was only recorded in one patient. Other symptoms included obstructive jaundice (3.8%), vomiting (1.9%) and weight loss (0.9%) (Table 2). In all patients the tumor marker CA 19.9 was normal.
| n = 106 (%) | |
| Age | |
| mean ± SD | 26.4 ± 13 |
| Sex | |
| Female | 95 (89.6) |
| Male | 11 (10.4) |
| Country | |
| Egypt | 48 (45.3) |
| India | 39 (36.8) |
| Poland | 5 (4.7) |
| France | 4 (3.8) |
| United Kingdom | 5 (4.7) |
| Romania | 3 (2.8) |
| Pakistan | 2 (1.9) |
| n (%) | |
| Symptoms | |
| Yes | 87 (82.1) |
| No | 19 (17.9) |
| Presentation | |
| Asymptomatic | 19 (17.9) |
| Abdominal pain | 80 (75.5) |
| Weight loss | 1 (0.9) |
| Obstructive jaundice | 4 (3.8) |
| Vomiting | 2 (1.9) |
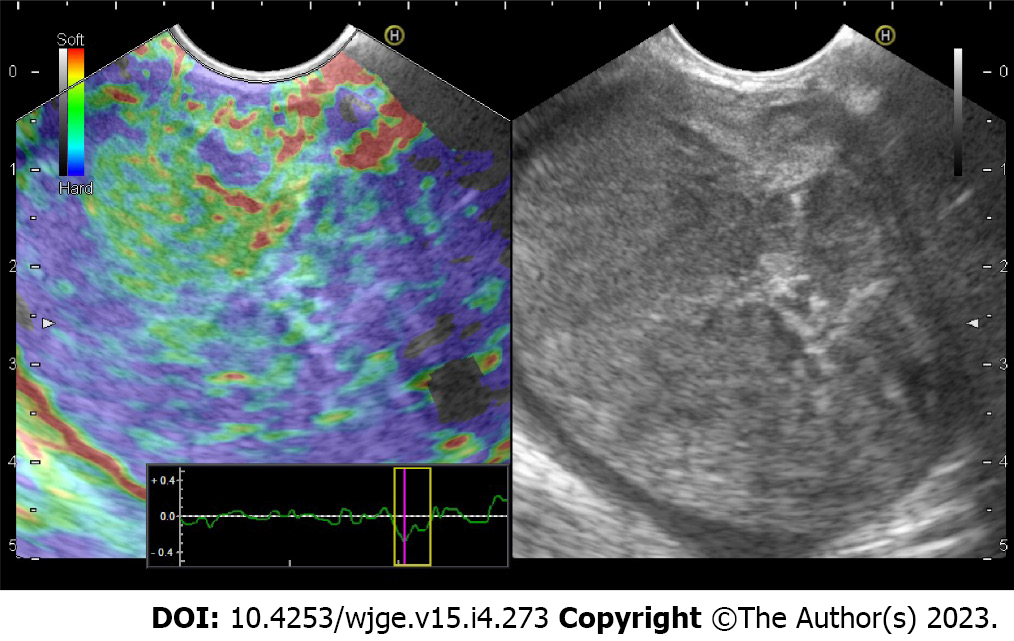
EUS characteristics of solid pseudopapillary tumors: The mean size of the lesion was 52.8 mm (range 15-130 mm), with the predominant location in the head of the pancreas (44/106; 41.5%). Detailed endoscopic ultrasound evaluation was performed identifying lesions with solid (60/106; 56.1%), mixed (43/106; 40.2%), and cystic (3/106; 2.8%) morphology (Figure 1). In terms of endosonographic echotexture, the tumors considered as solid were mainly hypoechoic, heterogeneous, well-demarcated with regular border (Figure 2A). In three cases presented as a solid mass, hyperechoic echotexture corresponding to calcification was observed (Figure 2B). Also, one tumor with pancreatic head location caused a portal vein confluence thrombosis due to external compression and expansile growth. Cystic lesions presented mainly with a multilocular appearance with septations but without mural nodules or honeycomb pattern (Figure 2C). In one case, circumferential calcification was observed. Mixed tumors included both components; however, the solid part demonstrated soft-tissue stiffness on EUS elastography assessment (Figure 3). In addition, Doppler assessment did not demonstrate significant intralesional vascularity or hypervascular infiltration of surrounding structures. Dilation of the main pancreatic duct (MPD) was reported in 2 cases (2/106; 1.9%) of solid SPN (mean size 46 mm; pancreatic head location) measuring up to 10 mm (mean 8.5 mm), while the common bile duct was dilated in 5 cases (5/106; 54.7%) of solid tumors (mean size 61.4 mm; pancreatic head location) with no previous cholecystectomy. Also, there was no correlation between size of the tumor, consistency and anatomical location. The results are summarized in Tables 3-4 and Figure 1.
| n (%) | |
| Location | |
| Head | 44 (41.5) |
| Body | 43 (40.6) |
| Tail | 19 (17.9) |
| Consistency | |
| Solid | 60 (56.1) |
| Mixed | 43 (40.2) |
| Cystic | 3 (2.8) |
| Additional findings | |
| Calcification | 4 (3.8) |
| CBD dilation | |
| Yes | 5 (4.7) |
| No | 101 (95.3) |
| PD dilation | |
| Yes | 2 (1.9) |
| No | 104 (98.1) |
| Size | |
| mean ± SD | 52.8 ± 23.1 |
| Median (range) | 50 (15-130) |
| Size | P value | |
| Consistency/appearance | 0.365 | |
| Cystic, median (range) | 52.5 (36-130) | |
| Solid, median (range) | 45 (15-120) | |
| Mixed, median (range) | 54 (17-95) | |
| Location | 0.4 | |
| Head, median (range) | 51.5 (15-130) | |
| Body, median (range) | 45 (19-100) | |
| Tail, median (range) | 60 (17-125) | |
Technical aspects of EUS-guided biopsy, therapeutic strategy: EUS guided biopsy was performed using three different types of needle: FNA in 67 (63.2%), FNB in 37 (34.9%), Trucut in 2 (1.9%) lesions. For the later, the size 18G and 22G were selected. The 22G and 19G size was mainly selected (94.0%) for the FNA needle type, and 22G for the FNB needle (89.2%). The mean number of passes was 2 and 3 for FNA and FNB needle respectively. Data regarding the needle type used are presented in Table 5.
| n(%) | |
| Needle type | |
| FNA | 67 (63.2) |
| FNB | 37 (34.9) |
| Tru cut | 2 (1.9) |
| Needle size | |
| 19 G | 9 (8.5) |
| 22 G | 88 (83) |
| 18 G | 1 (0.9) |
| 20 G | 2 (1.9) |
| 25 G | 6 (5.7) |
| No of passes | |
| Median (range) | 3 (1-3) |
| FNA | n = 67 (%) |
| Needle size | |
| 19 G | 9 (13.4) |
| 22 G | 54 (80.6) |
| 25 G | 4 (6) |
| No of passes | |
| Median (range) | 2 (1-3) |
| FNB | n = 37 (%) |
| Needle size | |
| 22 G | 33 (89.2) |
| 20 G | 1 (2.7) |
| 25 G | 3 (8.1) |
| No of passes | |
| Median (range) | 3 (1-3) |
| Trucut | n = 2 (%) |
| Needle size | |
| 22 G | 1 (50) |
| 18 G | 1 (50) |
| No of passes | 2 |
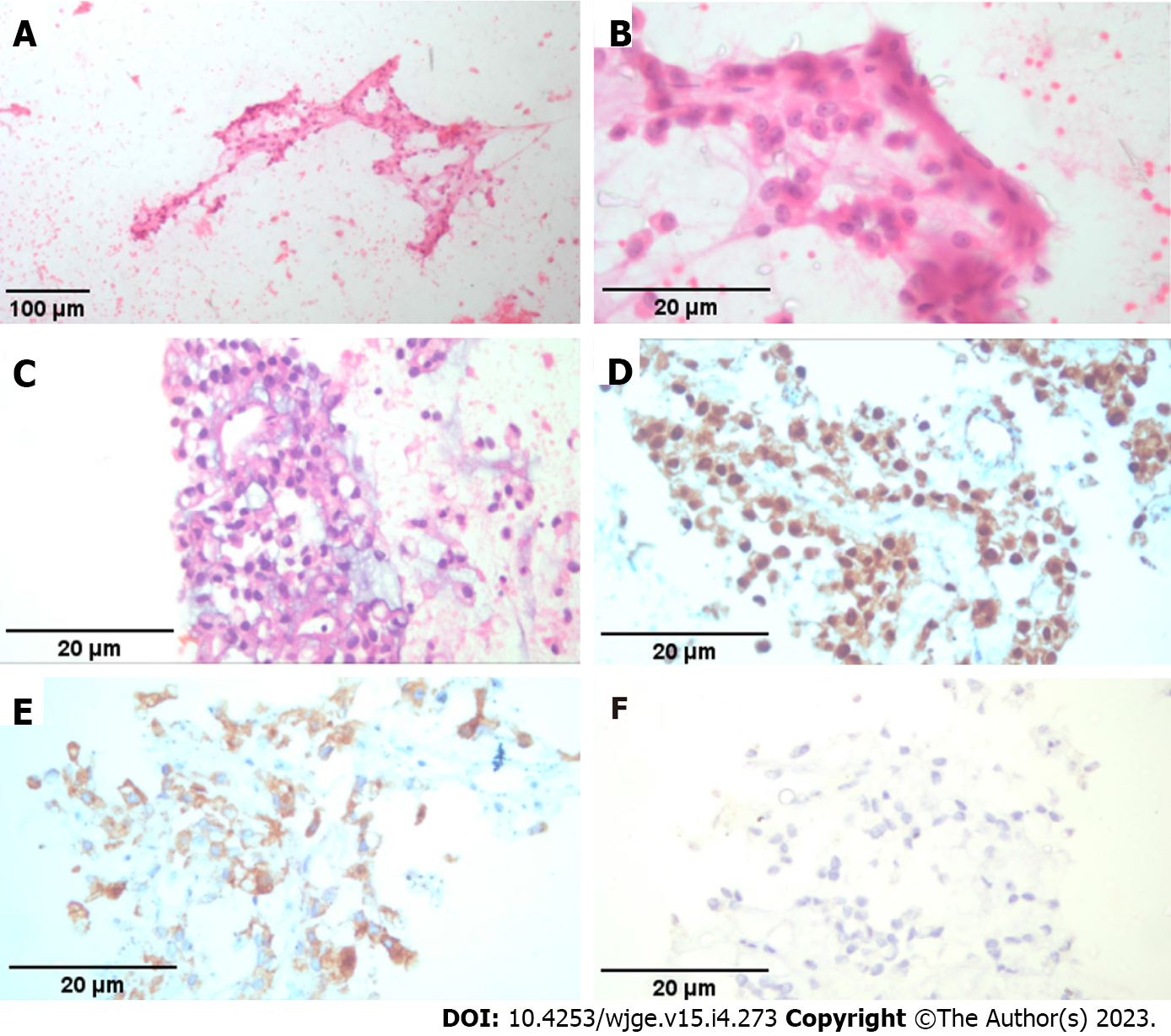
The tissue samples were conclusive in 103 (97.2%) cases (Figure 4). The mucin stain was negative in all cases. Three lesions without conclusive FNA (diagnosed nonspecifically as hemorrhagic material, inflammatory cells and neuroendocrine tumor suspicion) were definitively validated by surgical resection. Consequently a histological diagnosis was reached in all cases (Table 6).
| n (%) | |
| Cytopathology | |
| Non-diagnostic | 3 (2.8) |
| SPN (conclusive) | 103 (97.2) |
| Surgery | |
| Yes | 97 (91.5) |
| No | 9 (8.5) |
| Surgery | |
| Central pancreatectomy | 29 (27.4) |
| Distal pancreatosplenectomy | 21 (19.8) |
| Whipple's panceatoduodenectomy | 47 (44.3) |
| Refused | 9 (8.5) |
Ninety-seven (91.5%) patients were treated surgically (Figure 5). Whipple's/pancreatoduodenectomy was performed in 47 (44.3%), central pancreatectomy in 29 (27.4%) and distal pancreatosplenectomy in 21 (19.8%). Post-surgical SPN confirmation was determined in all cases. Follow up varied according to local protocol and within the 2 year research period, no cases of recurrence or metastatic disease were recorded.
In this study, we found that SPN presented as moderately large lesions without other clinically specific features or typical endosonographic appearance including the size, echotexture, impact on the main ducts (CBD and MPD), and growth pattern. In addition there was no consistent pattern evident in the ancillary EUS features of calcification, vascularity or stiffness (elastography).
The distribution of SPN was in all anatomic components of the pancreas, with a slight dominance in the pancreatic head (41.5%), followed by pancreatic body (40.6%), consistent with the previous published work which has been unable to conclusively demonstrate atypical location for SPN[2,10,11]. Interestingly, of the 44 cases presenting in the head of the pancreas, only four led to a local complication resulting in jaundice and double duct sign was only evident in one case. Importantly, some lesions grew to a significant size without significant symptoms. We performed logistic regression analysis and could not find any correlation between size and symptoms or tumor appearance. Additionally, even the largest tumor with pancreatic head location (130 mm) did not cause double duct sign and most of them did not infiltrate surrounding structures despite such large size, which was in agreement with previous literature[2,11].
In our study group, only one tumor located in the pancreatic head with the size of 42 mm had an expansile growth pattern leading to the compression of portal vein confluence and thrombosis, but without an impact on the bile duct or pancreatic duct. In our cohort, we did not observe infiltrative SPN nature. We believe that the lack of ductal changes may be due to the inherent parenchymal localization of the tumor, with specific growth dynamics that induces a preferential growth away from the pancreas and not towards the main pancreatic duct or bile duct.
Also, there were no typical findings regarding EUS imaging ancillary features such as elastography and Doppler assessment.
Confirming previous reports, we found that the majority of patients were female with a 9:1 ratio[12]. Other features, age, size, and tumor appearance were similar, with no statistical significance. Although variation was demonstrated between genders and lesion location (male – head, female – body predominance), these results were not statistically significant.
Previously, Marchegiani et al[13] found that expansive growth pattern had a statistically significant association with recurrence of SPN. However, during our period of assessment, no cases of local recurrence or metastatic disease were identified. Clearly ongoing surveillance of this group will be of interest.
Importantly, we found that EUS-guided tissue acquisition was an efficient and safe diagnostic tool regardless of biopsy needle type. Consistently a high preoperative diagnostic yield was achieved. We were able to reach a preoperative diagnosis in 97% the patients, confirmed by resected specimen.
Our study has potential shortcomings, including its retrospective design. In addition we did not perform a comparison between the needle type, size and number of passes in terms of the efficiency.
Finally, to our knowledge, this represents the largest multicenter study of SPN to date, with the advantage of varied international geographic location.
In conclusion, we found that SPN presented mainly as solid endosonographic lesions, with slight dominance of pancreatic head location without pathognomonic EUS features that would permit a definitive imaging diagnosis. Despite their large size, SPN do not tend to impinge on the pancreatic duct and more frequently demonstrate a parenchymatous growth. Importantly, we confirmed that EUS-guided biopsy is an efficient and safe diagnostic tool, regardless of needle type, with high preoperative diagnostic yield. We propose that a prospective international study of SPN would further improve our understanding of this rare tumor.
Solid pseudopapillary neoplasm (SPN) is an uncommon pathology of the pancreas with unpredictable malignant potential. Endoscopic ultrasound (EUS) assessment plays a vital role in lesion characterization and confirmation of the tissue diagnosis.
There is a paucity of data regarding the imaging assessment of these lesions.
To determine the characteristic EUS features of SPN and define its role in preoperative assessment.
This was an international, multicenter, retrospective, observational study of prospective cohorts from 7 large hepatopancreaticobiliary centers. All cases with postoperative histology of SPN were included in the study. Data collected included clinical, biochemical, histological and EUS characteristics.
One hundred and six patients with the diagnosis of SPN were included. The mean age was 26 years (range 9 to 70 years), with female predominance (89.6%). The most frequent clinical presentation was abdominal pain (80/106; 75.5%). The mean diameter of the lesion was 53.7 mm (range 15 to 130 mm), with the slight predominant location in the head of the pancreas (44/106; 41.5%). The majority of lesions presented with solid imaging features (59/106; 55.7%) although 33.0% (35/106) had mixed solid/cystic characteristics and 11.3% (12/106) had cystic morphology. Calcification was observed in only 4 (3.8%) cases. Main pancreatic duct dilation (MPD) was uncommon, evident in only 2 cases (1.9%), whilst common bile duct dilation was observed in 5 (11.3%) cases. One patient demonstrated a double duct sign at presentation. Elastography and Doppler evaluation demonstrated inconsistent appearances with no emergence of a predictable pattern. EUS guided biopsy was performed using three different types of needles: FNA (67/106; 63.2%), FNB (37/106; 34.9%), and Sonar Trucut (2/106; 1.9%). The diagnosis was conclusive in 103 (97.2%) cases. Ninety-seven patients were treated surgically (91.5%) and the post-surgical SPN diagnosis was confirmed in all cases. During the 2-year follow-up period, no recurrence was observed.
SPN presented primarily as a solid lesion on endosonographic assessment. The lesion tended to be located in the head or body of the pancreas. There was no consistent characteristic pattern apparent on either elastography or Doppler assessment. Similarly SPNs did not frequently cause stricture of the pancreatic duct or common bile duct. Importantly, we confirmed that EUS-guided biopsy was an efficient and safe diagnostic tool. The needle type used did appear to have a significant impact on the diagnostic yield. Overall SPN remains a challenging diagnosis based on EUS imaging with no pathognomonic features. EUS guided biopsy remains the gold standard in establishing the diagnosis.
We propose that a prospective international study of SPN would further improve our understanding of this rare tumor.
We would like to acknowledge our hospitals, and their workers, nurses and staff members, for all the support and help in this study and throughout our careers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugimoto M, Japan; Yildirim M, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | La Rosa S, Bongiovanni M. Pancreatic Solid Pseudopapillary Neoplasm: Key Pathologic and Genetic Features. Arch Pathol Lab Med. 2020;144:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 3. | Antoniou EA, Damaskos C, Garmpis N, Salakos C, Margonis GA, Kontzoglou K, Lahanis S, Spartalis E, Patsouras D, Kykalos S, Garmpi A, Andreatos N, Pawlik TM, Kouraklis G. Solid Pseudopapillary Tumor of the Pancreas: A Single-center Experience and Review of the Literature. In Vivo. 2017;31:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Roskell DE, Buley ID. Fine needle aspiration cytology in cancer diagnosis. BMJ. 2004;329:244-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Lakhtakia R, Al-Wahaibi K, Zahid KF, Malik KA, Burney IA. Solid pseudopapillary neoplasm of the pancreas: a case report with review of the diagnostic dilemmas and tumor behavior. Oman Med J. 2013;28:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Terris B, Cavard C. Diagnosis and molecular aspects of solid-pseudopapillary neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Mirminachi B, Farrokhzad S, Sharifi AH, Nikfam S, Nikmanesh A, Malekzadeh R, Pourshams A. Solid Pseudopapillary Neoplasm of Pancreas; A Case Series and Review Literature. Middle East J Dig Dis. 2016;8:102-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Sheikh ZA, Alali AA, Almousawi FAS, Das DK. Solid pseudo-papillary tumor of the pancreas: Diagnosis by endoscopic ultrasound-guided fine needle aspiration cytology and immunocytochemistry. Diagn Cytopathol. 2021;49:E242-E246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Lanke G, Ali FS, Lee JH. Clinical update on the management of pseudopapillary tumor of pancreas. World J Gastrointest Endosc. 2018;10:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 10. | Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 12. | Cruz MAA, Moutinho-Ribeiro P, Costa-Moreira P, Macedo G. Solid Pseudopapillary Neoplasm of the Pancreas: Unfolding an Intriguing Condition. GE Port J Gastroenterol. 2022;29:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Marchegiani G, Andrianello S, Massignani M, Malleo G, Maggino L, Paiella S, Ferrone CR, Luchini C, Scarpa A, Capelli P, Mino-Kenudson M, Lillemoe KD, Bassi C, Castillo CF, Salvia R. Solid pseudopapillary tumors of the pancreas: Specific pathological features predict the likelihood of postoperative recurrence. J Surg Oncol. 2016;114:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |