Published online Apr 16, 2023. doi: 10.4253/wjge.v15.i4.240
Peer-review started: November 26, 2022
First decision: January 23, 2023
Revised: February 2, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 16, 2023
Processing time: 138 Days and 19.5 Hours
Gastric signet-ring cell gastric carcinoma (GSRC) is an unfavorable subtype of gastric cancer (GC) that presents with greater invasiveness and poorer prognosis in advanced stage than other types of GC. However, GSRC in early stage is often considered an indicator of less lymph node metastasis and more satisfying clinical outcome compared to poorly differentiated GC. Therefore, the detection and diagnosis of GSRC at early stage undoubtedly play a crucial role in the mana
Core Tip: Gastric signet-ring cell gastric carcinoma (GSRC) represents a special subtype of gastric cancers with unique clinical and pathological characteristics. With the advancement of endoscopic technology, the diagnosis and curability of early GSRC have been substantially improved. This overview gives the latest update on the endoscopic diagnosis and treatment of early GSRC.
- Citation: Tang YH, Ren LL, Mao T. Update on diagnosis and treatment of early signet-ring cell gastric carcinoma: A literature review. World J Gastrointest Endosc 2023; 15(4): 240-247
- URL: https://www.wjgnet.com/1948-5190/full/v15/i4/240.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i4.240
Gastric signet ring cell carcinoma (GSRC) is a unique histological type of gastric cancer (GC). GSRC is mainly composed of more than 50% of signet ring cells (SRC) based on the World Health Organization (WHO) classification. The SRC is characterized by crescent-shaped nuclear distributed in the side of the cell and substantial cytoplasm filled with large mucin vacuole[1,2]. GSRC is associated with aggressive invasiveness and abdominal implantation metastasis due to E-cadherin down-regulation[3]. Most GSRC patients were diagnosed at an advanced stage in the past years due to the underdevelopment of screening technology, leading to poorer prognosis and decreased quality of life compared with patients with other types of GC. However, studies have shown that the clinical course of GSRC is significantly different between the early stage and advanced stage, indicating that treatment at an early stage can improve the prognosis of GSRC patients. Therefore, different treatment methods should be applied for early GSRC and advanced GSRC[4]. Conventional endoscopy may miss early GSRC due to its morphological traits. However, the diagnosis rate of GSRC has significantly increased due to the advancement of endoscopic imaging technology, including narrow-band imaging (NBI), magnifying endoscopy (ME), and endoscopic ultrasonography (EUS). The margin and size of the tumor can be determined using NBI, while the invasion depth and lymph node metastasis (LNM) can be detected using EUS[5].
Endoscopic resection (ER) is the current standard treatment for early GC that meets the indication for ER. ER includes endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)[6,7]. Nevertheless, ESD is the current standard treatment for early GC since it allows complete dissection of intramucosal lesions with better flexibility and effectiveness than EMR. Although some early GSRC patients undergoing ER experience local recurrence and LNM, they have better overall survival (OS) than patients with poorly differentiated adenocarcinoma (PDC)[8,9]. This article reviews the progress related to the endoscopic diagnosis and treatment of early GSRC in recent years and offers a different perspective on managing early GSRC patients.
GSRC is more common in women and young patients than non-SRCC (NSRC). Early-stage GSRC lacks typical clinical manifestations. Most patients with early GSRC complain of chronic gastritis-like symptoms, such as abdominal pain and abdominal distension, which can be misdiagnosed as gastritis and peptic ulcer. Although both SRC and PDC are grouped into the “undifferentiated type” (UD) based on Nakamura classification, the two subtypes may have different histological and clinical characteristics[6]. Unlike PDC which tends to infiltrate the submucosa, early GSRC is common in the mucus layer of the stomach, showing a comparably lower risk of LNM[4]. The intramucosal GSRC yields a more agreeable clinical course than PDC, possibly due to its subepithelial spreading pattern[10]. SRC spreads horizontally in the laminapropria without invading the gastric epithelium. As a result, it is difficult to accurately evaluate the size and margin of SRC since endoscopy may not detect the part of SRC hiding beneath the normal gastric epithelium.
Most intramucosal GSRCs have a double-layer structure (DLS)[11]. The upper layer of DLS is mainly composed of abundant mucins in the cytoplasm and an eccentric nucleus, while the lower layer mainly contains a few intracytoplasmic mucins and acidophilic cytoplasm. Proliferative cells disseminate between these two layers. DLS is defined if the lesion satisfies the following criteria in immunohistochemical staining: (1) MUC5AC is positive only in the superficial layer; (2) Ki-67 is positive only in the middle proliferative area; and (3) MUC6 is positive in the deep layer. The existence of DLS suggests that SRCs are in a low proliferative station, while the destruction or the absence of DLS indicates an activated phase of proliferation and invasion, resulting in a higher risk of LNM and submucosal infiltration.
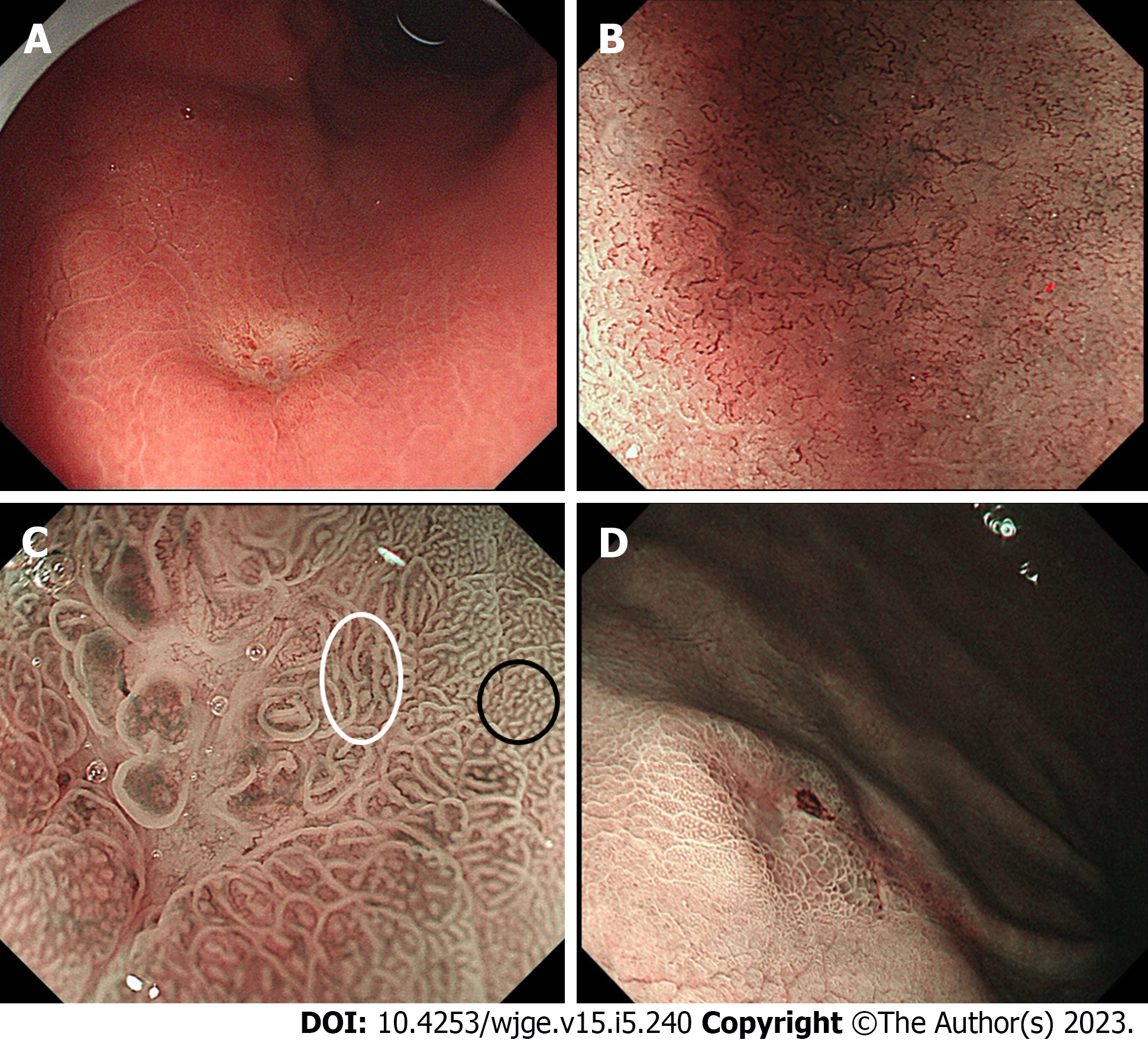
Early GSRC has similar endoscopic features to other types of undifferentiated carcinoma. Early GSRC is characterized by flat or depressed lesions with discoloration under traditional white light imaging (WLI) (Figure 1A). Most lesions are located in the middle 1/3 of the stomach, without signs of mucosal change, such as ulcers, depressions, or elevation[12,13]. Biopsies and further endoscopic imaging techniques should be actively used when lesions with the above characteristics are encountered under WLI to prevent missed diagnosis.
Magnifying endoscopy combined with narrow-band light imaging (ME-NBI) is crucial for diagnosing GC. Endoscopists can capture the microsurface structure (MS) and microvascular architecture (MV) of early GC through NBI without chemical staining[14]. Yao et al[14] Invented the “VS classification” based on the MS and MV pattern under ME-NBI and described the surface structure of early gastric cancer (EGC), and set the criteria for EGC that distinguishes it from noncancerous lesions. They found that most carcinomas under ME-NBI have either an irregular microvascular pattern or an irregular microsurface pattern with a demarcation line. Ok et al[15] showed that most early GSRC do not have MS, and have a corkscrew MV pattern similar to that of other undifferentiated carcinomas (Figure 1B). Phalanusitthepha et al[12] investigated the endoscopic features of SRC using ME-NBI and found that early GSRC has a specific "stretching sign" under NBI. The stretching sign indicates the stretching and dilatation of the irregular gastric glands and microvasculature that cannot be identified in non-SRC lesions (Figure 1C). However, further investigations with a larger sample are required to confirm the accuracy of this result. Meanwhile, ME-NBI can be used in the diagnosis and prediction of GSRC before the histological examination.
Although ME-NBI shows a clear demarcation line in most EGC patients that separates the lesion from the surrounding mucosa, studies have confirmed that ME-NBI cannot accurately identify the lateral extent of undifferentiated EGC as in the case of differentiated GC[14,16,17]. Watari et al[18] found that non-magnifying NBI (NM-NBI) can also detect early GSRC. GSRCs, including early GSRC have well-defined whitish lesion differentiating them from the surrounding brown mucosa (Figure 1D). Therefore, the NBI combined with magnifying endoscopy may improve the diagnosis of early GSRC and the accurate delineation of such lesions.
EUS is widely used to predict the depth of tumor invasion and LNM in GC patients. However, its efficacy in predicting the invasion depth of EGC is controversial (accuracy 76%-97%)[19,20]. Kuroki et al[19] reported that EUS has better sensitivity, specificity, and accuracy in terms of diagnosis of infiltration depth for mucosal tumors than conventional endoscopy. However, they also showed that the accuracy of EUS in diagnosing invasion depth is less comparable for lesions larger than 20 mm, or those with ulcerations[21]. Pei et al[20] indicated that the accuracy, sensitivity, and specificity of preoperative EUS in lymph node staging are about 65%-95%, 0.82 and 0.68, respectively. Therefore, further investigations should be conducted to assess the value of EUS in diagnosing invasion depth and LNM in GSRC patients.
ESD has become the optimal treatment for early GC due to the rapid development of endoscopic diagnosis and treatment. Besides, ESD is less invasive, less costly, and can maintain gastric function in patients[22]. Patients with undifferentiated cancer less than 2 cm, without ulcer, with a negative margin, and negative lymphovascular invasion (LVI) are included as the expanded indication of ESD based on the guidelines for GC treatment of Japanese Gastric Cancer Association (JGCA)[2]. Therefore, the clinicopathological features of the patients should be carefully evaluated before using ESD for the treatment of early GSRC. ESD could be a safe and effective treatment for patients with pure SRC who meet the absolute indications. Besides patients meeting the absolute indications, the LNM risk of intramucosal GSRC lesions between 20 mm and 40 mm is also relatively low[11,23]. The presence of DLS guarantees low LNM risk and prevents submucosal invasion. DLS can only serve as a predictive factor in curability evaluation after ESD since the determination of DLS presence depends on postoperative pathological specimens of ESD. Therefore, future studies should assess whether the presence of DLS can be identified based on preoperative pathology to extend the indication for early GSRC to lesions of 20-40 mm with positive DLS, without ulceration and without lymphovascular invasion. However, surgical gastrectomy and lymphectomy should be performed for early mixed GSRC with poorly differentiated components because of the higher LNM risk. Furthermore, a more strict surveillance strategy should be applied in such patients if they have been treated with ESD to prevent local recurrence.
ESD curative resection (CR) is defined as the complete removal of the lesion with no evidence of tumor invasion on both horizontal and vertical margins of the resected specimen[24]. Studies have found that early GSRC patients undergoing ESD have an unfavorable CR rate. For example, Kim et al[25] reported a CR rate of 70.7% for SRC patients, while Bang et al[26] detected a CR rate of only 36.4% for patients receiving CR after ESD. Kim et al[27] found that tumor size is a key risk factor for non-curative resection (NCR) in ESD patients. Among patients who received NCR, tumors in PDC patients had a higher tendency to infiltrate into the SM layer, creating a higher possibility of a positive vertical margin. In contrast, tumors in most early GSRC patients receiving NCR were positive at the horizontal margin[26].
This phenomenon could be due to the special growth pattern of GSRC. Tubular cervical dysplasia located in the lamina propria of the mucosa is the precursor of GSRC[28]. GSRC grows horizontally in the lamina propria, and its surface is covered by intact gastric foveolar epithelium. Therefore, the actual size of the tumor might be larger than the size measured by endoscopy, even if the boundary of GSRC can be clearly identified. Lee et al[10] evaluated the pathological specimens and background mucosa of 86 GSRC patients and found that 75% had a subepithelial growth pattern, especially in lesions with discoloration. Kim et al[13] discovered that subepithelial spreading is common in GSRCs surrounded by atrophic mucosa or intestinal metaplasia (IM). This growth pattern can make the tumor look small, leading to inaccurate delineation of the tumor, thus increasing the likelihood of tumor invasion on the horizontal margin and consequently resulting in NCR.
Kim et al[29] compared the endoscopic size and pathological size of GSRC patients after ESD and found no statistical difference. However, the pathological size of the tumor was larger than the endoscopic size by 0.2 mm. The results further confirmed that the tumor size was underestimated in more than 30% of total patients due to the subepithelial growth pattern of GSRC. Furthermore, the maximum difference between endoscopic size and pathological size reached 6 mm. Multivariate analysis showed that tumor size and atrophy of the surrounding mucosa are the risk factors for size underestimation.
Therefore, accurate evaluation of tumor size and determination of the resection margin is crucial for complete resection. Besides, four-quadrant biopsies should be performed, followed by ESD if all the biopsies are negative for tumor involvement. ME-NBI and NM-NBI should be used to decide on the size measurement and delineation of GSRC. The horizontal margin of the tumor should be at least 1 cm from the margin of the tumor to reduce the possibility of tumor involvement and improve the curability of GSRC, especially for patients with atrophic surrounding mucosa and discolored lesions or IM confirmed via endoscopic inspection.
Although gastrectomy is recommended for GSRC patients receiving NCR after ESD, most studies have shown that only a few patients are exposed to LNM after NCR[30]. The curability evaluation algorithm proposed by JGCA is widely used in decision-making after non-curative resection of ESD[2]. Patients are divided into four grades based on their postoperative pathological results (A/B/C1/C2). The GSRC corresponds to eCura B based on the eCura grade, indicating tumors less than 2 cm, without ulcer, negative on both margins, and with negative LVI, and are not recommended for additional surgery. However, eCura C patients with tumors over 2 cm, without ulcer, negative on both margins, and negative LVI require additional treatment strategy if the presence of DLS has been detected through immunohistochemistry. Furthermore, curative gastrectomy can be avoided in DLS-positive patients. Additional gastrectomy and lymph node dissection should be performed if the patients do not have the above conditions. Although Hatta et al[31] showed that the eCura system that was designed for risk stratification after ESD can guide EGC patients in choosing the appropriate treatment strategy after NCR, most patients have differentiated GC. However, a multi-center, prospective study is needed to verify the value of eCura system for UDC, especially early GSRC. Therefore, GSRC patients should make careful decision-making when using eCura system after NCR.
The indication of ER for undifferentiated carcinoma (UDC) is limited to tumors less than 2 cm, with ulceration (-), and without lymphovascular invasion due to the higher risk of LNM[2,32]. However, LNM risk varies in different subtypes of UDC. Early GSRC has a relatively lower risk of LNM than PDC. Lee et al[33] conducted a retrospective analysis of 1837 surgical resected UDC patients and showed that only 2.2% of SRC patients had LNM. No LNM was detected in GSRC patients with intramucosal tumors smaller than 1 cm. Jin et al[9] also found that GSRC patients have LNM similar to that of differentiated adenocarcinoma, but much lower than that of PDC patients. Therefore, the expansion of indication for early GSRC in endoscopic resection should be further discussed. Submucosal infiltration, tumor size, ulcer, and lymphovascular invasion are the common independent risk factors for LNM in GSRC patients[34].
Mixed GSRC is defined as GSRCs that contain less than 50% of the SRC component of the tumor. Notably, low risk of LNM does not apply to the mixed GSRC. Mixed GSRC is associated with increased invasiveness, increased risk of developing submucosal invasion, and larger tumors. Lee et al[33] showed that the LNM risk is higher (6.3%) in mixed-type GC than in both PDC and pure GSRC. Chen et al[35] divided mixed GC into four categories based on the predominant component of the tumor: differentiated-predominant type mixed with poorly differentiated component (MD-P), poorly differentiated-predominant type mixed with differentiated component (MP-D), differentiated-predominant type mixed with SRC component (MD-S), and poorly differentiated-predominant type mixed with SRC component (MP-S). The results showed that the LNM risk was higher in MP-S (24.5%) than in pure GSRC (11.3). The increased LNM risk is mainly due to the PD component of the tumor. The SRC component does not increase the LNM risk of mixed-type GC. The estimation of LNM risk and treatment strategies should be determined based on the predominant composition of mixed-type GSRC. The LMN risk is higher in MP-S than in other mixed types even if it is meeting the indication for ER, and thus should be carefully handled when deciding the treatment strategy.
The double-layer structure (DLS) of GSRC is closely related to LNM of GSRC. Takizawa et al[36] found that DLS serves as a protective factor against GSRC, indicating that its absence leads to an increased risk of submucosal invasion. Murai et al[11] showed that the absence of DLS, ulcers, tumors greater than 20 mm are independent risk factors for LNM in SRC. Yusuke Horiuchi et al. reviewed and analyzed the survival data of 1425 patients with undifferentiated cancer who underwent surgery and found that tumors > 40 mm is an independent risk factor of LNM. Besides, there was no risk of LNM in patients with tumors smaller than 40 mm, negative resected margins, without ulcer, and without LVI[23]. Notably, LNM was not detected in early intramucosal GSRC with DLS, even for tumors larger than 20 mm, indicating that the indications of ESD treatment could be expanded for patients with early GSRC. Besides, future studies should include DLS in the stratification of early GSRC patients before ESD and evaluation of ESD curability for a more personalized management strategy that improves the overall survival and quality of life.
The confirmative trial JCOG1009/1010 in Japan confirmed that the 5-year OS and RFS of patients with undifferentiated cancer after ESD resection are comparable to those of patients who received curative gastrectomy (98.6%, 98.5%)[36]. Ahn et al[37] conducted a retrospective study and showed that OS was not significantly different between 328 patients who underwent ESD and 383 patients who underwent surgery. Furthermore, the early GSRC patients had better OS than PDC patients. A propensity score-matched analysis compared the long-term outcomes between ESD and surgery in UDC patients and showed that OS is not significantly different between early GSRC patients undergoing ESD and those undergoing surgery. JGCA later changed the expanded indications into the absolute indications of ESD due to the satisfying prognostic outcome of patients who received ESD[35]. Therefore, ESD is a reliable and safe treatment for early GSRC patients who meets the expanded indications.
Early GSRC is characterized by unique biological behavior and favorable prognosis. It appears as flat or depressed lesions with discoloration under WLI, NM-NBI, and ME-NBI, which could further consolidate the diagnosis and delineation of the tumor. The depth of tumor invasion and LNM can be predicted using EUS. Pure GSRC lesions that meet the expanded indications of ESD are associated with a low risk of submucosal invasion and LNM, thus have desirable prognostic outcomes. As a result, ESD is the optimal treatment for such lesions instead of traditional surgical resection. However, tumor size may be underestimated due to the subepithelial spreading of SRC, thus limiting curative resection. Therefore, the preoperative biopsies in the surrounding mucosa of the lesion should be used to help determine the safe resection margin of the tumor. The eCura grade could serve as a reliable reference for the management of patients with non-curable resection after ESD. Particularly, early mixed GSRC or DLS-negative GSRC should be closely followed-up to prevent LNM and local recurrence. Additionally, ESD should be considered for tumors larger than 20 mm if an effective method can be developed to predict the presence of DLS before ESD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow WK, Taiwan; Ozden S, Turkey; Sano W, Japan S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 2. | Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1197] [Cited by in RCA: 1227] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 4. | Zhang C, Liu R, Zhang WH, Chen XZ, Liu K, Yang K, Chen XL, Zhao LY, Chen ZX, Zhou ZG, Hu JK. Difference Between Signet Ring Cell Gastric Cancers and Non-Signet Ring Cell Gastric Cancers: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:618477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Chen J, Zhou C, He M, Zhen Z, Wang J, Hu X. A Meta-Analysis And Systematic Review Of Accuracy Of Endoscopic Ultrasound For N Staging Of Gastric Cancers. Cancer Manag Res. 2019;11:8755-8764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 7. | Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Lee SH, Jee SR, Kim JH, Seol SY. Intramucosal gastric cancer: the rate of lymph node metastasis in signet ring cell carcinoma is as low as that in well-differentiated adenocarcinoma. Eur J Gastroenterol Hepatol. 2015;27:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Jin X, Wu W, Zhao J, Song S, Zhang C, Sun W, Lv B. Clinical Features and Risk Factors for Lymph Node Metastasis in Early Signet Ring Cell Gastric Cancer. Front Oncol. 2021;11:630675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Lee YM, Kang SH, Kim JS, Eun HS, Joo JS, Rou WS, Park JH, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY, Yeo MK, Song KS, Yoo HM. Subepithelial Spread of Early Gastric Signet Ring Cell Carcinoma: How Far They Can Reach? Dig Dis. 2020;38:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Murai K, Takizawa K, Shimoda T, Fujii S, Sugino T, Yoshida M, Kawata N, Tanaka M, Kakushima N, Terashima M, Ono H. Effect of double-layer structure in intramucosal gastric signet-ring cell carcinoma on lymph node metastasis: a retrospective, single-center study. Gastric Cancer. 2019;22:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Phalanusitthepha C, Grimes KL, Ikeda H, Sato H, Sato C, Hokierti C, Inoue H. Endoscopic features of early-stage signet-ring-cell carcinoma of the stomach. World J Gastrointest Endosc. 2015;7:741-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kim H, Kim JH, Lee YC, Kim H, Youn YH, Park H, Choi SH, Noh SH, Gotoda T. Growth Patterns of Signet Ring Cell Carcinoma of the Stomach for Endoscopic Resection. Gut Liver. 2015;9:720-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 15. | Ok KS, Kim GH, Park do Y, Lee HJ, Jeon HK, Baek DH, Lee BE, Song GA. Magnifying Endoscopy with Narrow Band Imaging of Early Gastric Cancer: Correlation with Histopathology and Mucin Phenotype. Gut Liver. 2016;10:532-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011;74:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 17. | Okada K, Fujisaki J, Kasuga A, Omae M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y, Tsuchida T, Nakajima A, Hoshino E, Igarashi M. Diagnosis of undifferentiated type early gastric cancers by magnification endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2011;26:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Watari J, Tomita T, Ikehara H, Taki M, Ogawa T, Yamasaki T, Kondo T, Toyoshima F, Sakurai J, Kono T, Tozawa K, Ohda Y, Oshima T, Fukui H, Hirota S, Miwa H. Diagnosis of small intramucosal signet ring cell carcinoma of the stomach by non-magnifying narrow-band imaging: A pilot study. World J Gastrointest Endosc. 2015;7:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kuroki K, Oka S, Tanaka S, Yorita N, Hata K, Kotachi T, Boda T, Arihiro K, Chayama K. Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer. 2021;24:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Pei Q, Wang L, Pan J, Ling T, Lv Y, Zou X. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol. 2015;30:1566-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Kim SJ, Lim CH, Lee BI. Accuracy of Endoscopic Ultrasonography for Determining the Depth of Invasion in Early Gastric Cancer. Turk J Gastroenterol. 2022;33:785-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Shiotsuki K, Takizawa K, Ono H. Indications of Endoscopic Submucosal Dissection for Undifferentiated Early Gastric Cancer: Current Status and Future Perspectives for Further Expansion. Digestion. 2022;103:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Horiuchi Y, Ida S, Yamamoto N, Nunobe S, Ishizuka N, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, Kumagai K, Ohashi M, Sano T, Fujisaki J. Feasibility of further expansion of the indications for endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Gastric Cancer. 2020;23:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Kim EH, Park JC, Song IJ, Kim YJ, Joh DH, Hahn KY, Lee YK, Kim HY, Chung H, Shin SK, Lee SK, Lee YC. Prediction model for non-curative resection of endoscopic submucosal dissection in patients with early gastric cancer. Gastrointest Endosc. 2017;85:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, Hyung WJ, Noh SH, Kim CB, Chung JB. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009;69:e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Bang CS, Park JM, Baik GH, Park JJ, Joo MK, Jang JY, Jeon SW, Choi SC, Sung JK, Cho KB. Therapeutic Outcomes of Endoscopic Resection of Early Gastric Cancer with Undifferentiated-Type Histology: A Korean ESD Registry Database Analysis. Clin Endosc. 2017;50:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Kim MN, Kim HK, Shim CN, Lee HJ, Lee H, Park JC, Shin SK, Lee SK, Lee YC. Tumour size is related to the curability of signet ring cell early gastric cancer with endoscopic submucosal dissection: a retrospective single centre study. Dig Liver Dis. 2014;46:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Kumarasinghe MP, Lim TK, Ooi CJ, Luman W, Tan SY, Koh M. Tubule neck dysplasia: precursor lesion of signet ring cell carcinoma and the immunohistochemical profile. Pathology. 2006;38:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Kim JS, Kang SH, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY. Accuracy of endoscopic size measurements of early gastric signet ring cell carcinoma. Surg Endosc. 2021;35:2324-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Nagano H, Ohyama S, Fukunaga T, Seto Y, Fujisaki J, Yamaguchi T, Yamamoto N, Kato Y, Yamaguchi A. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Nakaya N, Shimosegawa T. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? Gastric Cancer. 2018;21:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 33. | Lee IS, Lee S, Park YS, Gong CS, Yook JH, Kim BS. Applicability of endoscopic submucosal dissection for undifferentiated early gastric cancer: Mixed histology of poorly differentiated adenocarcinoma and signet ring cell carcinoma is a worse predictive factor of nodal metastasis. Surg Oncol. 2017;26:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Kim JY, Kim YY, Kim SJ, Park JC, Kwon YH, Jung MK, Kwon OK, Chung HY, Yu W, Park JY, Lee YK, Park SS, Jeon SW. Predictive factors for lymph node metastasis in signet ring cell gastric cancer and the feasibility of endoscopic submucosal dissection. J Gastric Cancer. 2013;13:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Chen JN, Wang QW, Zhang QW, Tang ZR, Li XB. Poorly differentiated is more significant than signet ring cell component for lymph node metastasis in mixed-type early gastric cancer: a retrospective study from a large-volume hospital. Surg Endosc. 2021;35:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M; Gastrointestinal Endoscopy Group (GIESG) and the Stomach Cancer Study Group (SCSG) of Japan Clinical Oncology Group. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer. 2021;24:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 37. | Ahn JY, Kim YI, Shin WG, Yang HJ, Nam SY, Min BH, Jang JY, Lim JH, Kim J-, Lee WS, Lee BE, Joo MK, Park JM, Lee HL, Gweon TG, Park MI, Choi J, Tae CH, Kim YW, Park B, Choi IIJ. Comparison between endoscopic submucosal resection and surgery for the curative resection of undifferentiated-type early gastric cancer within expanded indications: a nationwide multi-center study. Gastric Cancer. 2021;24:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |