Published online Apr 16, 2023. doi: 10.4253/wjge.v15.i4.265
Peer-review started: December 22, 2022
First decision: February 15, 2023
Revised: February 25, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 16, 2023
Processing time: 113 Days and 3.3 Hours
Endoscopic submucosal dissection (ESD) procedure has a longer procedure time and higher perforation rate than endoscopic mucosal resection owing to technical complications, including a poor field of vision and inadequate tension for the submucosal dissection plane. Various traction devices were developed to secure the visual field and provide adequate tension for the dissection plane. Two randomized controlled trials demonstrated that traction devices reduce colorectal ESD procedure time compared with conventional ESD (C-ESD), but they had limitations, including a single-center fashion. The CONNECT-C trial was the first multicenter randomized controlled trial comparing the C-ESD and traction device-assisted ESD (T-ESD) for colorectal tumors. In the T-ESD, one of the device-assisted traction methods (S–O clip, clip-with-line, and clip pulley) was chosen according to the operator’s discretion. The median ESD procedure time (primary endpoint) was not significantly different between C-ESD and T-ESD. For lesions ≥ 30 mm in diameter or in cases treated by nonexpert operators, the median ESD procedure time tended to be shorter in T-ESD than in C-ESD. Although T-ESD did not reduce ESD procedure time, the CONNECT-C trial results suggest that T-ESD is effective for larger lesions and nonexpert operators in colorectal ESD. Compared with esophageal and gastric ESD, colorectal ESD has some difficulties, including poor endoscope maneuverability, which may be associated with prolonged ESD procedure time. T-ESD may not effectively improve these issues, but a balloon-assisted endoscope and underwater ESD may be promising options and these methods can be combined with T-ESD.
Core Tip: Various traction devices were developed to overcome the challenges faced in endoscopic submucosal dissection (ESD). A multicenter randomized controlled trial in Japan compared the conventional ESD and traction device-assisted ESD (T-ESD) for colorectal tumors. Although T-ESD did not reduce ESD procedure time, the results of this study suggest that T-ESD is effective for larger lesions and nonexpert operators. A balloon-assisted endoscope and underwater ESD may be promising options and these methods can be combined with T-ESD.
- Citation: Nagata M. Device-assisted traction methods in colorectal endoscopic submucosal dissection and options for difficult cases. World J Gastrointest Endosc 2023; 15(4): 265-272
- URL: https://www.wjgnet.com/1948-5190/full/v15/i4/265.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i4.265
In endoscopic submucosal dissection (ESD), obtaining traction for lesions by hand is difficult. Gravity and the hood attached to the endoscope tip can be used to achieve traction while securing the visual field and tension for the dissection plane. However, these methods are occasionally insufficient, resulting in a prolonged procedure time and high perforation risk. Many traction devices have been developed to resolve these issues. Single-center randomized controlled trials (RCTs) reported that the colorectal ESD procedure time is shorter in traction device-assisted ESD (T-ESD) than in conventional ESD (C-ESD)[1,2]. However, no multicenter RCTs have been conducted to evaluate the effectiveness of T-ESD against C-ESD in colorectal ESD.
The CONNECT-C trial was the first multicenter RCT conducted at 10 institutions in Japan[3]. This study aimed to compare the C-ESD and T-ESD for treating colorectal lesions endoscopically diagnosed as early colorectal cancer (Tis-T1a) and measuring > 20 mm in diameter preoperatively. After excluding ineligible patients, 128 patients in the C-ESD group and 123 patients in the T-ESD group were finally included in the analysis.
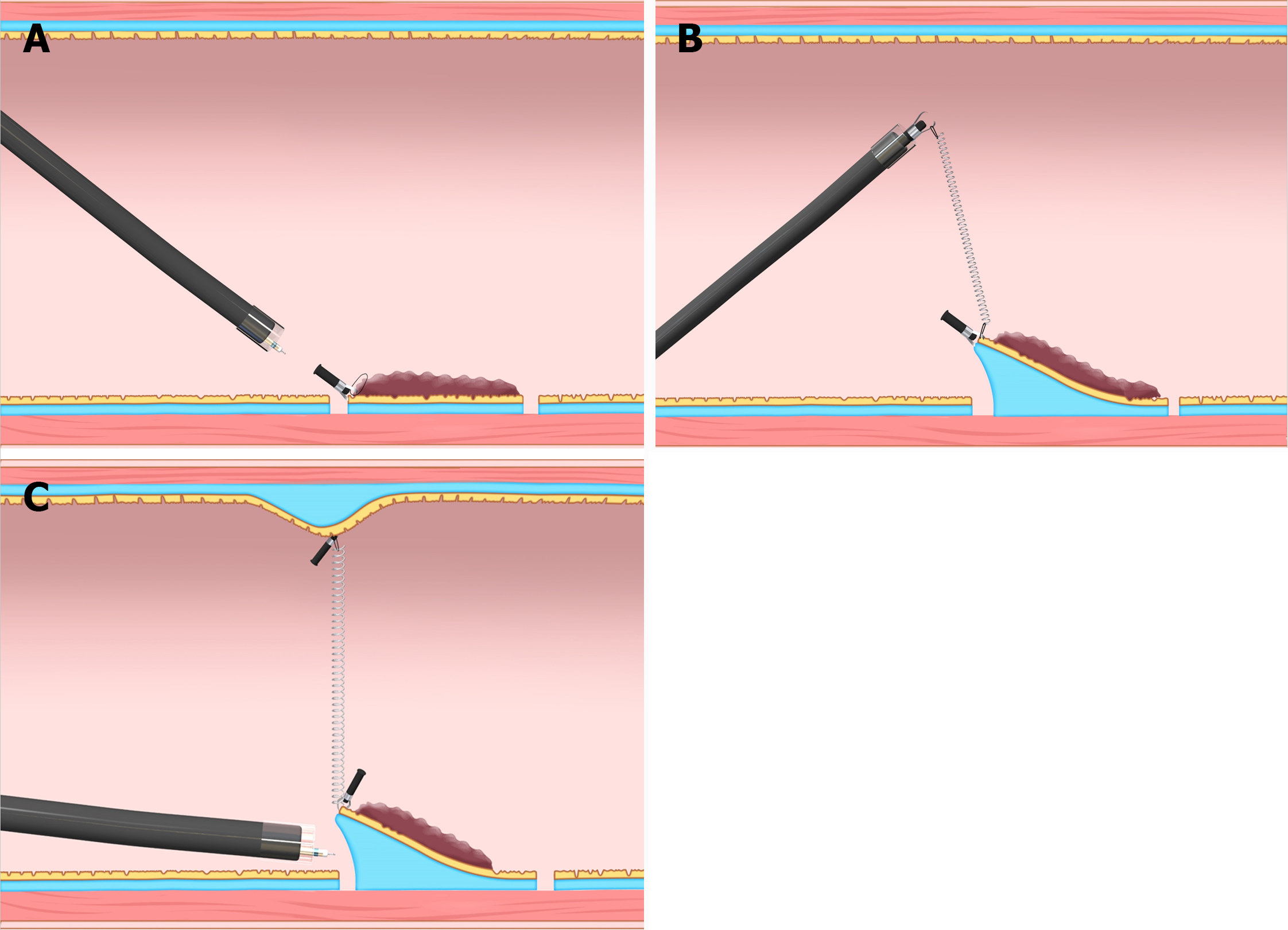
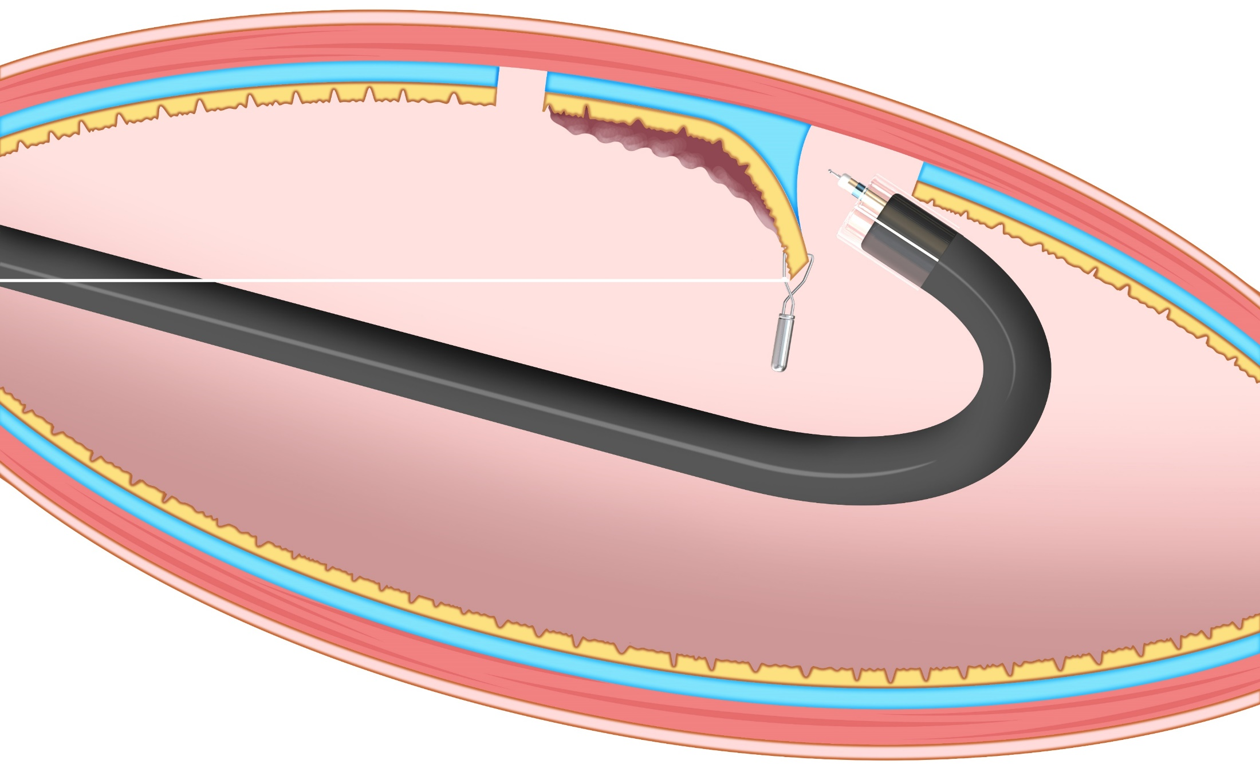
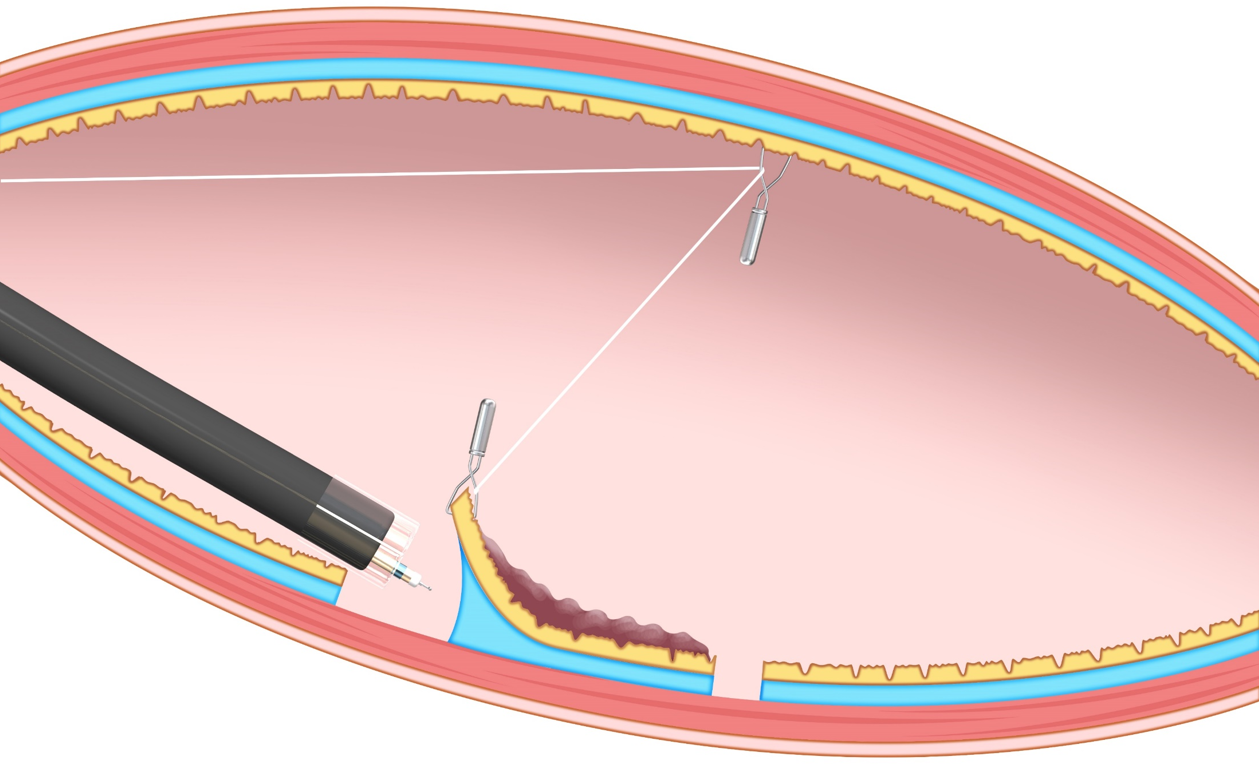
In the T-ESD group, one of the traction methods such as the clip-with-line (with modified preparation to omit the endoscope reinsertion)[4], S–O clip (Zeon Medical, Tokyo, Japan)[1], or the clip pulley[5] was chosen according to the operator’s discretion.
Endoscopists with an experience of 50 or more gastric or esophageal ESD cases were qualified as ESD operators, and those with an experience of 50 or more colon ESD cases were defined as experts. Handover to an expert was permitted if the ESD procedure was prolonged (≥ 60 and ≥ 90 min for lesions measuring < 40 and ≥ 40 mm, respectively).
The primary endpoint was the median ESD procedure time (from the start of the local injection to the end of lesion resection), which was 61 min in the C-ESD group and 53 min in the T-ESD group, showing no significant difference (P = 0.18). The secondary endpoints included the handover to an expert, en bloc resection rate, R0 resection rate, and adverse events; all of them were also not significantly different between the C-ESD and T-ESD groups.
Although not significant, the median ESD procedure time was shorter in the T-ESD group than in the C-ESD group for lesions ≥ 30 mm in diameter (69 vs 89 min, P = 0.05). In patients treated by nonexpert operators, the median ESD procedure time tended to be shorter in the T-ESD group than in the C-ESD group (64 vs 81 min, P = 0.07). These results suggest that T-ESD is effective for larger lesions and nonexpert operators.
In Japan, the multicenter RCTs CONNECT-E and CONNECT-G trials compared C-ESD and T-ESD in the esophagus and stomach, respectively. In the CONNECT-E trial, unlike C-ESD, T-ESD significantly reduced the esophageal ESD procedure time[6]. In the CONNECT-G trial, the gastric ESD procedure time showed no significant difference between the two procedures[7]. These discrepancies indicate that the effectiveness of T-ESD may differ according to how it works. Traction is a force, but it can be described as a vector, which includes size and direction. Therefore, traction direction is important for T-ESD efficiency. In addition, endoscopists need to perform a complicated procedure to change the traction direction during T-ESD. Thus, the impact of traction direction should be discussed.
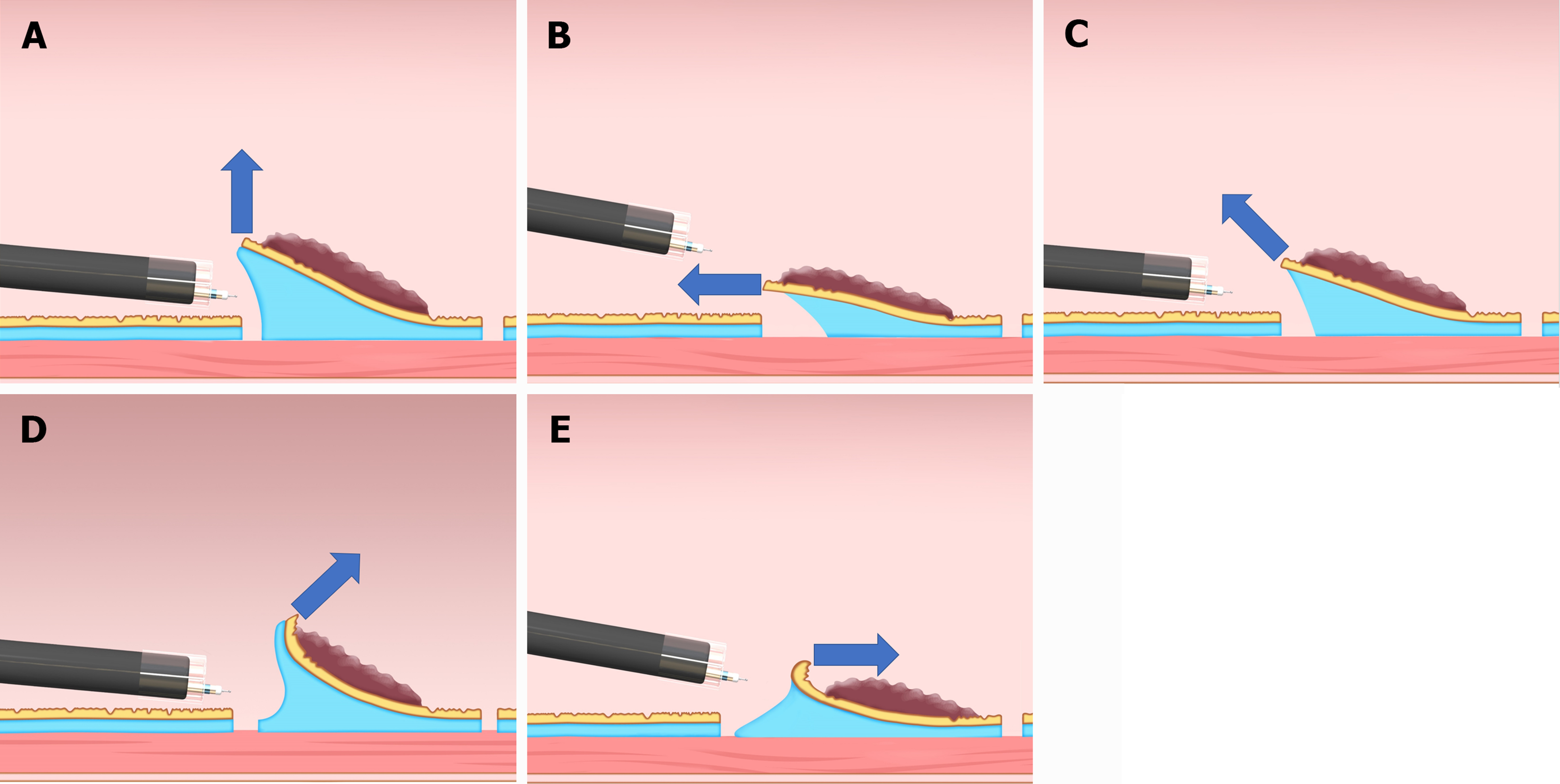
Traction direction can be divided into five categories: Proximal, diagonally proximal, vertical, diagonally distal, and distal direction (Figure 1)[8]. Although studies focusing on traction direction are limited, some study results indicated that traction direction affects T-ESD efficiency. The CONNECT-G trial implied that the vertical traction (Figure 1A) is the most efficient among the five traction directions[9]. A single-center RCT compared C-ESD with T-ESD, which used a multidirectional traction device (S–O clip) for superficial gastric neoplasms; results showed that the vertical traction can reduce the gastric ESD procedure time[10]. The CONNECT-E trial suggested that when the endoscope tip can approach the lesion parallel to the gastrointestinal wall, the esophageal ESD procedure time is shorter in the proximal traction (Figure 1B) than in the C-ESD[6].
Although the effectiveness of diagonally proximal traction (Figure 1C), diagonally distal traction (Figure 1D), and distal traction (Figure 1E) is unclear due to a lack of research on these traction directions, distal traction might be the most ineffective option, because it can make the submucosal dissection plane fall distally when the submucosal dissection progresses and result in thinner submucosa; this may result in accidental dissection of the muscle layer or mucosa due to misrecognition of the layer. Furthermore, distal traction may decrease the effective tension for submucosal dissection, thereby decreasing the dissection speed and prolonging the ESD procedure time. Diagonally distal traction could be advantageous during the initial stages of submucosal dissection, as the traction force aids in widening the incised mucosa and exposing the submucosa, thereby facilitating submucosal dissection. Nevertheless, as submucosal dissection progresses, the dissection plane slowly falls distally from the endoscope tip and becomes thin, making it difficult to provide proper tension for the dissection plane. Diagonally proximal traction may be as effective as the vertical traction, because the dissection plane does not fall distally even when the submucosal dissection progresses, which help in maintaining appropriate tension on the dissection plane.
In the CONNECT-C trial, the three different traction methods, namely, S–O clip (Figure 2), clip-with-line (Figure 3), and clip pulley (Figure 4), were applied in T-ESD according to the operator’s discretion, with 75, 31, and 22 cases analyzed and a corresponding procedure time of 52, 64, and 51 min, respectively. Although not significant, the clip-with-line method tended to have a longer ESD procedure time than the two other methods (P = 0.25). These methods are the same in terms of pulling definition, but they differ in the manner of pulling. The S–O clip and clip pulley can control traction direction. In contrast, the clip-with-line cannot control the traction direction, thereby making submucosal dissection efficiency worse in some cases. Indeed, propensity score matching analysis (42 pairs) showed that the S–O clip method had a shorter median ESD procedure time (28.3 min vs 51.0 min; P = 0.022) and higher dissection speed (24.8 mm2/min vs 17.1 mm2/min, P = 0.001) compared with the clip-with-line method, although this study was conducted in gastric ESD[11]. All traction directions in the S–O clip method were vertical in this study, whereas only 16.7% tractions were vertical in the clip-with-line method. These findings indicate that vertical traction can reduce the gastric ESD procedure time and increase the dissection speed compared with other traction directions. Traction direction may influence the effectiveness of device-assisted traction methods in colorectal ESD, and its impact in traction device-assisted colorectal ESD should be investigated.
Colorectal ESD occasionally faces endoscope maneuverability difficulty (e.g., paradoxical movement) compared with esophageal and gastric ESD. Poor endoscope maneuverability can be a risk factor for ESD interruption, piecemeal resection, and perforation[12]. Moreover, poor endoscope maneuverability may cause a prolonged colorectal ESD procedure time. The three device-assisted traction methods (clip-with-line, S–O clip, and clip pulley) cannot improve endoscope maneuverability. Therefore, other options should be considered.
The balloon-assisted endoscope (BAE) is reportedly effective for colorectal ESD with poor endoscope maneuverability. A single-center retrospective study evaluated 83 deep-colon (from descending colon to cecum) ESD cases showing poor endoscope maneuverability preoperatively and treated with BAE (BAE group; n = 54) or without BAE (non-BAE group; n = 29)[13]. The ESD procedure time or dissection speed showed no significant differences between the two groups, but subgroup analysis showed the dissection speed in cecum and ascending colon cases was significantly faster in the BAE group than in the non-BAE group (22.3 and 11.3 mm2/min, respectively; P = 0.037). BAE can improve the endoscope maneuverability; thus, the endoscope can approach the target lesion stably and improve ESD efficiency despite poor endoscope maneuverability. BAE may reduce the risk of intraoperative perforation. A propensity score matching analysis comparing BAE-ESD and C-ESD in the proximal colon revealed that BAE-ESD significantly decreased intraoperative perforation for lesions ≥ 40 mm in diameter (0% vs 24%, P = 0.0188), although there were no significant differences in the en bloc resection rate (95% vs 99%, P = 0.17), R0 resection rate (92% vs 96%, P = 0.30), mean dissection speed (16 mm2/min vs 16 mm2/min, P = 0.53), and intraoperative perforation (5% vs 6%, P = 0.73) between BAE-ESD and C-ESD[14]. Combining T-ESD with BAE may improve procedure-related outcomes in colorectal ESD cases with poor endoscope maneuverability. However, the effectiveness of this combination has not been comprehensively investigated. Therefore, future studies should focus on counteracting this issue.
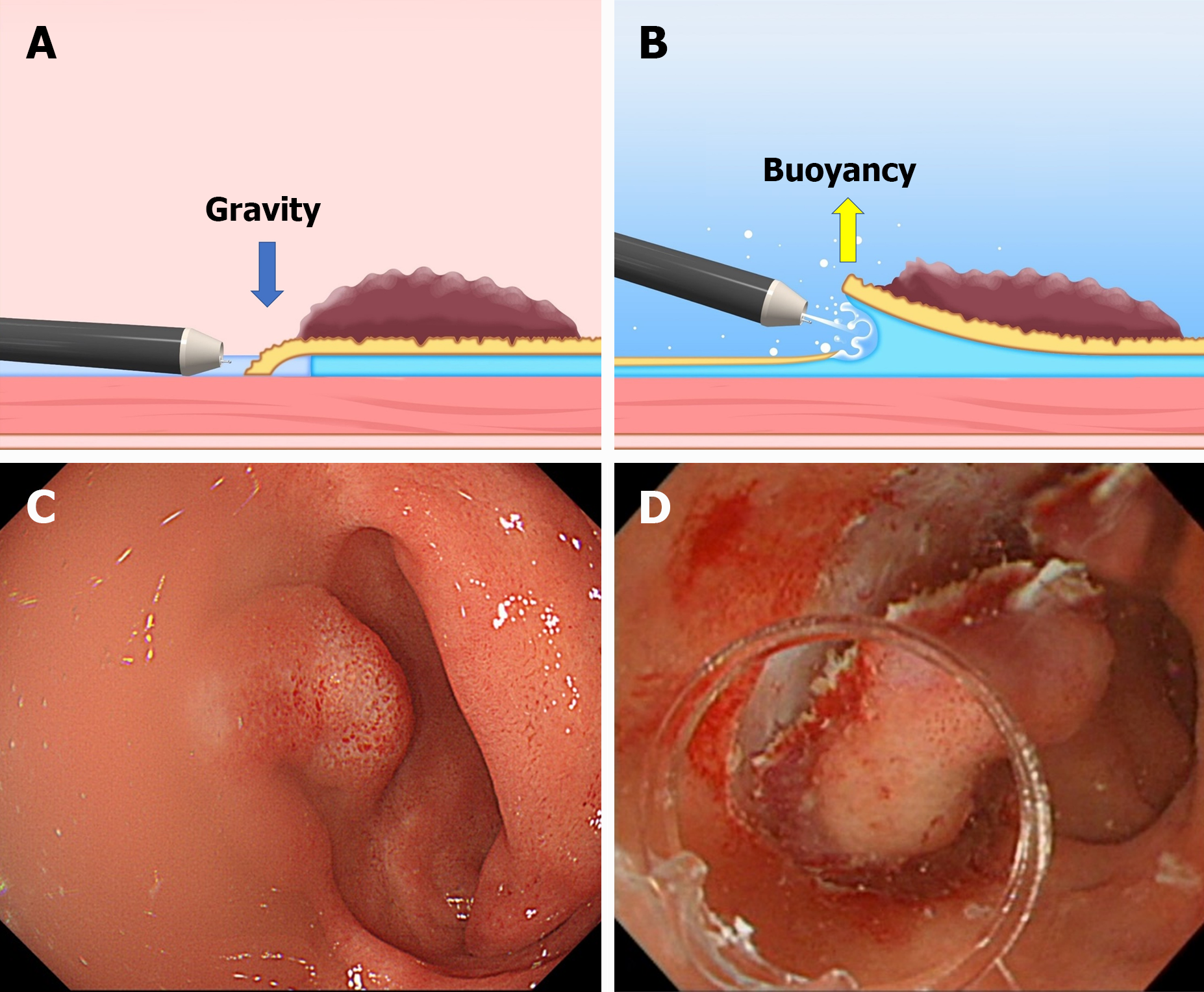
The advantages of U-ESD are as follows: A clear visual field without halation; buoyancy; easy use of water pressure for opening the mucosal cutting edge; and the heat-sink effect, which can be useful for cases with severe fibrosis, abundant fat tissue, and gravitational lower side (Figure 5)[15]. U-ESD may also improve the endoscope maneuverability. A single-center retrospective study reported that U-ESD is beneficial for difficult colorectal lesions, including lesions with poor endoscope maneuverability[16]. In U-ESD, even in a collapsed colorectal lumen resulting from degassing, an underwater condition can help secure the field of vision, improving the operability of the endoscope. Moreover, U-ESD can be combined with T-ESD[17-19]. However, commercially available devices for ESD (e.g., hood, electrosurgical knife, and hemostatic forceps) are basically designed to be used under gas insufflation, and some of them are not suitable for use in underwater conditions. In most U-ESD reports, a straight needle knife (e.g., Dual Knife; Olympus, Tokyo, Japan) was used as the electrosurgical knife. A long bent-type knife (Hook Knife; Olympus, Tokyo, Japan) can also be used with U-ESD, but the setting of the electrosurgical unit needs to be changed appropriately for use in underwater conditions[20].
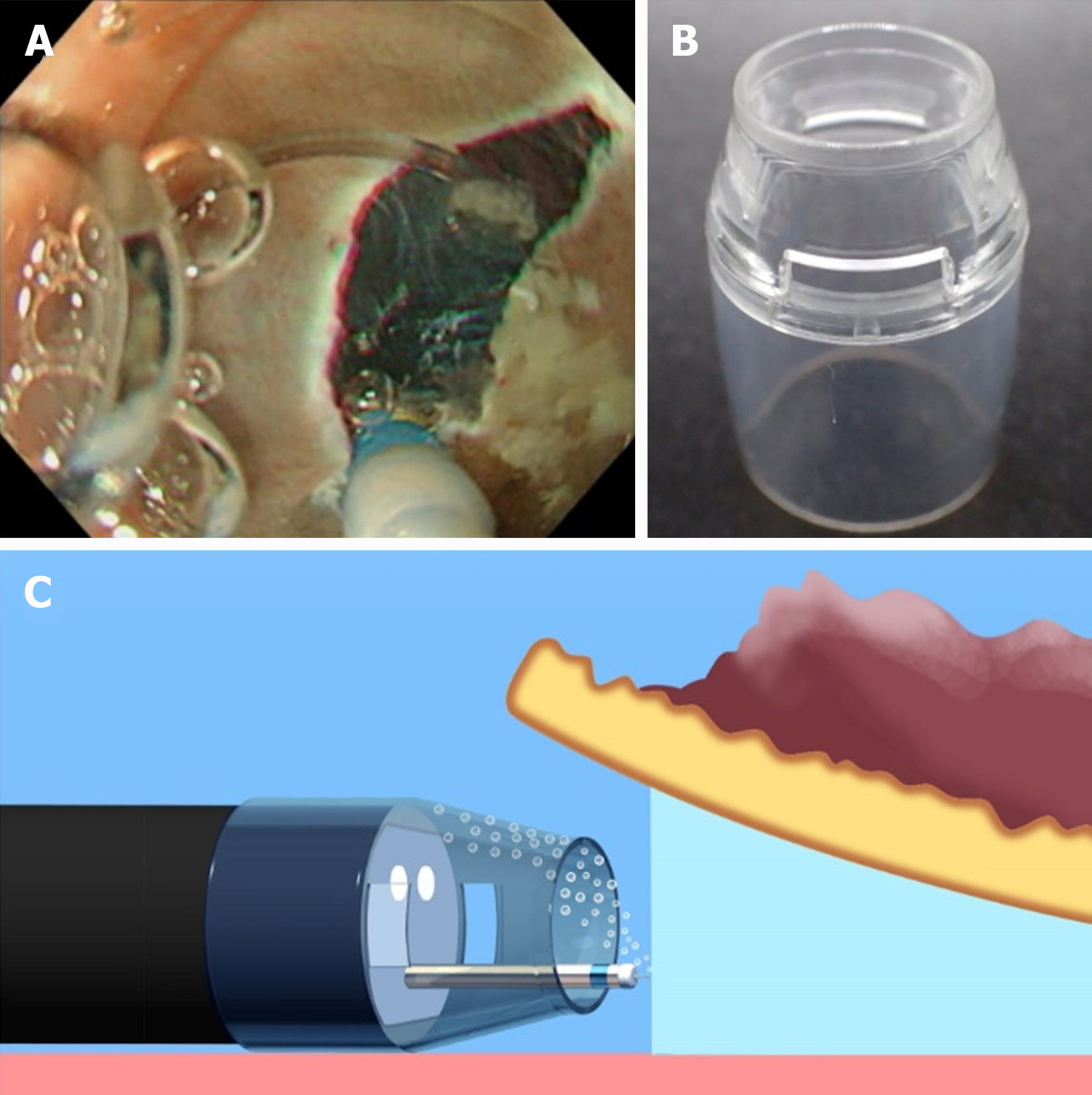
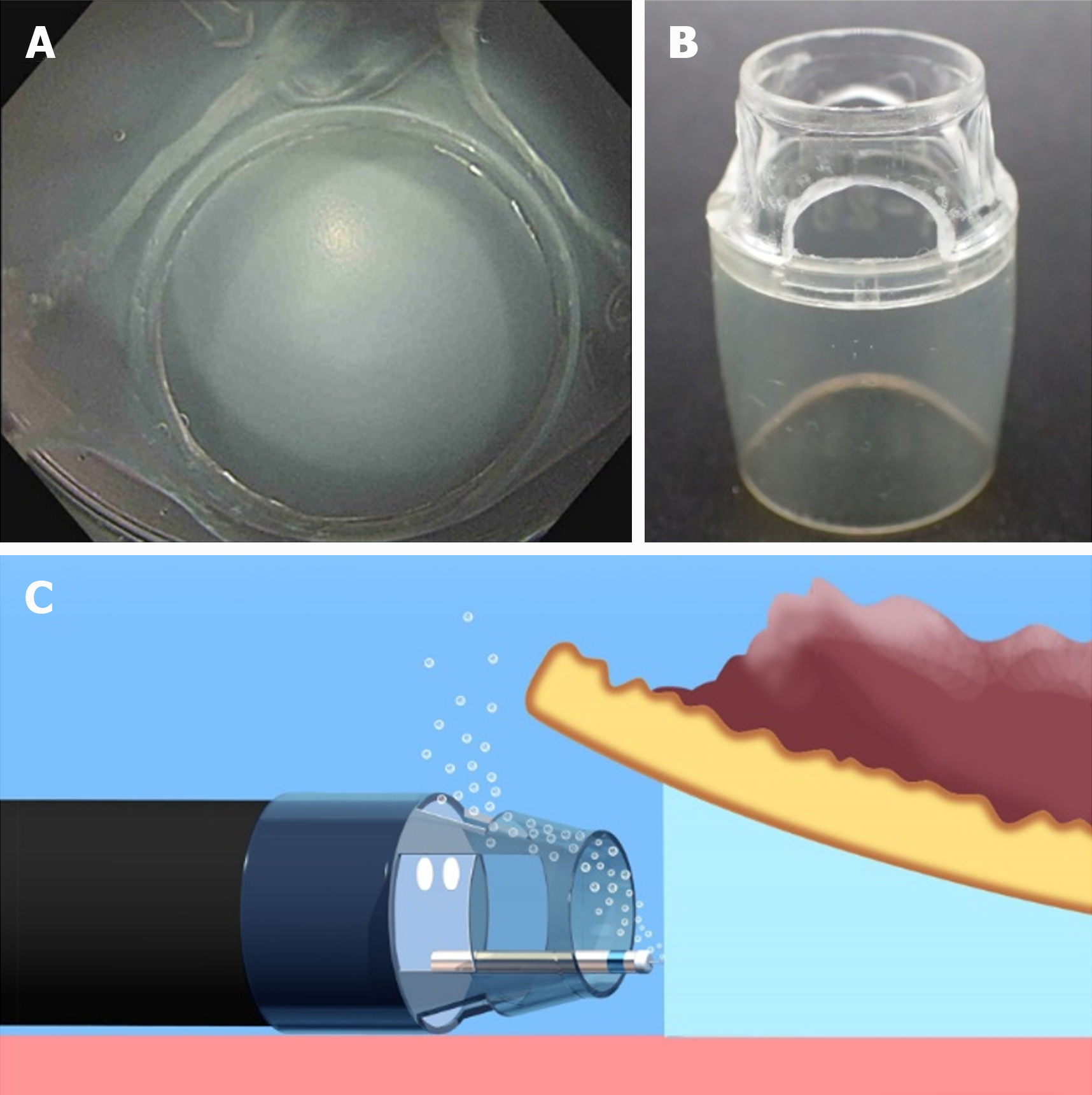
Conversely, U-ESD has two major disadvantages: visual field loss due to air bubble formation and active bleeding. In most U-ESD reports, a tapered hood, e.g., ST Hood Short Type (Fujifilm Medical, Tokyo, Japan), was used to facilitate the endoscope tip entering into the submucosal space. Air bubbles can be generated during the U-ESD procedures and accumulated in the tapered hood attached to the endoscope tip, resulting in visual field impairment (Figure 6). Recently, a novel tapered hood was reported to be efficient in removing air bubbles from the inside of the tapered hood, thereby maintaining the visual field during U-ESD (Figure 7)[21]. Regarding active bleeding, gel immersion endoscopy can be used to secure the visual field[22,23]. However, most of the U-ESD reports are mainly case reports and case series studies. The feasibility of U-ESD needs further investigation. Moreover, devices that are suitable for use in U-ESD should be developed.
The CONNECT-C trial suggests that T-ESD is effective for larger lesions and nonexpert operators in colorectal ESD. Compared with esophageal and gastric ESD, colorectal ESD faces some difficulties, such as poor endoscope maneuverability and strong angulated lumen, which are possibly associated with a prolonged ESD procedure time. These issues may not be effectively improved by device-assisted traction methods, but BAE and U-ESD may be promising options and these methods can be combined with T-ESD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Katayama Y, Japan; Panarese A, Italy S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Ritsuno H, Sakamoto N, Osada T, Goto SP, Murakami T, Ueyama H, Mori H, Matsumoto K, Beppu K, Shibuya T, Nagahara A, Ogihara T, Watanabe S. Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S-O clip. Surg Endosc. 2014;28:3143-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Yamasaki Y, Takeuchi Y, Uedo N, Kanesaka T, Kato M, Hamada K, Tonai Y, Matsuura N, Akasaka T, Hanaoka N, Higashino K, Ishihara R, Okada H, Iishi H. Efficacy of traction-assisted colorectal endoscopic submucosal dissection using a clip-and-thread technique: A prospective randomized study. Dig Endosc. 2018;30:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Ichijima R, Ikehara H, Sumida Y, Inada T, Nemoto D, Nakajima Y, Minagawa T, Sumiyoshi T, Inoki K, Yoshida N, Inoue K, Fukuzawa M, Minoda Y, Tsutsumi K, Esaki M, Gotoda T. Randomized controlled trial comparing conventional and traction endoscopic submucosal dissection for early colon tumor (CONNECT-C trial). Dig Endosc. 2023;35:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Yamasaki Y, Takeuchi Y, Hanaoka N, Higashino K, Uedo N, Ishihara R, Iishi H. A novel traction method using an endoclip attached to a nylon string during colonic endoscopic submucosal dissection. Endoscopy. 2015;47 Suppl 1:E238-E239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Ikehara H, Kirtane T, Gotoda T. Endoscopic submucosal dissection by use of a modified dental floss clip method for recurrent tumor in the cecum. VideoGIE. 2017;2:356-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Yoshida M, Takizawa K, Nonaka S, Shichijo S, Suzuki S, Sato C, Komori H, Minagawa T, Oda I, Uedo N, Hirasawa K, Matsumoto K, Sumiyoshi T, Mori K, Gotoda T, Ono H; CONNECT-E Study Group. Conventional vs traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc. 2020;91:55-65.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 7. | Yoshida M, Takizawa K, Suzuki S, Koike Y, Nonaka S, Yamasaki Y, Minagawa T, Sato C, Takeuchi C, Watanabe K, Kanzaki H, Morimoto H, Yano T, Sudo K, Mori K, Gotoda T, Ono H; CONNECT-G Study Group. Conventional vs traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc. 2018;87:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Nagata M. Advances in traction methods for endoscopic submucosal dissection: What is the best traction method and traction direction? World J Gastroenterol. 2022;28:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 9. | Nagata M. Optimal traction direction in traction-assisted gastric endoscopic submucosal dissection. World J Gastrointest Endosc. 2022;14:667-671. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc. 2021;93:1097-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Nagata M, Namiki M, Fujikawa T, Munakata H. Impact of Traction Direction in Traction-Assisted Gastric Endoscopic Submucosal Dissection (with Videos). Dig Dis Sci. 2023:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kamigaichi Y, Oka S, Tanaka S, Nagata S, Kunihiro M, Kuwai T, Hiraga Y, Furudoi A, Onogawa S, Okanobu H, Mizumoto T, Miwata T, Okamoto S, Yoshimura K, Chayama K. Factors for conversion risk of colorectal endoscopic submucosal dissection: a multicenter study. Surg Endosc. 2022;36:5698-5709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Kuroki Y, Asonuma K, Uehara N, Endo T, Suzuki R, Yamamoto Y, Nagahama M. Efficacy and suitable indication of colorectal endoscopic submucosal dissection using a balloon-assisted endoscope. JGH Open. 2020;4:185-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Tanaka H, Oka S, Tsuboi A, Kamigaichi Y, Tamari H, Sumioka A, Shimohara Y, Nishimura T, Inagaki K, Okamoto Y, Iio S, Yamashita K, Sumimoto K, Tanaka S. Efficacy of single-balloon overtube for endoscopic submucosal dissection in the proximal colon: A propensity score-matched analysis. DEN Open. 2022;2:e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos). Gastrointest Endosc. 2018;87:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Cecinato P, Lucarini M, Campanale C, Azzolini F, Bassi F, Sassatelli R. Underwater endoscopic submucosal dissection and hybrid endoscopic submucosal dissection as rescue therapy in difficult colorectal cases. Endosc Int Open. 2022;10:E1225-E1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Mavrogenis G, Mavrogenis I, Anastasiadis S, Bazerbachi F. Underwater endoscopic submucosal dissection in saline solution with rubber-band countertraction for a cecal polyp extending into a diverticulum. Ann Gastroenterol. 2019;32:527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kono M, Nagami Y, Kitagawa D, Manabe T, Ominami M, Fukunaga S, Fujiwara Y. Underwater endoscopic submucosal dissection for a duodenal neuroendocrine tumor using pocket creation and ring-shaped thread countertraction methods. Endoscopy. 2021;53:E110-E111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Hamada K, Horikawa Y, Shiwa Y, Techigawara K, Ishikawa M, Nishino N, Honda M. Underwater and traction-assisted endoscopic submucosal dissection in the gastric fundus using a multibending endoscope. Endoscopy. 2023;55:E312-E313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 20. | Nagata M. Underwater endoscopic submucosal dissection in saline solution using a bent-type knife for duodenal tumor. VideoGIE. 2018;3:375-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Nagata M. Tapered hood with wide holes in its sides for efficient air bubble removal during underwater endoscopic submucosal dissection. Dig Endosc. 2022;34:654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Yano T, Nemoto D, Ono K, Miyata Y, Numao N, Iwashita C, Nagayama M, Takahashi H, Lefor AK, Yamamoto H. Gel immersion endoscopy: a novel method to secure the visual field during endoscopy in bleeding patients (with videos). Gastrointest Endosc. 2016;83:809-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Akasaka T, Takeuchi Y, Ishida H, Mita E. A novel gel immersion technique using a bipolar needle-knife in endoscopic submucosal dissection for superficial gastrointestinal neoplasms. Ann Gastroenterol. 2018;31:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |